In this study, we tested the potential of restored areas to maintain biodiversity in the scope of a recently proposed category of protected area called “Restoration Reserves”. To accomplish this, we compared bird richness and functional group structure of two small forest fragments (<250ha) with adjacent recently reforested areas (9 and 7 years of reforestation). Reforested areas had equal or higher bird richness and similar functional group structure. These results indicate that reforested areas are capable of maintaining current levels of biodiversity and reducing species extinction debt in small forest fragments, which is the main purpose of “Restoration Reserves”. However, when we compared a large forest fragment with an old adjacent reforested area (20 years of reforestation), we found that it was of limited value for certain functional groups. Therefore, “Restoration Reserves” could provide essential additional habitat in highly fragmented landscapes that consists mainly of small forest fragments.
Intensified human land use has resulted in landscapes consisting of several forest fragments, immersed in a matrix of urban and rural areas (Turner, 1990). Deforestation and forest fragmentation is so severe in the tropics that present landscapes are highly fragmented in small and isolated forest fragments (Melo et al., 2013). For example, 83.4% of remaining forest fragments in Brazil's Atlantic Forest are smaller than 50ha (Ribeiro et al., 2009). Because of this dramatic situation, Brancalion et al. (2013) recently advocated for the creation of a new category of protected area entitled “Restoration Reserves”, as a tool to increment natural forest cover and support biodiversity conservation. The idea behind “Restoration Reserves” is to combine both the protection and restoration efforts of small forest fragments in a landscape ecology perspective, with the aim of reducing species extinction debt (Brancalion et al., 2013). Since many species and populations have a delayed response in relation to environmental disturbances, such as habitat loss and degradation, future extinction of local population and species (i.e., species extinction debt) is expected (Tilman et al., 1994; Kuussaari et al., 2009). However, there is the possibility to reverse this trend if conservation actions, like habitat restoration are employed to try to increase both habitat availability and connectivity (Kuussaari et al., 2009; Brancalion et al., 2013). The restoration of areas next to forest fragments should reduce edge effects as well as provide additional habitat, which should result in an increase in population size for several species, reducing the chances of future extinction. A small number of cases have demonstrated that restored areas can indeed provide additional suitable habitat for forest species (Donner et al., 2010; Reid et al., 2014), but more empirical data is needed to support the idea that “Restoration Reserves” are capable of mitigating species extinction debt.
For bird species, habitat heterogeneity of primary forest is a strong predictor for the occurrence of species with different ecological requirements (MacArthur and MacArthur, 1961). In the Neotropics, the occurrence of understory and terrestrial insectivores birds is correlated with vegetation density (i.e., lianas, hedges and bushes) of the understory (e.g. Volpato et al., 2006; Stratford and Stouffer, 2013; Marques and Anjos, 2014). However, reforested areas generally lack variability in vegetation structure, particularly when reforestation was implemented recently (Donner et al., 2010). In this scenario, the importance of reforested areas for bird species with unique ecological requirements is unclear (Gibson et al., 2011), but there is an indication that reforested areas in Australia, with complex vegetation structure are able to maintain high richness of forest dependent bird species (Munro et al., 2011). In the Amazon, terrestrial insectivore forest birds are sensitive to habitat modification and forest fragmentation (Robinson, 1999; Stratford and Stouffer, 1999), being rarely found in secondary regenerated forests (Borges and Stouffer, 1999; Blake and Loiselle, 2001; Stratford and Stouffer, 2013). Moreover, some species with very specific ecological requirements are only found in regenerated areas after 30 years (Powell et al., 2013).
Considering that reforested areas contain only a subset of the original species because of differences in vegetation (structure, complexity and richness) and that species composition, at least for birds, generally changes with the age of the reforested area (Catterrall et al., 2012), we could consider that reforested areas work as a habitat filter. If this process of habitat filtering occurs in a non-random manner, it is possible to identify which ecological characteristics are sensitive to reforested areas (Mouillot et al., 2013), thus, providing important information for future conservation strategies. For example, if certain functional groups are lost or in low abundance (number of species) in reforested areas, active management strategies need to be developed to circumvent this loss. Birds are an interesting model group to study these aspects, because they play important ecological functions such as: seed dispersal, seed predation, pollination, predation (of animals), scavenging and some species are even considered to be ecosystem engineers (Whelan et al., 2008).
The aim of this study was to evaluate differences in bird richness and functional group structure between forest fragments and their adjacent reforestation areas, as well as how these differences are affected by the size of the forest fragment. We predict that reforested areas, next to small forest fragments, will harbour a higher proportion of the bird fauna of its adjacent forest fragments when compared with reforested areas that are next to a large pristine old growth forest fragment. If this is the case, it is an indication that: (1) “Restoration Reserves” could provide essential additional habitat that could reduce species extinction debt in highly fragmented landscapes that consist mainly of small forest fragments and (2) bird species of pristine old forest with specific ecological requirements would have limited potential do colonize “Restoration Reserves”. We also aim to evaluate which groups of species have a limited potential of being encountered in reforestation areas and discuss our results in the context of “Restoration Reserves” (Brancalion et al., 2013).
Material and methodsStudy areaWe selected three different areas of seasonal semidecidous forest in the north of Paraná that consist of a forest fragment and a neighbouring reforested area of native plant species (Fig. S1). The study areas were: Parque Estadual Mata dos Godoy (PEMG); Reserva do Patrimônio Particular Natural Matas do Cici (RPMC); and Fazenda Congonhas (FCON). The forests remnants are late successional and suffered limited timber extraction in the early 1980s.
PEMG (22K 475,143.87m E; 7,406,363.26m S; site PG) is located in the municipality of Londrina (PR) and has an area of 656ha inserted into a larger area of 2397.5ha. Adjacent to the park there is a reforested area of 20ha (site RG, Fig. S1), which was implemented in 1991 using the following native plants: Peltophorum dubium (Fabaceae – Caesalpinoideae), Parapiptadenia rigida (Fabaceae – Mimosoideae), Handroanthus impetiginosus (Bignoniaceae), Cordia trichotoma (Boragninaceae) and Colubrina glandulosa (Rhamnaceae) (J.D. Torezan pers. comm.). Even after over 20 years, this reforested area is in the initial phase of ecological succession, with the presence of several regenerating tree species in areas where the canopy is more closed, whereas in other areas of the reforested area the presence of the invasive grass Megathrsus maximus (Jacq.) dominates (Mantoani et al., 2012).
FCON (22K 480,790.30m E; 7,476,589.92m S) comprises of a forest fragment (site FC) of 104.8ha and an adjacent reforested area (site RC, Fig. S1) of approximately 13ha. Forest fragment FC suffered selective logging during the 1970s, but afterwards became a Legal Reserve. Reforestation was implemented in RC in 2002 using 67 species of native plants with the following predominant tree species: Guazuma ulmifolia (Malvaceae), Schinus terebinthifolius (Anacardiaceae), Heliocarpus popayanensis (Malvaceae), Cecropia pachystachya (Urticaceae) and Trema micrantha (Cannabaceae) (J.D. Torezan pers. comm.).
RPMC (22K 505,643.33m E; 7,456,061.65m S) contains a forest fragment (site FA) of 134.1ha and an adjacent reforestation area (site RA, Fig. S1) of approximately 11ha. Forest fragment FA also suffered from selective logging in the past and comprises of clearings and thick tangles of lianas. However, floristic composition of FA is similar to FC. RA was implanted in 2004 using 70 native plant species and is connected to the northern portion of FA (see Fig. S1). Plant species composition of RA is also similar to FA. See supplementary methods for more details on reforestation procedure, landscape metrics (Table S1) and climate of study area.
Bird sampling methodsPoint counts of limited distance were performed from September to December 2011, period in which birds are most conspicuous, thus, increasing the chances of detecting rare species (Esquivel and Peris, 2008). Sampling in forests fragments were conducted on pre-established research trails, while in reforested areas we took care to avoid as much as possible the edge of the fragment. For each study site, 12 point counts were spread out over 100m intervals. Each group of 12 point counts were considered as one study site. In each point count, individuals belonging to the same species were counted, taking care to sample the same individual only once (Anjos et al., 2010). Sampling started just after sunrise, time period in which birds are most active (Esquivel and Peris, 2008), and each point count was sampled for 15min, which is sufficient time to detect most birds in tropical regions (Cavarzere et al., 2013).
Sampling took place during six different days for each study site. Therefore, the total number of point counts for each area was 72, giving a total of 432-point counts for the six different sites. Point counts were performed by PCSJ. Bird identification was aided by binoculars (Nikon Monarch 10.5mm×45mm) and a sound recorder (Sony ICD-SX712). Recorded vocalizations were compared with the sound collection from Universidade Estadual de Londrina (Laboratório de Ornitologia e Bioacústica).
Determination of functional groupsBird taxonomy followed American Ornithologists’ Union (Remsen et al., 2014). Each bird species was categorized into a functional group according to the literature (Table S2) and field observations. Diet and foraging stratum were used to define functional groups, but we also used weight for frugivores (Dunning, 2008). The following categories were used: bamboo insectivores (BIN), carnivores (CAR), canopy insectivores (CIN), edge granivores (EGR), large frugivores (mass >80.1g; LFR), small frugivores (mass ≤80.1g; SFR), leaf insectivores (LIN), nectarivores/insectivores (NEC), nocturnal insectivores (NOI), omnivores/insectivores (OIN), omnivores (OMN), terrestrial granivores (TGR), terrestrial insectivores (TIN), trunk and twig insectivores (TTI) and understory insectivores (UIN).
Statistical analysisRarefication curves were obtained by plotting the randomized bird richness against the sampling sites (point counts). Randomized richness was obtained by bootstrap using the package “rich” in R (Rossi, 2011). We also used the “rich” package to test for differences in bird richness among study sites using the function “c2cv”. The “c2cv” function compares the observed difference in species richness to differences in richness obtained after randomizing samples between communities. Randomization was done 1000 times. The observed difference in bird richness was compared to the quantiles of the corresponding randomized values of a 0.05% probability level (i.e., quantiles 0.025 and 0.975; see Rossi, 2011 for more details). For this analysis, all forests fragments were compared with PG. Comparisons were also made between the small forests fragments (FA and FC) and their respective adjacent reforested areas (RA and RC). We also show the difference in bird species per habitat preference type. To test if reforested areas would group with their respective forest fragment, we used cluster analysis with a single linkage agglomerative clustering (Boccard et al., 2011), using Jaccard dissimilarity index as pairwise distances among study sites. Functional group structure was analyzed using a principal component analysis, which used the number of species of each functional group present in each point count sample. This allowed the visualization of changes in the composition of functional groups.
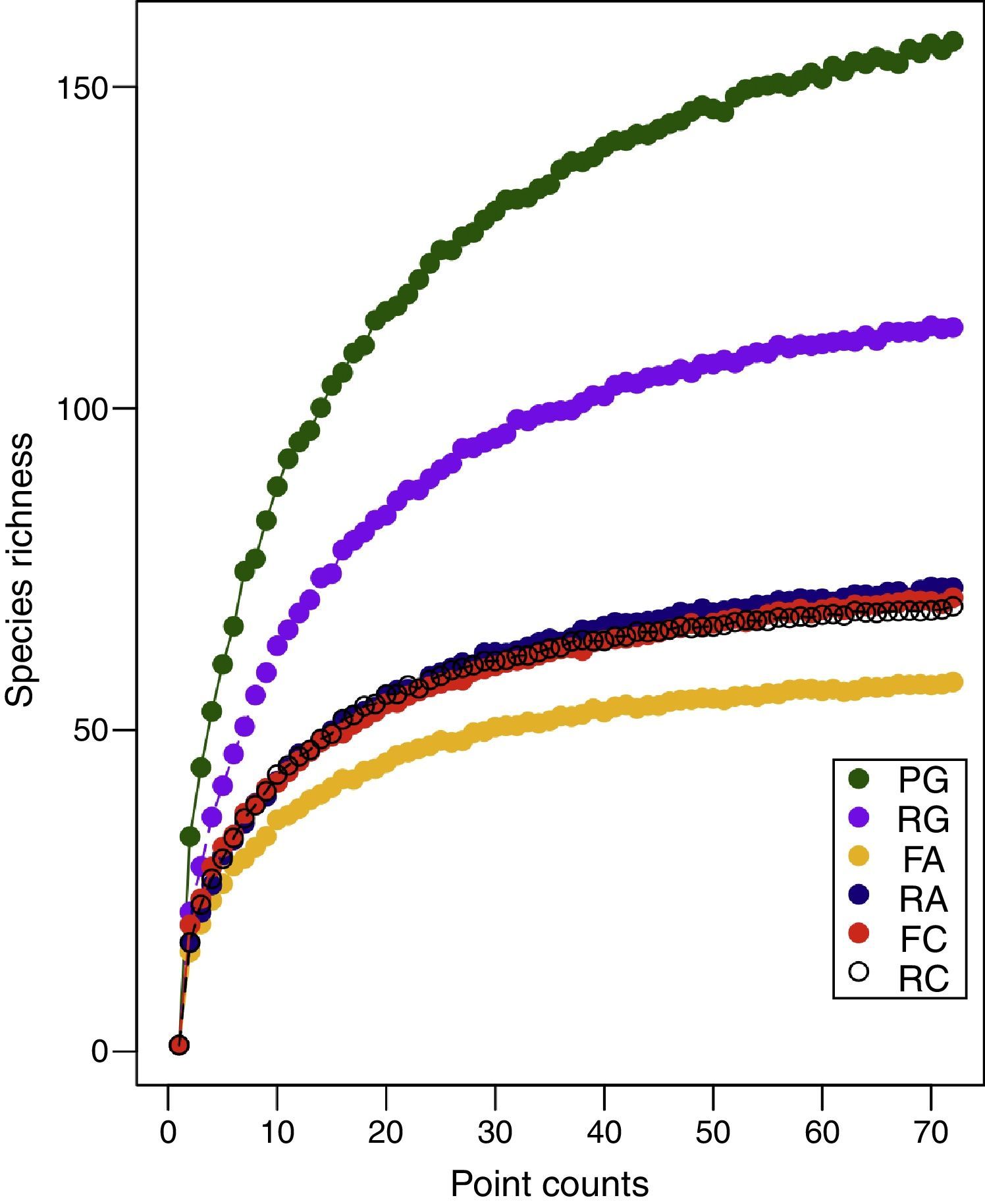
ResultsA total of 225 bird species were recorded for the six study sites (Table S2). Rarefaction curve analysis indicated that forest fragment FA had a significant lower number of bird species when compared with its adjacent reforested area RA, however, values found for FA were similar to those found in FC and reforested area RC (Fig. 1 and Table 1). PG had the highest number of species followed by its adjacent reforested area (Fig. 1). Indeed, permutation analysis indicated that PG had a significant higher number of bird species when compared with all other study sites (Table 1).
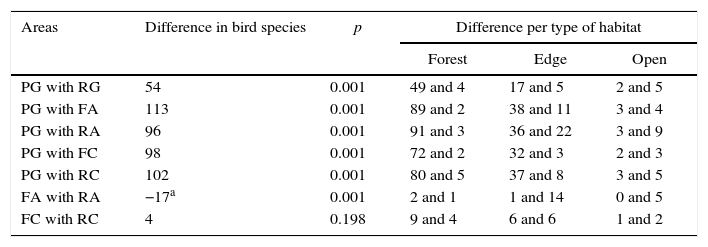
Permutation analysis of bird richness between Parque Estadual da Mata dos Godoy (PG) with its adjoining reforestation area (RG), forest fragments (FA and FC) and reforestation areas (RA and RC). Comparisons between forest fragments (FA and FC) and their respective adjoining reforestation areas (RA and RC). Differences in bird species per preferred habitat type are the number of bird species present in area one (i.e., PG) and not in area two (i.e., RG), as well as the number of bird species present in area two (i.e., RG) and not in area one (i.e. PG). Therefore, to get the total difference one must add all differences of area one, all the differences of area two and subtract them (for example, in the case of PG with RG: (49+17+2)−(4+5+5)=54).
| Areas | Difference in bird species | p | Difference per type of habitat | ||
|---|---|---|---|---|---|
| Forest | Edge | Open | |||
| PG with RG | 54 | 0.001 | 49 and 4 | 17 and 5 | 2 and 5 |
| PG with FA | 113 | 0.001 | 89 and 2 | 38 and 11 | 3 and 4 |
| PG with RA | 96 | 0.001 | 91 and 3 | 36 and 22 | 3 and 9 |
| PG with FC | 98 | 0.001 | 72 and 2 | 32 and 3 | 2 and 3 |
| PG with RC | 102 | 0.001 | 80 and 5 | 37 and 8 | 3 and 5 |
| FA with RA | −17a | 0.001 | 2 and 1 | 1 and 14 | 0 and 5 |
| FC with RC | 4 | 0.198 | 9 and 4 | 6 and 6 | 1 and 2 |
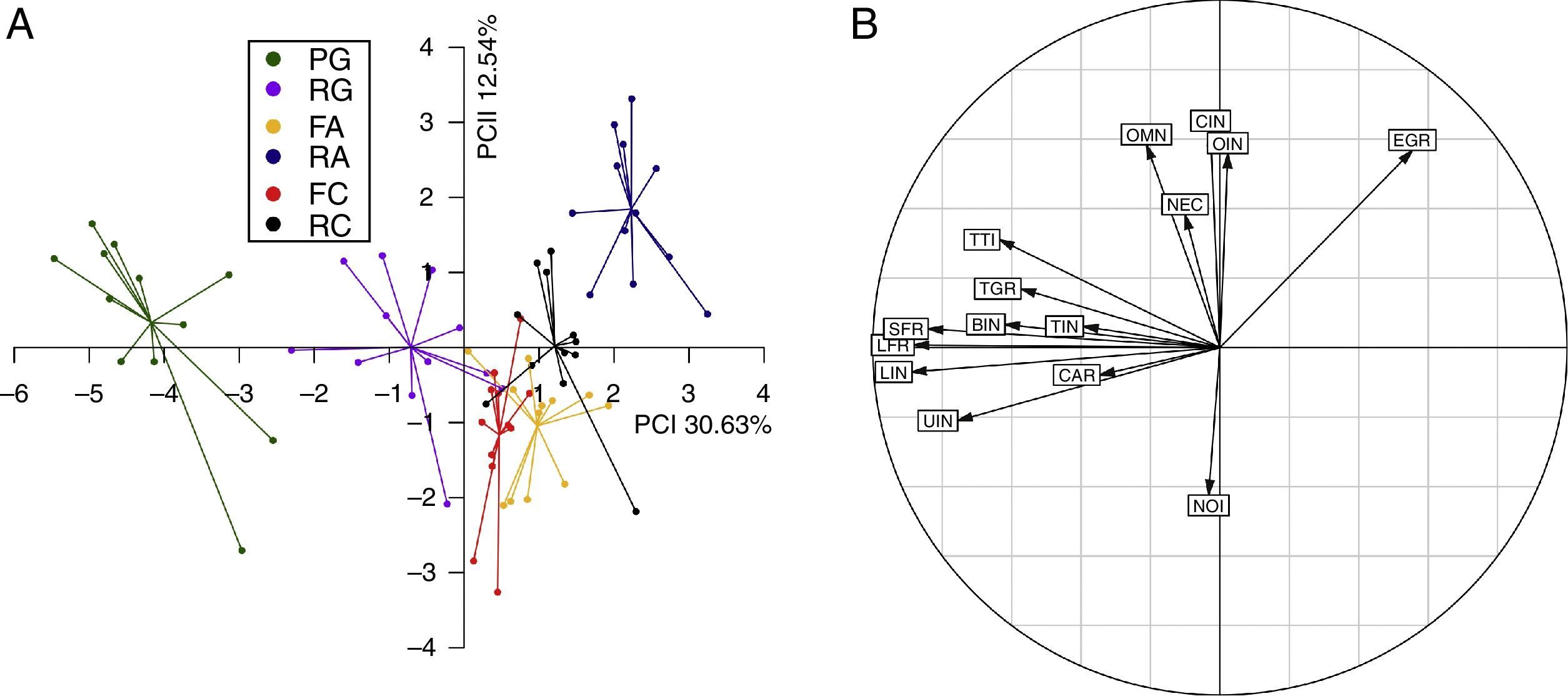
In all three studied cases the reforested areas were grouped with their respective forest fragments (Fig. 2), which indicates that birds from local forest fragments are capable of colonizing adjacent reforested areas. Principal component analysis revealed two important axis of functional group structure that jointly explained 43.17% of the variance (Fig. 3 and Table S3). Negative values in axis one are associated with sites that have a higher number of species belonging to the following functional groups: large frugivores, small frugivores, leaf insectivorous and understory insectivores; while positive values in axis one was associated with sites having a higher number of bird species belonging to the functional group edge granivores. Negative values in axis two were associated with sites having a higher number of nocturnal insectivores species, while positive values were associated with sites that have a higher number of omnivores/insectivores, omnivores species, canopy insectivores and edge granivores (Fig. 3B and Table S2). Areas FC, RC, FA and RA lacked any species representing the functional groups “bamboo insectivores” and “terrestrial insectivores” (Table S2), which were represented by few species found in sites PG and RG (Table S2).
(A) Principal component analysis of the structure of bird functional groups for the three forest fragments (PG, FA and FC) and their respective adjacent reforested areas (RG, RA and RC). (B) The correlation circle indicates the importance of each functional group on the first and second principal component axis, which jointly explained 43.17% of the variation. Functional groups are coded as: bamboo insectivores (BIN), carnivores (CAR), canopy insectivores (CIN), edge granivores (EGR), large frugivores (mass >80.1g; LFR), small frugivores (mass ≤80.1g; SFR), leaf insectivores (LIN), nectarivores/insectivores (NEC), nocturnal insectivores (NOI), omnivores/insectivores (OIN), omnivores (OMN), terrestrial granivores (TGR), terrestrial insectivores (TIN), trunk and twig insectivores (TTI) and understory insectivores (UIN).
Because most Atlantic forest fragments are smaller than 250ha (Ribeiro et al., 2009), our study sites represent a realistic scenario. If we consider bird richness, reforested areas adjacent to small forest fragments had equal (RC) or higher (RA) bird richness. Functional group structure was also similar. However, Jaccard dissimilarity indexes were higher than 0.5 among forest fragments and neighbouring reforested areas, which indicate that not all bird species were able to recolonize reforested areas. These results suggest that reforested areas were capable of increasing habitat availability, but only for a specific selection of bird species. Reforested areas usually have a lower number of bird species that prefer forest habitats and recolonizing bird species tend to be opportunists, as well as generalist species (Critescu et al., 2012). Supporting this idea, our data shows that reforested areas had a higher number of species that preferred either open or edge habitats (Table 1). One of the ideas of “Restoration Reserves” is to mitigate species extinction debt by increasing habitat availability (Brancalion et al., 2013). However, our data suggest that only a subset of local species will have the potential of increasing their population size with the additional area provided by reforestation. It is important to emphasize that reforested areas RC and RA were implemented recently (<10 years), and bird composition changes with reforestation age (Catterrall et al., 2012). Therefore, it would be interesting to monitor these sites to evaluate if other species (i.e., forest dependent species) will be able to colonize these reforested areas in the near future.
In general, there is a lack of studies on the recovery of wildlife in reforested areas (Block et al., 2011), possibly because it is assumed that if the flora is re-established then wildlife will return to the reforested areas (Thompson and Thompson, 2004). However, animals provide important ecosystem function and if Restoration Reserves are to be implemented to reduce habitat loss and improve biodiversity, reforested areas also need to provide appropriate habitat to native fauna. Birds tend to respond well to reforestation and reforested areas will usually present similar bird richness to reference forest areas, however, bird composition will usually be different (Munro et al., 2011; Catterrall et al., 2012; Freeman et al., 2015). In contrast, other animal taxa have more difficulty in recolonizing reforested areas. Data on recovered mining areas in Australia show that mammals, reptiles, amphibians and arthropods tend to have lower species richness and abundance in reforested areas (reviewed in Critescu et al., 2012). In the Atlantic Forest, ant richness was similar between reforested sites and a secondary forest site, but ant composition differed because colonization sources of reforested areas were from nearby agro-ecosystems (Gomes et al., 2014). Overall, fauna composition of reforested areas is not very similar prior to the disturbance in the area, mainly because several forest specialists are usually absent. A possible reason could be the fact that specific microhabitats may take time to develop in reforested areas (Stanturf et al., 2014). Therefore, Restoration Reserves on its own may have some difficulty in restoring ecosystem function. However, active management of the area could aid the recolonization of habitat specialists by manually adding the unavailable microhabitats (Christie et al., 2013). These microhabitats could be artificially built, such as nest boxes to guarantee recolonization of cavity nesting species, or the simple introduction of microhabitat, such as hollow logs, wood debris and rocks. For example, after the introduction of small piles of woody debris in reforested sites, the reptile species Napoleon's skink (Egernia napoleonis) managed to colonize reforested sites in Jarrah forests in south-western Australia (Christie et al., 2013). The addition of nest boxes in both logged and primary subtropical Atlantic Forest resulted in an increase of nesting density (Cockle et al., 2010), thus, indicating that this procedure could be an important tool in increasing the presence of cavity nesting species in reforested areas. However, active management will not always help restore the local fauna, as was the case with bats in Costa Rica, where the introduction of artificial bat roosts in pasture land had a small effect in forest regeneration because bats rarely visited the roosts (Reid et al., 2013).
In the case of the larger studied forest fragment, PG and its adjacent restored area RG, we found that RG had a lower number of bird species and differed regarding bird functional group structure. However, RG was the reforested area that most resembled PG (Fig. 3A). This result was expected because RG was adjacent to PG and also because reforestation in this study site began in 1991. Moreover, it is unreasonable to expect RC and RA to be similar to PG because of the age of reforested sites (<10 years), distance to PG and size of adjacent forest fragments. A more correct form of evaluation is to compare each reforested area with its neighbouring forest fragment. In this case, our data indicate that reforested sites were capable of maintaining good levels of current bird biodiversity and functional structure when reforested areas neighboured small forest fragments. But reforestation seemed less efficient when reforested site was next to a large pristine old growth forest, mainly because RG had a lower number of bird species that preferred forest habitats. This reinforces the case that appropriate microhabitat for forest dependent species takes time to develop (Stanturf et al., 2014) and appropriate managing techniques need to be implemented if reforested sites are to increase habitat availability for forest dependent species, even when reforestation site is close to a well-preserved large forest fragment.
Small forest fragments (FA and FC) and restoration areas (RG, RA and RC) had a low number of species that belonged to the following functional groups: frugivores, leaf insectivores and understory insectivores. A necessary requirement for both the insectivores groups is the presence of vegetation structure, such as high density of understory vegetation and leaf litter (e.g. Volpato et al., 2006; Stratford and Stouffer, 2013; Marques and Anjos, 2014). In the case of frugivores, reforested areas probably had lower diversity of tree species that should translate into low availability of fruit resources throughout the year. Moreover, cavity nesting species such as woodpeckers and parrots usually require large trees for nesting, which were probably missing in reforested areas and small forest fragments. Therefore, with the exception of site PG, all studied sites lacked the necessary vegetation structure and plant diversity to sustain species of these three functional groups. We suggest that restoration strategies should be improved to increase the potential colonization of bird species that belong to these three functional groups. For example, active management to increase understory vegetation density to attract bird species belonging to the two insectivore functional groups and planting of specific fruit trees to attract frugivores, or possibly the use of nest boxes to attract large frugivores. Terrestrial insectivore and bamboo insectivore species were missing in forest fragments FA and FC and their neighbouring reforested areas. However, even in PG only a few number of species that belong to these groups occur there, all of which have small population sizes (Volpato et al., 2006). The reason being that terrestrial insectivores and bamboo insectivores are very sensitive and require very specific microhabitats that will rarely be present in small forest fragments and reforested areas.
Our data suggest that reforested areas were important for the maintenance of bird diversity in highly fragmented landscapes containing small forest fragments (i.e., <250ha), because reforested areas provided appropriate habitat for several bird species. However, bird species that preferred forest habitats were less frequently encountered in reforested sites, even when a large forest fragment was next to the reforested site. An important finding was that bird functional structure was similar between small forest fragments and their neighbouring reforested areas, even though reforested areas were very young (<10 years). This indicates that restoration can be an effective method of increasing habitat availability for birds. We advocate for the use of “Restoration Reserves” as an important conservation strategy in landscapes that are dominated by small forest fragments. However, because of our limited sample size and use of only one animal group, more studies are needed that incorporate a larger number of fragments, larger spectrum of fragment size, connectivity among fragments and reforested sites, and different taxa (e.g. other vertebrates as well as invertebrates).
Conflicts of interestThe authors declare no conflicts of interest.
CNPq supported PCSJ and FCM. MRL was awarded a post-doc fellowship from CAPES (PNPD 2013/2257). CNPq provided a research grant to LdA (302694/2010-2). We thank two anonymous reviewers and JP Metzger for valuables comments on a previous version of this paper and CRL for English and style revision of this manuscript. We thank Instituto Ambiental do Paraná (IAP) for conceding access to Parque Estadual Mata dos Godoy (study area PG and RG; permit n° 254/10) and also to the proprietors of Fazenda Congonhas (study area FC and RC) and Reserva do Patrimônio Particular Natural Matas do Cici (study area FA and RA) that allowed us to carry out our study in their land. We thank T Zaiden and ALBoesing for field assistance.