Using a long-term dataset, we tested whether beta diversity of the zooplankton community in the Upper Paraná River floodplain increases during periods of high environmental heterogeneity and productivity and decreases with increases in water level (when there is higher connectivity between sites). We detected temporal trends of increasing beta diversity. A temporal decrease in species occupancy was more frequent than a temporal increase. Our results do not support a generalized association between beta diversity and environmental heterogeneity or productivity. Water level variation was an important explanatory variable only for rotifers and in the opposite direction expected. Taken as whole, our results suggest that we are far from a comprehensive understanding of the processes underlying variation in beta diversity. We speculate that the large number of dams built upstream from the study area may account for the positive trend in beta diversity.
The search for biodiversity patterns across different spatial and temporal scales and for the mechanisms behind these patterns is a central goal in ecology. In this context, an ever-growing number of studies have focused on β-diversity patterns (Anderson et al., 2011). However, few studies have modeled temporal changes in β-diversity (see review in McGill et al., 2015). This scarcity is related to financial and logistical constraints involved in conducting long-term studies at multiple sites with standardized methods (Magurran et al., 2010).
Studies of β-diversity over time may be based on at least two approaches. The first considers changes in species composition between different periods (similarity decay in time; Korhonen et al., 2010). The second considers the magnitude of changes in species composition between sites over time. Therefore, for this latter approach, data must be obtained from n sites and t times (Langenheder et al., 2012; Bonecker et al., 2013; Dornelas et al., 2014).
Changes in species composition are driven by local extinctions, disturbances, species colonization/invasion and environmental variability. The relative importance of these processes may change over time. Thus, the proximate cause of temporal variation in β-diversity can be related to the levels of synchronicity of the processes cited above. For example, the invasion of the same species in pairs of local communities with different species compositions would decrease the β-diversity between sites from time t (before invasion) to time t+1 (after invasion). Similarly, local extinctions of different rare species would also decrease β-diversity between pairs of local communities. In contrast, the colonization of different species at each site would increase β-diversity. These processes are also dependent on the level of connectivity between local communities and on the dispersal ability of the species. For example, a decrease in β-diversity would occur in periods of high connectivity between sites due to the increase in dispersal rates (Gonzalez, 2009).
An increase in β-diversity with primary productivity has been detected. For instance, Chase (2010) found a positive relationship between β-diversity and productivity and attributed it to an increased role of stochastic assembly processes (e.g., dispersal limitation, ecological drift), when compared to deterministic processes (e.g., species sorting and priority effect), in more productive environments. Other empirical evidence suggests that environmental heterogeneity is positively correlated with β-diversity (Veech and Crist, 2007). Sites presenting higher rather than lower environmental heterogeneity increase the probability that different species from the regional pool find suitable conditions according to their environmental requirements.
Floods markedly affect the levels of connectivity between the habitats of floodplain systems. An increase in hydrological connectivity occurs during flood periods, reducing environmental heterogeneity and β-diversity. In contrast, during low water seasons (when floodplain habitats are more isolated), local driving forces cause an increase in environmental and biological heterogeneity (Thomaz et al., 2007). For instance, while a small lagoon may be drying out, another may contain a high density of predatory fish and a third may have a high rate of deoxygenation. Thus, it is expected a more idiosyncratic dynamic of the environmental characteristics and structure of local communities, increasing the environmental and biological heterogeneity of the system. However, an increased number of hydroelectric dams upstream the floodplain systems may be disrupting these patterns.
In this study, we examined long-term data (2000–2011) on zooplankton communities from 12 sites in the Upper Paraná River floodplain (Brazil) to evaluate the following predictions: zooplankton β-diversity (spatial variation in species composition) should be positively correlated with environmental heterogeneity and primary production and negatively correlated with water level.
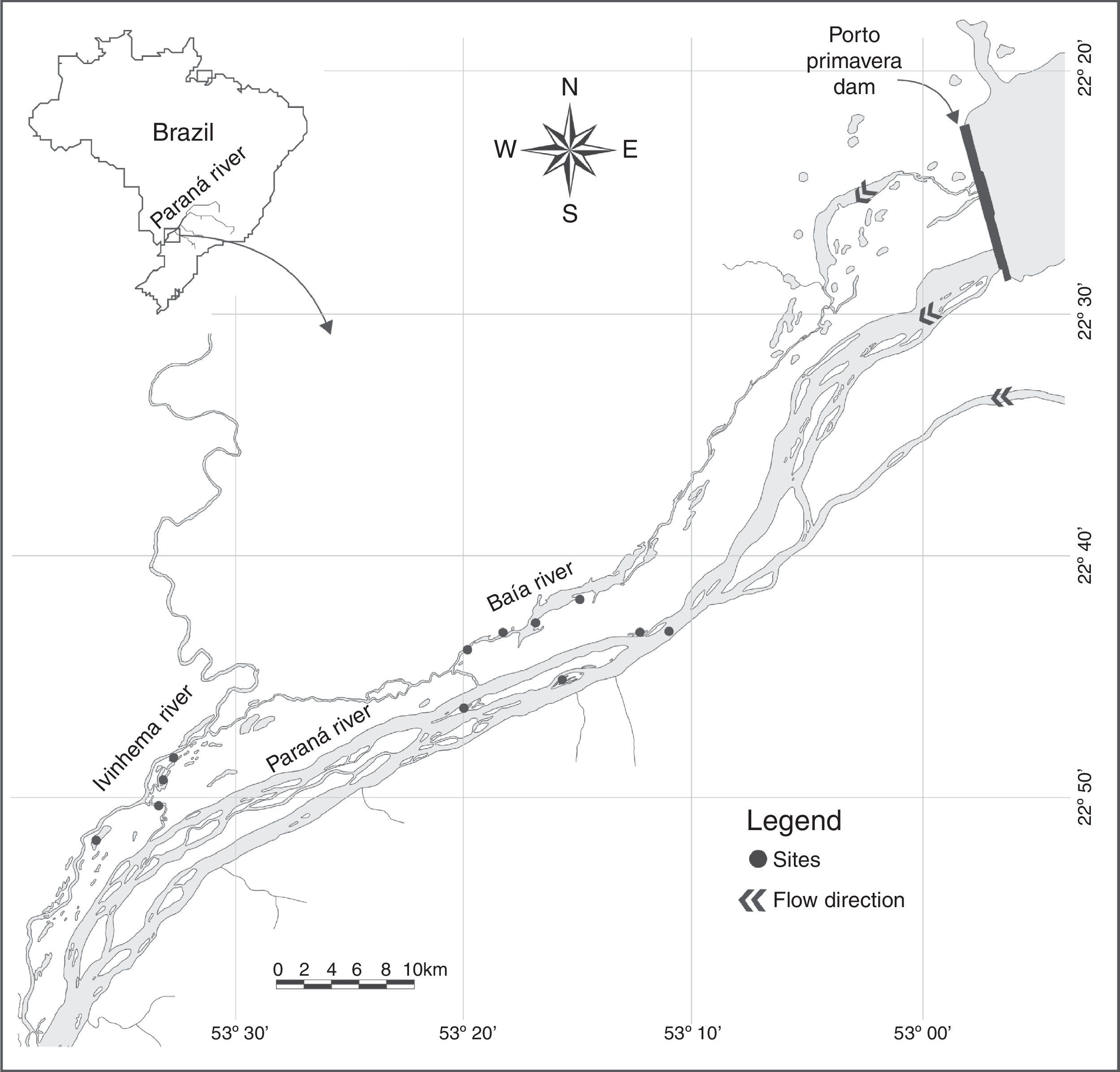
Materials and methodsOur sampling sites were distributed over the Upper Paraná River floodplain, which represents the last area without dams in the Brazilian portion of the Paraná River Basin (Fig. 1). Samples were collected during 12 years (from 2000 to 2011) from 12 permanent sites. Standardized fieldwork was performed every three months except in 2001, when samples were collected only twice. Zooplankton samples were collected in the limnetic zone of each site by filtering 600L of water through a plankton net of 68μm mesh size. The samples were preserved in formaldehyde (4%, buffered with calcium carbonate), microscopically counted and identified in the laboratory following Bottrell et al. (1976).
Water level in the Paraná River (Porto Rico Municipality) was measured daily with a meter stick. We also measured the following environmental variables according to APHA (2005): water temperature, dissolved oxygen, pH, conductivity, water transparency, turbidity, total suspended solids, total alkalinity, chlorophyll-a, total nitrogen and total phosphorus.
Statistical analysisWe estimated β-diversity by calculating the Simpson multiple-site dissimilarity index (βSIM) at each time t (Baselga, 2013). βSIM was calculated for the whole zooplankton community and separately for rotifers and microcrustaceans, given the differences in the life histories between these broad taxonomic groups (e.g., parthenogenesis in the case of rotifers). In turn, these differences may account for different patterns of β-diversity.
To model the temporal variation in β-diversity (i.e., βSIM for the whole zooplankton community, rotifers and microcrustaceans), we used a Generalized Least Squares (GLS) model incorporating an autoregressive structure (Zuur et al., 2009). The response variable was β-diversity (βSIM as estimated for total zooplankton, rotifers and microcrustaceans), and it was modeled as a function of mean water level (considering the mean values estimated with data from 10, 20, 30, 40 and 50 days before sampling), mean chlorophyll-a concentration, mean total phosphorus (total P) concentration (both as proxies for primary production) and environmental heterogeneity. Thomaz et al. (2007) show that there are time lags between hydrological variation and is effects on biological communities. This was the reason for estimating mean water level according to different temporal windows. Our first measure of environmental heterogeneity (dc) consisted of the average dissimilarity between individual sampling sites and their group centroid (as defined by the sampling dates; Anderson et al., 2006). Second, for each variable, we calculated the coefficient of variation over the sites. The resultant coefficients of variation were then averaged for each month (cv).
The set of candidate models (20 in total) included time, one measure of water level (considering different time lags: 10, 20, 30, 40 or 50 days before the sampling), one proxy for primary production (chlorophyll-a or total P concentrations) and one measure of environmental heterogeneity (dc or cv). We used Akaike's Information Criterion (AIC), AIC differences (delta AIC) over all candidate models in the set and Akaike's weight of each model (wi) to select the best approximating model for our data (Burnham and Anderson, 1998). To further explore the proximate causes of temporal patterns in community structure, we used the Mann–Kendall test to evaluate trends in species richness and in the “occupancy” of each species (i.e., the number of sites occupied in a given month) that occurred in more than 4 months. All statistical analyses were performed in R (R Core Team, 2013).
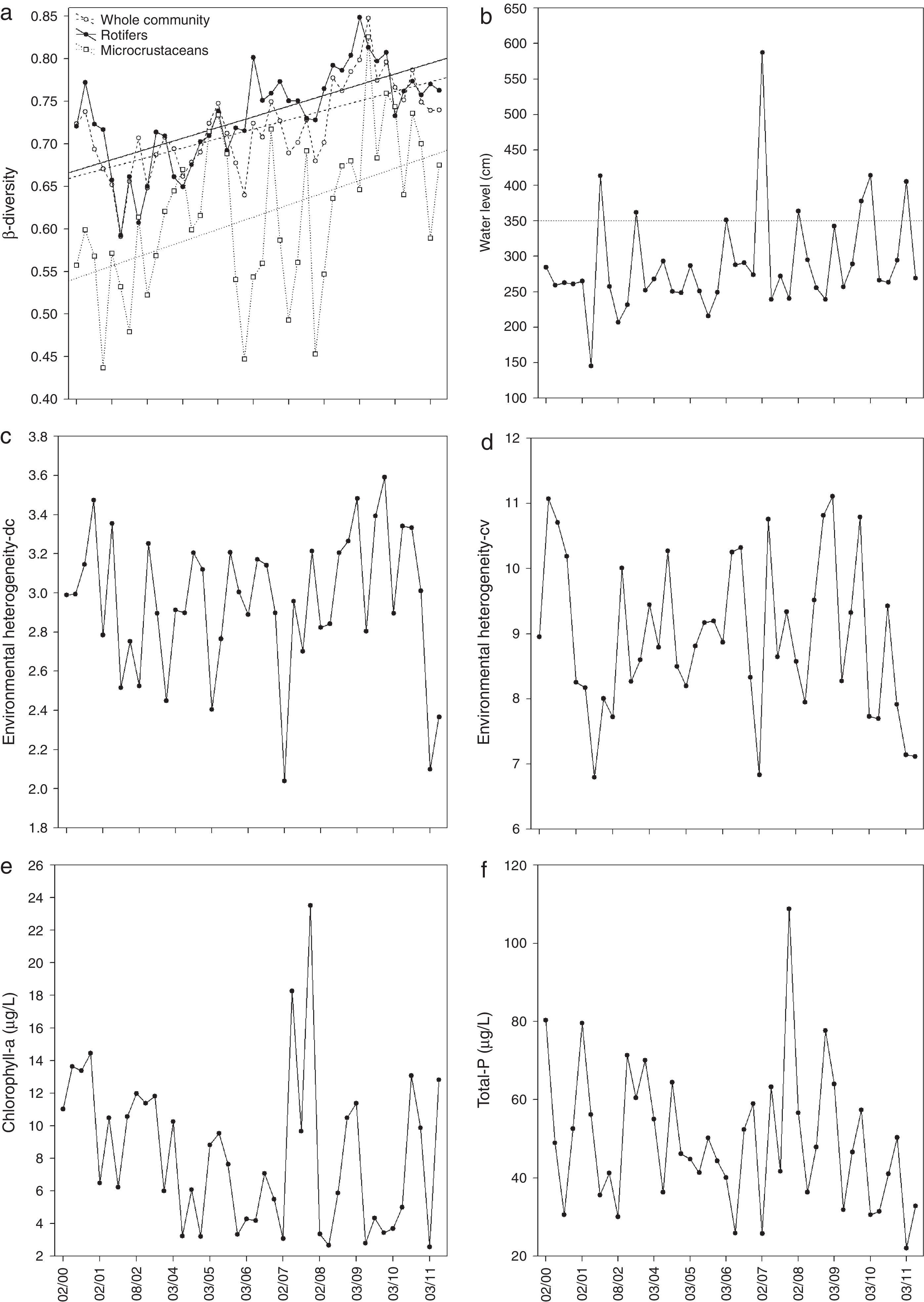
ResultsWe found wide variation in most of the variables (Table S1). For example, as assessed by total phosphorus, trophic status ranged from oligotrophic to hypereutrophic. Sites and months were also conspicuously different, for instance, in terms of water transparency, pH and chlorophyll-a (Table S1). Monthly mean species richness ranged from 14.6 to 54.5 for the whole community, from 8.2 to 36.2 for rotifers and from 5.7 to 17.4 for microcrustaceans. We also found a large variation in our response and explanatory variables (Fig. 2).
Time series of β-diversity and of the explanatory variables included in the regression models. (a) β-Diversity was estimated separately for the whole zooplankton community, rotifers and microcrustaceans; (b) water level (the horizontal dashed line indicates the maximum water level before flooding); (c) environmental heterogeneity (as estimated by Anderson et al., 2006); (d) environmental heterogeneity (based on the coefficient of variation); (e) mean concentration of chlorophyll-a; (f) mean concentration of total phosphorus.
The best approximating model for β-diversity of the whole community, included the following variables: water level (average of 40 days preceding sampling), environmental heterogeneity (dc) and chlorophyll-a. The next-best models were very similar to the best model, differing only in terms of the period window used to calculate water level (Table S2). The effect sizes (regression coefficient/standard error) suggest that time was the main predictor of β-diversity, while the other variables have low effect sizes (Table S3). Results for microcrustaceans were similar to those of the whole community and, in addition to the high uncertainty in model selection, the effect sizes of the best-ranked models indicated that time was the most important variable in predicting β-diversity (Tables S4 and S6). For rotifers, the effect sizes of the best-ranked models (Table S6) suggested an increase in β-diversity with time, water level (average of the 50 days preceding sampling) and environmental heterogeneity (cv; Table S7). In all models, the effect sizes of chlorophyll-a and total P were negligible.
Despite the large variation, mean species richness was found to decrease over time (Kendall's correlation coefficients for the whole community, rotifers and microcrustaceans=−0.35, P=0.001; −0.39, P<0.001; and −0.21, P<0.001, respectively). From the 190 species analyzed (i.e., those occurring in more than 4 months), we found that: 130 species showed negative trends in occupancy; from these, 46 species (13 microcrustaceans and 33 rotifers) showed significant negative trends; and only 60 species showed positive trends; from these, 14 species (7 of each group) showed significant positive trends (Fig. S1).
DiscussionOur results for the whole zooplankton community and for microcrustaceans do not support an association between β-diversity and environmental heterogeneity (for similar results see Heino et al., 2013; Bini et al., 2014). This result could be due to the omission of key environmental variables, which would result in an inadequate measure of environmental heterogeneity (Heino et al., 2013). However, we doubt this explanation because we measured a number of variables thought to be influential in structuring zooplankton communities. A low environmental variability as an explanation is also unlikely because we found that the monthly coefficients of variation ranged widely (Fig. 2). In short, despite being expected, a positive relationship between β-diversity and environmental heterogeneity has not gained indisputable empirical support.
Also, we did not detect the expected relationship between β-diversity and chlorophyll-a (a proxy for primary production; see Chase, 2010). According to monthly average chlorophyll-a concentration, trophic state in the study area ranged from oligotrophic to mesotrophic. Thus, missing the final part of the trophic gradient (i.e., eutrophic conditions) might explain the lack of relationship between β-diversity and productivity. To further examine this possibility, we used total P concentration, in place of total chlorophyll-a, in our models. Total P concentrations ranged from 22μg/L to 109μg/L (Fig. 2f). Therefore, the trophic status gradient was much wider according to this indicator. However, the empirical support for the relationship between total P and β-diversity was even lower than that for the relationship with chlorophyll-a (for a contrasting result, see Langenheder et al., 2012). In other studies, the relationship between β-diversity and productivity was hump-shaped (Chalcraft et al., 2004) or negative (Astorga et al., 2014). We then contend that even the form of the relationship between β-diversity and productivity is debatable and likely scale-dependent, which is similar to the uncertainties regarding the species richness-productivity relationship (Whittaker, 2010).
Despite the recognized importance of water level in the control of several ecological patterns (e.g., species richness and dominance) and biogeochemical processes (e.g., decomposition rates) in floodplain systems (Junk et al., 1989; Thomaz et al., 2007), we did not detect a substantial correlation between water level and β-diversity (except for rotifers, but in a direction opposite to the one expected). Thus, again, our results cast doubt on some common predictions, at least in terms of temporal variation in β-diversity.
One could argue that high levels of spatial autocorrelation would decrease our ability to detect strong relationships with the environmental data due to the lack of variability in the response variable. Low beta diversity would then result due to the proximity between the sites. Although our study covers a spatial extent of ca. 41km, this possibility is worth testing because plankton patch size varies widely and the different samples that we took may belong to the same community. Thus, for each month, we calculated a pairwise (between-sites) Simpson dissimilarity matrix and evaluated this possibility. The results suggest, however, that the lack of variability was not a problem in our study (Fig. S2). Also, and surprisingly, in most months (36, 33 and 39 out of 42 months, for the whole community, rotifers and microcrustaceans, respectively), the relationship between pairwise dissimilarities and geographic distances was not significant, indicating negligible levels of spatial autocorrelation (Fig. S3).
Surprisingly, given the (few) evidences available (McGill et al., 2015), we found a positive temporal trend in β-diversity. Hence, as time passed, the compositional dissimilarities between pairs of sites increased. To some extent, a temporal increase in β-diversity parallels other studies showing a temporal decrease in the predictability of community structure (Bengtsson et al., 1997). We can offer at least three non-exclusive explanations for the temporal increase in β-diversity in the floodplain of the Upper Paraná River. First, changes in sampling methods and taxonomic determinations may be important in accounting for trends in biodiversity patterns (Straile et al., 2013). We believe that this explanation is unlikely in our case because the same group of researchers was responsible for sampling, counting and taxonomic work over the study period. Second, an increase in β-diversity could be accounted for by the arrival of species from the upstream reservoirs (Bovo-Scomparin et al., 2013). However, this hypothesis is also unlikely because average species richness decreased over time. As a third explanation, we may speculate that this trend is related to the cumulative impacts caused by the construction of dams in the upstream reaches of the study area. Indeed, several dams have been built in this region during the past 60 years. However, the construction of Porto Primavera Reservoir in the late 90s was crucial for increasing the control of the river flow (Stevaux et al., 2009; see results from a interrupted time series analysis of water level in Table S8 and Fig. S4 and S5). As a result, we believe that the following scenario can be hypothesized: after water level regulation (especially following the construction of the Porto Primavera Reservoir), temporal variation in local species composition became more idiosyncratic as the large floods, which previously tended to homogenize species compositions (i.e., decreasing β-diversity; see Thomaz et al., 2007), became increasingly rare. It is not easy to detect local extinction, for instance, due to problems of detectability (e.g. Fisher and Blomberg, 2011). However, although we cannot assert unequivocally whether β-diversity patterns were driven by local extinctions, we found that most significant trends in species occupancy were negative. This is consistent with the expectation that a decrease in dispersal rates or, as in our case, floodplain connectivity (because floods are becoming less frequent), should leads to a decrease in local species richness and an increase in β-diversity (see Fig. 2 in Gonzalez, 2009).
We emphasize that the inferences related to the effects of water level regulation on β-diversity are speculative and that our data are not suitable to test a “dam effect”. We recognized the following hindrances to test this effect per se: (i) lack of longer time series (specially including data from before dam construction) and (ii) high noise levels in plankton data. Other approaches are, therefore, necessary to test our hypothesis that an increase in β-diversity can be explained by the cumulative effects of low flows, which “reduce, limit, or eliminate river–floodplain connectivity” (Rolls et al., 2012).
In general, it is expected that anthropogenic impacts would produce a decrease in α- and β-diversity. Nevertheless, if our hypothesis proves correct, it may sound strange to suggest that a decrease in β-diversity would be, in general, the goal of restoration efforts aiming to mitigate the impacts from upstream reservoirs. To achieve this goal, one could suggest changing the operating rules of upstream dams to restore the homogenizing effect of seasonal flood pulses (i.e., by increasing the release of water during summer). At least, it would be advisable under the precautionary principle (see Cooney, 2004). However, the solution to this conundrum is far from trivial in view of the increase in energy demand and the high operational challenge of controlling the flow rates of cascade reservoirs.
Conflicts of interestThe authors have no conflict of interest to declare.
CNPq and CAPES have continuously supported our research group.