Deforestation has carved tropical forest landscapes into millions of different-sized patches worldwide. Research has focused on the effect of patch size on biodiversity, while neglecting patch quality effects and leaving geographic and interspecific variance to patch attributes largely unexamined. Here, we assess how patch size and quality affect the encounter rate (ER) and immature-to-female ratio (IFR) of three endangered primates – Mexican mantled howler monkey (Alouatta palliata mexicana), black howler monkey (Alouatta pigra), and spider monkey (Ateles geoffroyi) – in three rainforest regions with different forest cover. ER of spider monkeys in the best preserved region (50% forest cover) was positively related to both patch size and quality (i.e., species richness of top food species). ER of mantled howlers in the 17% forest cover region was also positively related to patch size. Yet, both ER and IFR of black howlers in the 50% forest cover region and mantled howlers in the 5% forest cover region were mainly related to patch quality. Our results demonstrate that conservation actions in human-modified landscapes must go beyond considering the effect of patch size, as animal populations can be better predicted by patch quality in some regions.
Land use change has converted natural contiguous forests into patches of different size, threating the maintenance of global biodiversity on an unprecedented scale and rate (Newbold et al., 2016; Taubert et al., 2018). This situation is of particular importance in the tropics, where deforestation rates are the highest worldwide and increasing (Hansen et al., 2013). Understanding how these changes affect species in human-modified landscapes is needed to improve conservation strategies (Arroyo-Rodríguez et al., 2020). However, research in fragmented forests focus on only a few patch attributes, such as patch size (Fahrig, 2003), overlooking the effect of other important variables, such as patch quality. Also, there is a paucity of research assessing the effect of the regional context (e.g., land use history, regional deforestation) and interspecific variance in responses to land use changes (Pardini et al., 2010; Villard and Metzger, 2014). This can lead to incomplete or inaccurate conclusions, and the adoption of inappropriate conservation measures.
The research bias towards patch size effects is related to the strong influence of island biogeography theory and metapopulation theory in ecology and conservation biology (Fahrig, 2013; Haila, 2002). However, an increased number of studies have documented that biodiversity patterns in fragmented landscapes are not related to patch size everywhere (Laurance, 2008; Onderdonk and Chapman, 2000; Watling et al., 2020). Although patch size is positively related to population abundance and species richness in some regions (Mohandass et al., 2017), it is a poor predictor of species presence, abundance, and richness in other regions (Mellink et al., 2017; Onderdonk and Chapman, 2000; Pardini et al., 2010). For instance, Onderdonk and Chapman (2000) did not find a relationship between patch area and the presence of primates in Uganda. Mellink et al. (2017) also found that bird communities depend on the presence of trees and not on patch size in a semi-arid region of central Mexico. Thus, other variables, such as patch quality, can play important roles for biodiversity maintenance in fragmented landscapes.
Patch quality for arboreal mammals is often indexed using variables such as tree density and size, canopy height, vegetation type, and disturbance level (Arroyo-Rodríguez and Mandujano, 2009). Tree size, for instance, is a good indicator of food availability (Grant et al., 1992; Chapman et al., 1992) and has been proven to be an important predictor of forest species distribution in fragmented landscapes, as has patch tree species richness (Korboulewsky et al., 2016). Yet, which ecological variable bests predicts success in fragmented landscapes varies among species and their dietary flexibility (Dunlop et al., 2017; Hannibal et al., 2020; Marsh and Chapman, 2013). Therefore, our understanding of this topic is far from complete.
Primates are involved in numerous ecological processes, such as herbivory, seed dispersal, predation, and pest control, representing an important component of forest ecosystems (Andresen et al., 2018; Chapman et al., 2013; Estrada et al., 2017). Yet, as most primate species are forest dwellers (Galán-Acedo et al., 2019), they are particularly vulnerable to deforestation (Marsh and Chapman, 2013). In fact, ∼60% of the world's primate species are threatened with extinction (Estrada et al., 2017). The loss of primate species will have significant implications as they are of critical cultural and ecological importance (Estrada et al., 2017).
Here we assessed the effect of patch size and quality on the encounter rate (ER) and immature-to-female ratio (IFR, a proxy of reproductive output) of three endangered and forest-specialist primates: Mexican mantled howler monkey (Alouatta palliata mexicana), black howler monkey (Alouatta pigra), and Geoffroy's spider monkey (Ateles geoffroyi). We sampled 36 forest patches from three regions with different forest cover (50%, 17% and 5% forest cover). As patch colonization increases with patch size, and larger patches usually have more food resources (Arroyo-Rodríguez and Mandujano, 2006a) and lower anthropogenic pressures (Chapman et al., 2006; Peres, 2001), we predicted that both ER and IFR would increase with patch size in most regions. Yet, following the fragmentation threshold hypothesis (sensuPardini et al., 2010), patch size effects are predicted to be weaker (or absent) in the 5% forest cover region, as the lack of landscape connectivity in this region limit inter-patch animal movements, making populations more dependent to the local conditions in the patches (also see Villard and Metzger, 2014). Thus, patch quality effects are predicted to be stronger in this region. In particular, ER and IFR of both primate species are predicted to be positively related to total tree basal area (a proxy of tree size and forest maturity positively related to food availability, Arroyo-Rodríguez and Mandujano, 2006b; Chapman et al., 1992) and basal area and species richness of top food species. Finally, although howler monkeys can consume many early successional (pioneer) tree species in disturbed habitats (Arroyo-Rodríguez and Dias, 2010), basal area of pioneer species is indicative of forest disturbance. Thus, we expect that basal area of pioneer trees will be negatively related to ER and IFR of both species.
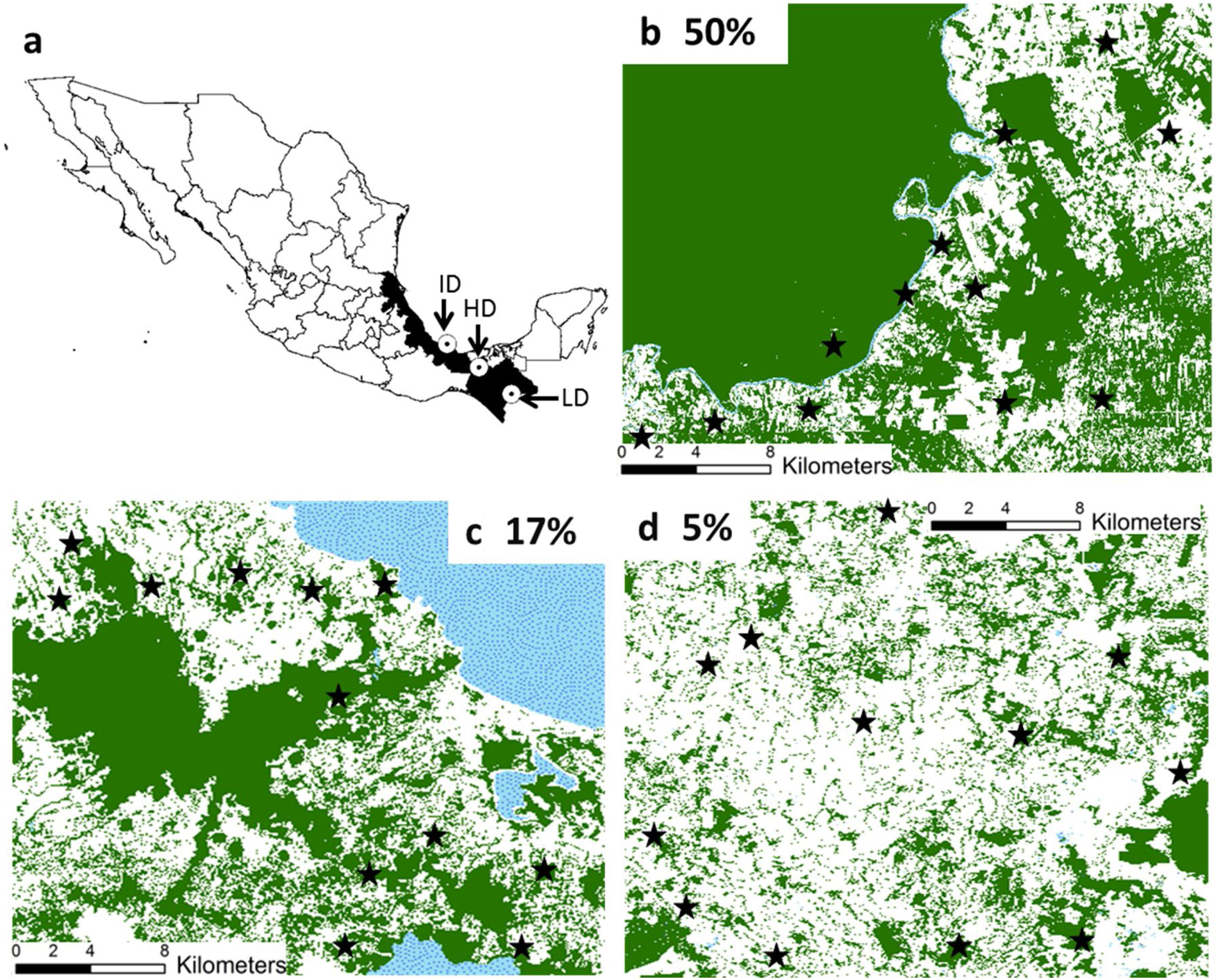
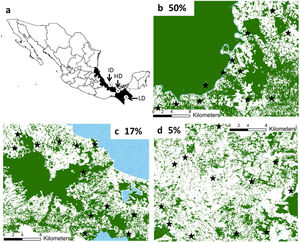
Materials and methodsStudy regionsWe worked in three regions from southeastern Mexico with similar climate and vegetation (rainforest), but with different patterns and histories of land-use (Fig. 1). These regions are described elsewhere (Galán-Acedo et al., 2018), but a brief overview is given here. The 50% forest cover region is in the Marqués de Comillas municipality, Chiapas (90°41′W 16°2′N). The 17% of forest cover region is in Los Tuxtlas rainforest, Veracruz (98°38′W 18°03′N). Finally, the 5% forest cover region is in the 8th North region, Chiapas (93°08′W 17°45′N) (Fig. 1). Mean annual temperature in all regions ranges from 22 to 25°C. Mean annual precipitation is 2143mm in the 50% forest cover region, 4900mm in the 17% forest cover region, and 2600mm in the 5% forest cover region. Deforestation began earliest in the 5% forest cover region (1950s), then in the 17% forest cover region (1960s), and finally in the 50% forest cover region (late 1970s). The dominant anthropogenic matrix surrounding the remaining forest in the 50% forest cover region are cattle pastures, arboreal and annuals crops, while in the other two regions are mainly pastures.
Location of the three study regions in southeastern Mexico (a). We indicate the remaining forest cover (in percentage) in each region. The study forest patches are indicated in black stars. The remaining forest cover is in dark green, anthropogenic matrix in white, and water bodies in dotted blue. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Mexico is the northern limit distribution of three Neotropical primate species: Ateles geoffroyi (Geoffroy's spider monkeys), Alouatta pigra (black howler monkeys), and Alouatta palliata mexicana (mantled howler monkeys) (Rylands et al., 2006). The three species are diurnal, arboreal, and forest specialists, but spider monkeys are mostly frugivorous, while both howler monkeys have a folivore-frugivore diet (Galán-Acedo et al., 2019). Spider monkeys have large home ranges (mean ∼200ha), a mean body mass of 7.5kg (Galán-Acedo et al., 2019), and high degree of group fission–fusion dynamics (van Roosmalen and Klein, 1988). Black howler monkeys have a mean home range of 9.2ha and a body mass of 6.6kg and Mexican mantled howler monkeys have a mean home range of 13ha and a body mass of 5.1kg (Galán-Acedo et al., 2019). Due to deforestation, these three primate species are declining in Mexico, and are currently classified as Endangered (IUCN, 2010). Of the regions studied, Ateles geoffroyi and Alouatta pigra inhabit the 50% forest cover region, while Alouatta palliata is found in the 17% and 5% forest cover region. The natural distribution of Alouatta palliata in Mexico does not include the 50% forest cover region, and Ateles geoffroyi historically inhabited 17% and 5% forest cover region, but is now locally extinct.
Forest patches and primate surveysWe randomly selected 12 old-growth forest patches in each region. We focused on old-growth forests because this is the main habitat of the study species. Although these primates can use food resources from second-growth (secondary) forests, this forest type is only temporary used by these primates (e.g., Ramos-Fernández and Ayala-Orozco, 2003; Arroyo-Rodríguez and Dias, 2010; Arroyo-Rodríguez et al., 2017). Focal patches were separated by ≥ 2.7km in three size class categories: 6 patches of 1-10ha, 4 of 10-50ha, and 2 of 50-100ha (36 patches in total) (Galán-Acedo et al., 2018). Because of the lack of large patches in HD, here we selected 10 small and 2 medium patches. We calculated patch size using Google Earth Pro. As detailed in Galán-Acedo et al. (2018), primates were sampled using a protocol widely used in studies of primates. In particular, we walked slowly (∼1-2km/ha) inside and around each patch from 6 am to 5 pm, when primates are active, which increases detection likelihood. We only worked during sunny days in the 2015 and 2016 dry seasons to avoid biases caused by bad weather conditions. In addition and complementarily to direct observations, we used howler monkeys long distance vocalizations and spider vocalizations to detect presence and locate groups. We also interviewed residents about primate presence in the focal patches, which also contributed to avoid underestimating patch occupancy. When we made visual contact with a group, we counted all individuals and categorized them by age/sex class (adult males, adult females, juveniles, and infants). Search time per patch was relative to its size, with three consecutive days dedicated to survey small patches, 5 days in medium-sized patches, and 7 days in large patches. Although search time was not proportional to patch size, we avoided potential underestimations of primate abundance in large patches by including sampling effort (i.e., km walked) in our estimations of encounter rate (see below). Also, each day we sampled different areas of the patch, covering the complete patch area. As spider monkeys have a high degree of group fission–fusion dynamics it is difficult to avoid double counting individuals. Thus, we used the encounter rate (ER - number of individuals per km walked) as the response variable, which is a common measure of relative abundance in studies of spider monkeys (dos Santos-Filho et al., 2017; Ortiz-Martinez et al., 2008; Ramos-Fernández and Ayala-Orozco, 2003). We also considered the immature-to-female ratio (IFR) by dividing the number of adult females by the number of immatures (juveniles+infants). IFR is widely used as a proxy of reproductive output (or ‘replacement rate’, Wilson and Bossert, 1971; Galán-Acedo et al., 2018).
Vegetation samplingWe estimated four vegetation variables using a modification of the Gentry's (1982) protocol: total basal area (i.e., all trees with a diameter at breast height>10cm), basal area of top food species, basal area of pioneer species, and top food species richness. To this end, we recorded all trees in ten 50×2-m plots (i.e., 0.1ha per site). Plots were placed parallel to each other (maintaining an inter-plot distance of 10m) to control for the potential confounding effects that differences in inter-plot isolation distance may have on β-diversity among plots, and consequently, on the accumulated number of species within each forest site. To estimate the basal area and species richness of top food species we used the lists of top food plant species (i.e., species comprising most of the diet of each species) published in several review studies (spider monkeys: González-Zamora et al., 2009; mantled howler monkeys: Arroyo-Rodríguez and Mandujano, 2006a; Cristóbal-Azkarate and Arroyo-Rodríguez, 2007; black howlers: Righini et al., 2017; Rivera and Calmé, 2006). All plants not identified in the field were collected and identified at the MEXU herbarium (Universidad Nacional Autónoma de México, Mexico City). Plant nomenclature followed the updated database “Tropicos”, Missouri Botanical Garden.
Statistical analysesWe used R 3.0.1 for analyses (R Core Team, 2013). We assessed collinearity among patch size and vegetation variables with the variance inflation factor (VIF) using the ‘car’ package. We did not detect significant collinearity between predictors, as all VIF values were<3.45. We then assessed the relative importance of each explanatory variable (patch size and four vegetation variables) in predicting each response variable with generalized lineal models (GLMs), using the ‘glmulti’ package (Calcagno and de Mazancourt, 2010). As suggested for continuous response variables (Crawley, 2007), we analyzed the GLMs with a Gaussian distribution error, after verifying that the complete model did not violate the normality assumption using a Shapiro–Wilk test (p>0.10, in six out of eight responses). The two responses for which the normality assumption was not met where the models for IFR of Mantled howler monkeys in the 17% forest cover region, and IFR of spider monkeys in the 50% forest cover region. This was likely related to the absence of immatures in some occupied patches, which increased the amount of zeros in the database (i.e., zero IFRs=9 of 12 sites in the first case, and 7 of 12 sites in the second case). Therefore, to avoid potential biases in parameter estimates and their associated measures of uncertainty related to ‘zero inflated’ modeling (see Martin et al., 2005), we decided excluding these two response variables from our analyses. We keep the other responses in the analyses because the amount of zeros was small enough that the data readily fitted the normal distribution (Martin et al., 2005).
The ‘glmulti’ package uses an information-theoretic approach and multimodel inference to assess the relative effect of each predictor on each response variable (Burnham and Anderson, 2002). For each response variable, we constructed 26 models, which combined up to three predictor variables and the null model (which only includes the intercept). We limited the maximum number of terms in the models to three to increase the power of our hypotheses tests, and thus decrease type II statistical error. For each model we computed Akaike's information criterion corrected for small samples (AICc) and we ranked the models from best to worst. Finally, we used Akaike weights (wi) to evaluate the importance of each predictor and produce model-averaged parameter estimates (Anderson, 2007). Hence, we summed wi of ranked models until the total was >0.95 (Whittingham et al., 2005). The set of models for which ∑wi is 0.95 represents a set that has 95% probability of containing the true best model (Whittingham et al., 2005). Following Crawley (2007), the goodness-of-fit of the models was estimated as: (explained deviance by the complete model x 100)/total deviance (i.e., deviance of the null model). To be more conservative, a given landscape variable was considered a relatively important predictor for a given response if the following four criteria were met: (i) the complete model in which such predictor appeared has a relatively high goodness-of-fit; (ii) the predictor shows a relatively high Σwi (i.e., considering all candidate model in which it appeared); (iii) it is present in at least one of the most plausible models (i.e., ΔAICc<2); and (iv) the model-averaged parameter estimate was higher than its unconditional variance (i.e., it did not include zero).
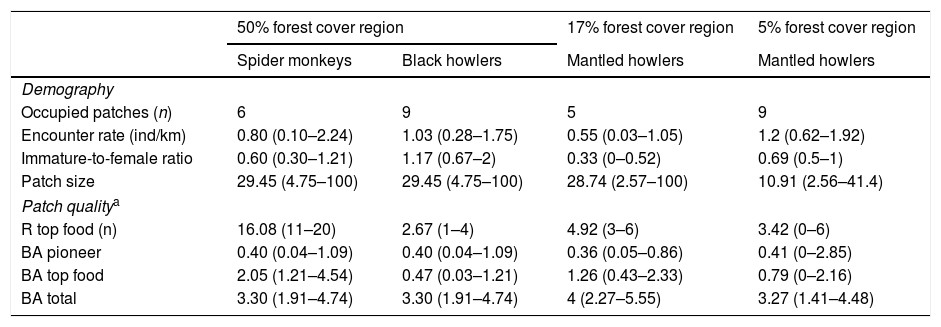
ResultsIn the 50% forest cover region, spider monkeys were recorded in 6 of 12 patches and black howler monkeys in 9 of 12 patches (Table 1). The encounter rate (ER) of spider monkeys averaged 0.8ind/km (range among patches=0.1–2.24ind/km) and mean immature-to-female ration (IFR) averaged 0.6 (range=0.3–1.21). Mean ER of black howlers was 1.03 (range=0.28–1.75ind/km) and mean IFR was 1.17 (range=0.67–2). In the 17% forest cover region, we only recorded mantled howler monkeys, which were present in 5 of 12 patches, with a mean ER of 0.55ind/km (range=0.03–1.05ind/km) and a IFR averaging 0.44 (range=0.38–0.52). Finally, in the 5% forest cover region, we only recorded mantled howlers in 9 of 12 patches and they averaged 1.2ind/km (range=0.62–1.92ind/km) and 0.69 immatures per female (range=0.5–1).
Demographic attributes of three primate species in three rainforest regions with different remaining forest cover (50%, 17% and 5% forest cover) from southeastern Mexico. The mean values (range in parenthesis) of forest patch size (n=12 patches per region) and vegetation characteristics per region are indicated.
| 50% forest cover region | 17% forest cover region | 5% forest cover region | ||
|---|---|---|---|---|
| Spider monkeys | Black howlers | Mantled howlers | Mantled howlers | |
| Demography | ||||
| Occupied patches (n) | 6 | 9 | 5 | 9 |
| Encounter rate (ind/km) | 0.80 (0.10–2.24) | 1.03 (0.28–1.75) | 0.55 (0.03–1.05) | 1.2 (0.62–1.92) |
| Immature-to-female ratio | 0.60 (0.30–1.21) | 1.17 (0.67–2) | 0.33 (0–0.52) | 0.69 (0.5–1) |
| Patch size | 29.45 (4.75–100) | 29.45 (4.75–100) | 28.74 (2.57–100) | 10.91 (2.56–41.4) |
| Patch qualitya | ||||
| R top food (n) | 16.08 (11–20) | 2.67 (1–4) | 4.92 (3–6) | 3.42 (0–6) |
| BA pioneer | 0.40 (0.04–1.09) | 0.40 (0.04–1.09) | 0.36 (0.05–0.86) | 0.41 (0–2.85) |
| BA top food | 2.05 (1.21–4.54) | 0.47 (0.03–1.21) | 1.26 (0.43–2.33) | 0.79 (0–2.16) |
| BA total | 3.30 (1.91–4.74) | 3.30 (1.91–4.74) | 4 (2.27–5.55) | 3.27 (1.41–4.48) |
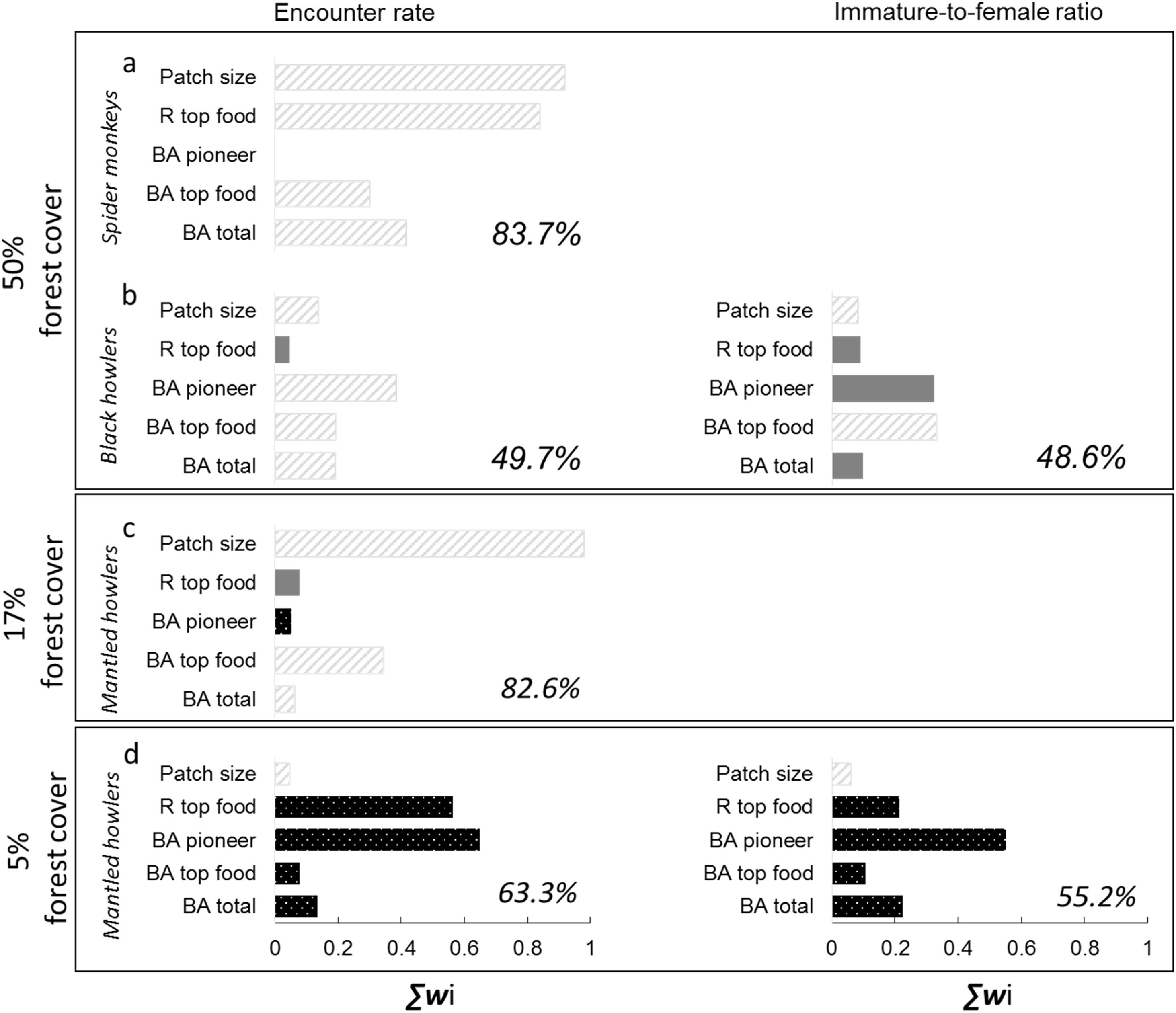
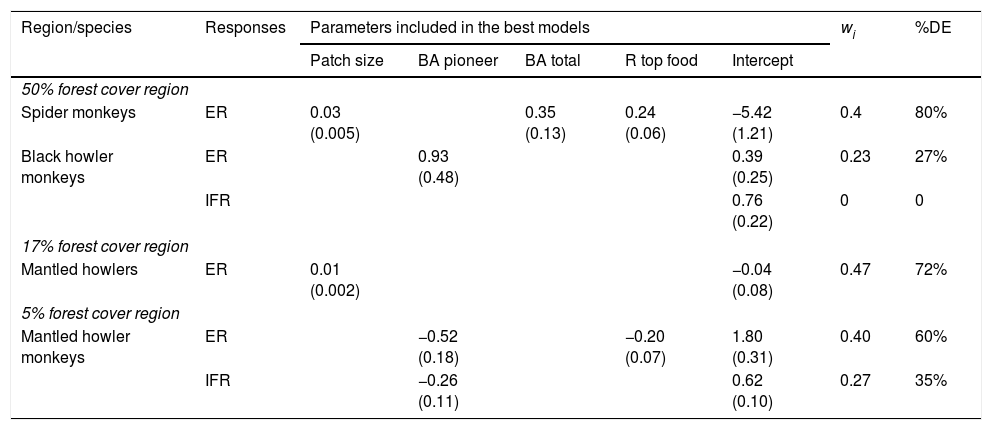
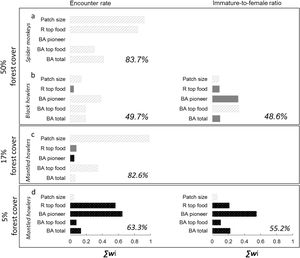
The predictor variables explained >48% of deviance of all response variables (Fig. 2). Its predictive power was higher for spider monkeys in the 50% forest cover region (Fig. 2a) and for mantled howlers in the 17% forest cover region (Fig. 2c). In the 50% forest cover region, ER of spider monkeys (∑wi=0.9) was mainly and positively related to patch size, although it was also associated with the richness of top food species and total basal area (Fig. 2a; Tables 2 and 3). Patch size was also the most important predictor of mantled howler populations, positively affecting ER (∑wi=0.96) in the 17% forest cover region (Fig. 2c; Tables 2 and 3).
Predictor variables included in 95% set of models (bars) selected for the two response variables (encounter rate and immature-to-female ratio) of three primate species in three rainforest regions with different forest cover. The importance of each variable is shown by the sum of Akaike weights (∑wi). We assessed the impact of patch size and four vegetation attributes: richness of top food species (R top food), basal area of pioneer species (BA pioneer), basal area of top food species (BA top food), and total tree basal area (BA total). The percentage of deviance explained by each complete model is indicated in each panel as a measure of goodness-of-fit of each complete model. Positive (striped grey bars) or negative (black bars) responses to each predictor are indicated. Filled grey bars represent the cases in which the unconditional variance was higher than the model-averaged parameter estimate, indicating that such parameter can include zero (i.e., a null effect; see parameter estimates and unconditional variances in Table 2).
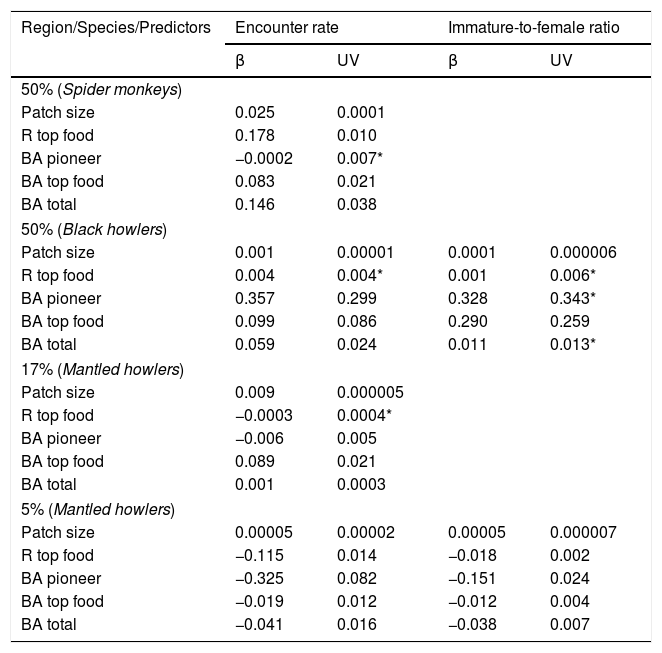
Values of model-averaged parameter estimates (β) and unconditional variance (UV) of information-theoretic-based model selection and multimodel inference for both response variables (encounter rate and immature-to-femal ratio of three primate species) related to five predictor variables in three rainforest regions with different forest cover (50%, 17% and 5% forest cover) (see all model sets in Appendix A in Supplementary material). Abbreviations: R top food=richness of top food species; BA pioneer=basal area of pioneer species; BA top food=basal area of top food species; BA total=total tree basal area. Asterisks indicate the cases in which the unconditional variance (UV) is higher than the model-averaged parameter estimates (β).
| Region/Species/Predictors | Encounter rate | Immature-to-female ratio | ||
|---|---|---|---|---|
| β | UV | β | UV | |
| 50% (Spider monkeys) | ||||
| Patch size | 0.025 | 0.0001 | ||
| R top food | 0.178 | 0.010 | ||
| BA pioneer | −0.0002 | 0.007* | ||
| BA top food | 0.083 | 0.021 | ||
| BA total | 0.146 | 0.038 | ||
| 50% (Black howlers) | ||||
| Patch size | 0.001 | 0.00001 | 0.0001 | 0.000006 |
| R top food | 0.004 | 0.004* | 0.001 | 0.006* |
| BA pioneer | 0.357 | 0.299 | 0.328 | 0.343* |
| BA top food | 0.099 | 0.086 | 0.290 | 0.259 |
| BA total | 0.059 | 0.024 | 0.011 | 0.013* |
| 17% (Mantled howlers) | ||||
| Patch size | 0.009 | 0.000005 | ||
| R top food | −0.0003 | 0.0004* | ||
| BA pioneer | −0.006 | 0.005 | ||
| BA top food | 0.089 | 0.021 | ||
| BA total | 0.001 | 0.0003 | ||
| 5% (Mantled howlers) | ||||
| Patch size | 0.00005 | 0.00002 | 0.00005 | 0.000007 |
| R top food | −0.115 | 0.014 | −0.018 | 0.002 |
| BA pioneer | −0.325 | 0.082 | −0.151 | 0.024 |
| BA top food | −0.019 | 0.012 | −0.012 | 0.004 |
| BA total | −0.041 | 0.016 | −0.038 | 0.007 |
The most plausible models describing the effect of patch size and quality on the encounter rate (ER) and immature-to-female ratio (IFR) of three primate species in three regions with different forest cover (50%, 17% and 5% forest cover). The parameter estimate (and standard error) of the predictor is indicated, as is the Akaike weight (wi) and percentage of deviance explained (%DE) of each model. Predictors of patch quality: R top food=richness of top food species; BA pioneer=basal area of pioneer species; BA total=total tree basal area.
| Region/species | Responses | Parameters included in the best models | wi | %DE | ||||
|---|---|---|---|---|---|---|---|---|
| Patch size | BA pioneer | BA total | R top food | Intercept | ||||
| 50% forest cover region | ||||||||
| Spider monkeys | ER | 0.03 (0.005) | 0.35 (0.13) | 0.24 (0.06) | −5.42 (1.21) | 0.4 | 80% | |
| Black howler monkeys | ER | 0.93 (0.48) | 0.39 (0.25) | 0.23 | 27% | |||
| IFR | 0.76 (0.22) | 0 | 0 | |||||
| 17% forest cover region | ||||||||
| Mantled howlers | ER | 0.01 (0.002) | −0.04 (0.08) | 0.47 | 72% | |||
| 5% forest cover region | ||||||||
| Mantled howler monkeys | ER | −0.52 (0.18) | −0.20 (0.07) | 1.80 (0.31) | 0.40 | 60% | ||
| IFR | −0.26 (0.11) | 0.62 (0.10) | 0.27 | 35% | ||||
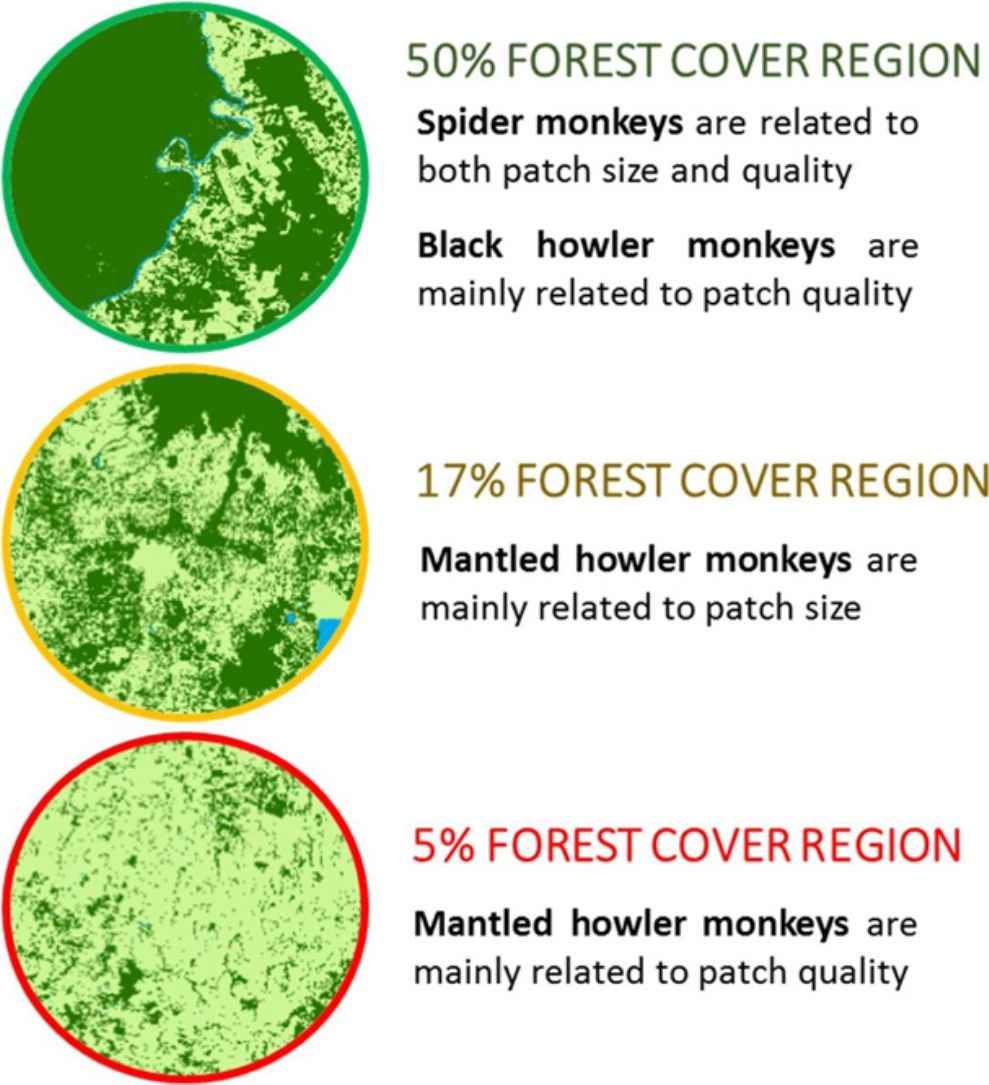
Patch quality was important for black howler monkeys in the 50% forest cover region (Fig. 2b) and for mantled howlers in the 5% forest cover region (Fig. 2d). In the first case, ER of black howler monkeys was mainly positively related to basal area of pioneer species (∑wi=0.38), but total basal area (∑wi=0.19) and basal area of top food species (∑wi=0.19) were also positively related to ER (Fig. 2c; Tables 2 and 3). IFR of black howlers was also positively related to basal area of top food species in this region (∑wi=0.32; Fig. 2b); yet, this relationship is weak, as the most plausible model included only the intercept (i.e., null model; Table 3). Regarding mantled howlers in the 5% forest cover region, the basal area of pioneer species was the best predictor of ER (∑wi=0.65) and IFR (∑wi=0.55), negatively affecting both responses (Fig. 2d; Table 3). Surprisingly, ER of mantled howlers was also negatively related to top food species richness (∑wi=0.56) in this region.
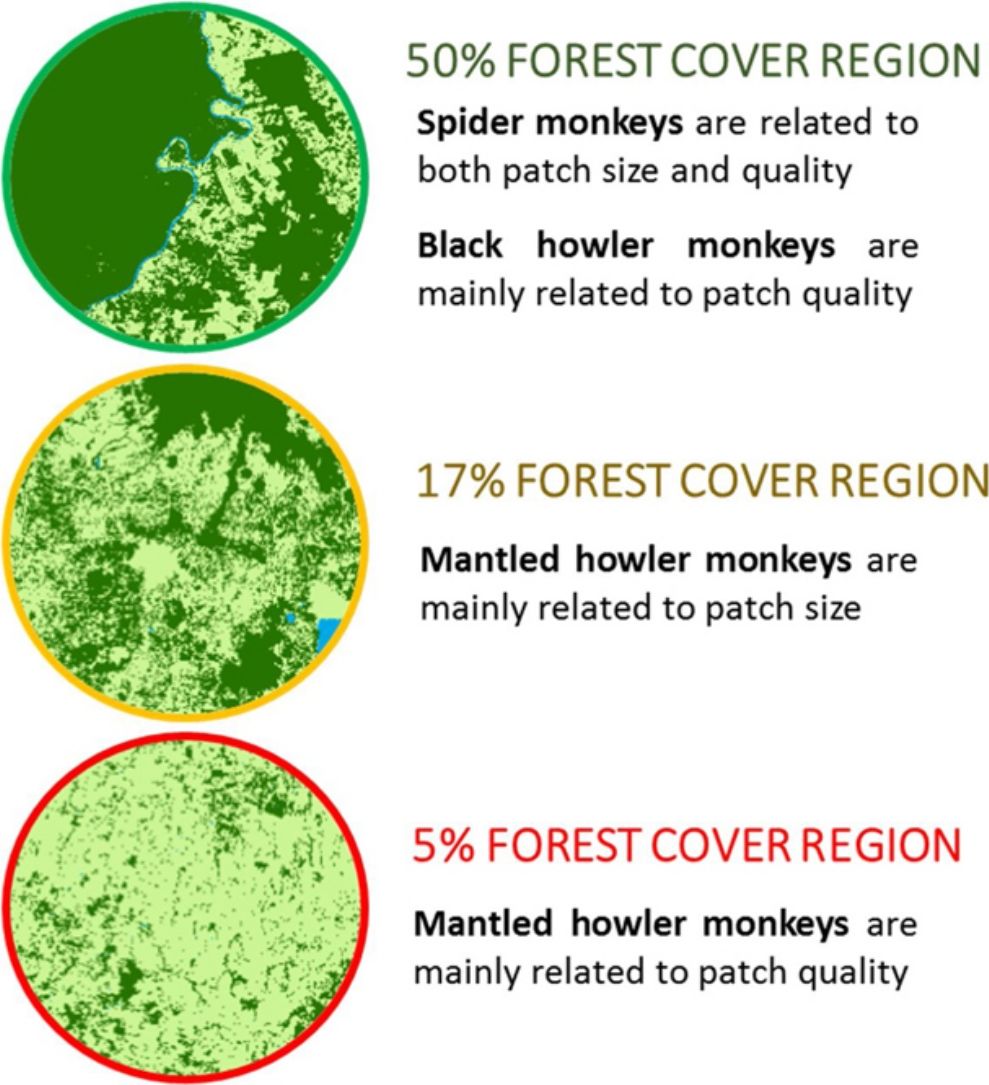
DiscussionThis study assesses the effect of patch size and quality on the encounter rate (ER) and immature-to-female ratio (IFR) of three endangered Mexican primates in three rainforest regions with different deforestation level. Our findings indicate that the effects of patch size and quality are regional and species specific. Patch size and quality (i.e., species richness of top food species) was the best predictor of spider monkey populations in the 50% forest cover region, positively affecting the encounter rate (ER). Patch size was also the best predictor of mantled howler monkeys, positively affecting ER in the 17% forest cover region. However, ER and IFR of black howler monkeys in the 50% forest cover region and mantled howlers in the 5% forest cover region were mainly related to vegetation attributes, thus indicating that patch quality can be the main driver of primates in some regions.
As expected, patch size is an important driver of primate populations in two of three regions. The ER of two species increased with patch size in the 50% forest cover (spider monkeys) and 17% forest cover (mantled howler monkeys) regions. This is not surprising, as patch area is viewed to be generally positively related to food availability and patch connectivity (Hanski, 1998), positively influencing patch occupancy, population abundance, and species richness (Harcourt and Doherty, 2005; Mohandass et al., 2017). Species persistence in large patches can also be enhanced by the fact that hunting pressure and diseases are usually lower in these patches (Arroyo-Rodríguez and Dias, 2010). However, consistent with previous studies (Mellink et al., 2017; Onderdonk and Chapman, 2000), our results indicate that patch size is not always the best predictor of primate populations in fragmented forests.
Consistent with our hypothesis, patch size was a poor predictor of mantled howler monkeys in the 5% forest cover region, possibly because the remaining forest patches were small, scarce, and highly isolated between each other. Together, these conditions can increase the exposition of patches to hunting and predation (Chapman et al., 2006; Peres, 2001). Also, the anthropogenic matrix in this region is dominated by open areas (i.e., cattle pastures), which can significantly limit the interchange of individuals between forest patches. Thus, following the fragmentation threshold hypothesis, such limited availability of habitat and limited flow of individuals between patches (i.e., low landscape connectivity) can decrease the abundance of individuals in both small and large patches, especially the abundance of forest dependent mammals (Pardini et al., 2010; Villard and Metzger, 2014). Therefore, it is not surprising that the encounter rate of mantled howlers in the 5% forest cover region does not depend on patch size.
Patch size was also a poor predictor of black howler monkeys in the 50% forest cover region. This finding also follows the predictions of the fragmentation threshold hypothesis, which predicts weak associations between patch size and abundance in high forested regions because the remaining patches in these regions are usually very large, and very close to each other (Pardini et al., 2010). Under this context, there can be enough flow of individuals to maintain populations in small and large patches, which can potentially weaken the association between population structure and patch size. However, patch size was an important predictor of spider monkeys in the 50% forest cover region. This could be related to the notably larger home range size requirements of spider monkeys, which is 20 times larger than that of black howlers. This large spatial requirement can strengthen the dependency of this and potentially other mammal species with similar home range requirements (e.g., tapir: 564ha home range, peccary: 4097ha home range; Jones et al., 2009) on patch size, even in high forested regions.
Our findings also indicate that patch quality plays a key role in maintaining primate populations in fragmented forests. In particular, vegetation structure drove the ER of spider monkeys and the ER and IFR of black howler monkeys in the 50% forest cover region. Also, vegetation structure affected mantled howler monkey responses in the 5% forest cover region. This is consistent with previous studies showing the importance of vegetation structure for the presence and abundance of different species, including primates (Arroyo-Rodríguez et al., 2007; Bohenek et al., 2017; Gardiner et al., 2018). In particular, we found a positive association between the ER of spider monkeys and species richness of top food species. This is probably related to the need of a nutritional balance in the macronutrient composition of their diets. Spider monkeys require a protein and non-protein (e.g., lipids) balance to allow their energetic intake (Felton et al., 2008). Thus, patches with higher richness of top food species will offer a better nutritional balance, increasing the survival and maintenance of spider monkeys.
Interestingly, the basal area of pioneer tree species has contrasting effects on howler monkeys in different regions. As predicted, this variable was negatively related to ER and IFR of mantled howler monkeys in the 5% forest cover region. Nevertheless, contrary to our expectations, it was positively related to the ER of black howler monkeys in the 50% forest cover region. This variable usually increases in more disturbed forests, so it can be considered a proxy of forest disturbance (Bongers et al., 2009). Thus, in the 5% forest cover region, where inter-patch individual movements may be strongly limited, a high basal area of pioneer tree species likely indicates that the patch is very disturbed and that resource availability can be limited because there are fewer large trees from preferred food species (Arroyo-Rodríguez and Mandujano, 2006a). However, in landscapes with 50% forest cover, animals can easily move between fragments. Thus, even though the resident patch is disturbed (i.e., with high basal area of pioneer trees), the monkeys can make use of resources from other patches. Furthermore, pioneer trees can also represent valuable resources for howler monkeys in fragments (Arroyo-Rodríguez and Dias, 2010; Milton et al., 2019), as the fast-growing leaves of these trees typically have higher protein and lower fiber and phenolic levels than those of old-growth shade-tolerant tree species (Coley, 1987). Therefore, the positive response of howler monkeys to basal area of pioneer trees in landscapes with 50% forest cover can be, at least partially, explained by the food resources that these trees provide to primates.
Another surprising result is the mantled howler monkeys’ negative association between ER and IFR and top food species richness in the 5% forest cover region. This is counterintuitive and we cannot find a reasonable explanation. It may be the result of uncontrolled confounding factors, such as human pressures or unknown historical events (e.g., disease). Such factors are difficult to identify in regions deforested long ago (∼70 years of human disturbance).
Also puzzlingly is the fact that this region has the highest number of inhabited patches (together with the 50% forest cover region) and the second highest regional abundance. This finding suggests that monkeys can survive on the few available resources, supporting claims of very high behavioral flexibility of this species (reviewed by Arroyo-Rodríguez and Dias, 2010). However, this may also reflect an extinction debt: in long-lived species such as howler monkeys, there can be a time lag in species extinctions (Cowlishaw, 1999; Lira et al., 2012; Metzger et al., 2009). Therefore, collecting long-term data, over a relatively large spatial scale is needed to better understand and predict population trends in this highly deforested region.
We conclude that both patch size and quality must be considered in conservation plans. Prioritizing the preservation of large patches is likely the most effective in low deforested regions (∼50% forest cover), especially for terrestrial mammal species with large home ranges. However, for species with dietary flexibility (e.g., Eulemur sp., Sato et al., 2016; Chlorocebus djamdjamensis, Mekonnen et al., 2018), conserving landscapes with multiple patches and preventing further vegetation disturbance may be the most appropriate strategy in high forested regions. In regions with intermediate level of deforestation (e.g., ∼17% forest cover), patch size may be more for primates and other mammal species (Garmendia et al., 2013; Magioli et al., 2015; Norris et al., 2010); thus, conservation strategies in these regions should rely on the preservation of large patches. Finally, in strongly deforested regions (e.g., ∼5% forest cover), where populations are isolated and dependent on local vegetation conditions, conservation and management plans should focus on forest restoration. However, given the potential extinction debts in the study landscapes (Cowlishaw, 1999; Metzger et al., 2009), additional long-term monitoring studies are needed to better understand population trends, and thus improve conservation plans in these landscapes. Such a conservation planing can also be improved by better understanding the role of other arboreal land covers (e.g., secondary forests, living fences, isolated standing trees; see Arroyo-Rodríguez et al., 2020), not only old-growth forests, in biodiversity conservation.
Declaration of interestsNone.
We thank the landowners of Marqués de Comillas, Los Tuxtlas and 8th North regions for allowing us to collect data on their properties. CGA thanks DGAPA-UNAM for her postdoctoral scholarship. We thank Manuel A. Hernandez-Ruedas for collecting the vegetation data. We also thank the support (infrastructure, logistics and administration team) provided by the Instituto de Investigaciones en Ecosistemas y Sustentabilidad (IIES-UNAM). H. Ferreira, A. Valencia and A. López provided technical support. This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT) (Project 2015-253946) and the Rufford Conservation Grant (18689-1).