Plants and pollinators might respond differently to changes in climate, and thus plant-pollinator relationships are vulnerable to spatial, temporal, morphological and recognition mismatches. Although the effects of climate change on pollinators and pollination services are expected to be greater in the tropics than in other latitudes, these effects remain poorly documented. Herein, we assessed the spatial distribution of nine species (of five genera) of Colombian stingless bees used in meliponiculture under present and future climate scenarios. Stingless bees are major pollinators in tropical areas and their use in managed pollination, to produce high-value honey, and as recreation is increasingly popular worldwide. Our models indicate that most species of stingless bees exhibit restricted distributions to ecosystems within the continental natural regions of Colombia. Using intermediate (RCP 4.5) and high (RCP 8.5) greenhouse gas emission scenarios, our models predict that seven of the nine species would experience a significant reduction in their climatically suitable areas, and thus will likely influence agriculture and rural livelihoods. These results are critical to developing new conservation policies and climate adaptation strategies that include restrictions in the relocation of colonies, as well as monitoring programs that help beekeepers to shift to other species in areas where our models predicted a likely reduction or loss of habitat suitability.
Climate change is one of the main drivers of human-related alterations on ecosystems and biodiversity (Parmesan, 2006; Feehan et al., 2009). Temperatures and CO2 levels are expected to rise during the 21st century, as well as the intensity, frequency, and duration of climatic events, such as rainfall and drought. These changes in climate will directly influence livelihoods, food supply, and human infrastructures (IPCC, 2019), as well as the geographical distribution of species and their interactions with other organisms in the ecosystem (e.g., Rodríguez-Castañeda et al., 2017).
Climate change will disrupt beneficial, mutualistic relationships between plants and insects, such as pollination. The IPCC (2013) estimates that during this century, temperatures will rise between 1.1 °C and 6.4 °C worldwide, resulting in mismatches between the emergence time of pollinators and the blooming period of plants, as well as latitudinal and elevational shifts in the distribution of plants and pollinators (Feehan et al., 2009). Such disruptions in plant-pollinator interactions might threaten our food security, as about 75% of global food production depends on animal pollination (Klein et al., 2007).
Although the magnitude of the impact of climate change will vary depending on the location and season, rising temperatures will more strongly affect tropical pollinators than those living at other latitudes. This is because tropical insects are already living close to the maximum temperature they tolerate, and they have a narrower thermal tolerance than insects at higher latitudes (Deutsch et al., 2008). Thus, the effects of climate change on the pollination and pollinators of tropical crops will be greater than crops at higher latitudes.
Bees are the most important pollinators for both wild and cultivated plants. Bees play an important role in ecosystem health and plant reproduction and, therefore, in general food security. There are more than 20,000 bee species worldwide (Michener, 2007), but stingless bees (Apidae: Meliponini) are perhaps one of the most ecologically, economically, and culturally significant of all of them. Stingless bees, a group consisting of about 400 species, live in colonies and produce honey and wax, similar to honey bees (Michener, 2007). However, they do not have a stinger and are native to tropical and subtropical areas of the world. Stingless bees are major pollinators of many native, introduced, and cultivated plants, and some species are managed to promote pollination of diverse crops (e.g., Cauich et al., 2006; Slaa et al., 2006). Indigenous and non-Indigenous populations in many regions of the world use the honey, pollen, cerumen, and propolis of numerous species for diverse purposes, including food, medicine, and crafts. In some cases, these bee products represent unique or additional sources of income or alternative medicines (e.g., Gonzalez et al., 2018a; Quezada-Euán et al., 2018).
As in other tropical countries, some Colombian farmers and indigenous people also depend on stingless bees and their products, which are highly valued for their medicinal honey and pollen (Nates-Parra, 2001; Engel et al., 2019). More than 100 species of stingless bees occur in all natural regions of Colombia, from sea level up to 3400 m in the Andes (Nates-Parra, 2001; Gonzalez and Engel, 2004). Although records indicate that about 28% of Colombia’s stingless bee species are used in beekeeping or meliponiculture (Nates-Parra and Rosso-Londoño, 2013), the popularity of stingless beekeeping has increased dramatically in recent years, particularly since November 2016 with the Peace Accords between the FARC (Fuerzas Armadas Revolucionarias de Colombia or Armed Revolutionary Forces of Colombia) and the Colombian government.
After nearly 60 years of conflict, Colombia is now facing new social and environmental challenges, such as providing for the social welfare of ex-combatants and displaced peoples, as well as an increase in deforestation, illegal mining, and coca plantations (Baptiste et al., 2017; Salazar et al., 2018; Suarez et al., 2018). During this transition to peace, the Colombian government and several national and international organizations have promoted and supported several environmentally sustainable practices that include small-scale agriculture of value crops, such as cocoa and coffee, and stingless bee keeping. In particular, beekeeping is a recognized poverty alleviating practice, as honey represents a readily available source of calories for children and sick people. It also improves food security while enhancing mental and physical health, and family and community relationships (e.g., Amulen et al., 2017; Chanthayod et al., 2017).
The recent popularity of stingless bees in Colombia has resulted in many small-scale stingless bee keeping projects across the country, has promoted the creation of private companies, and bolstered informal marketing, as well as the extraction and relocation of nests, sometimes from outside their native range (V.H. Gonzalez, personal obs.). Anthropogenic movement of stingless bee nests has potentially negative consequences to local populations and the success of sustainable management practices. Moving nests to locations outside bees’ native range with unsuitable habitats might lead to low rates of colony establishment or total loss, thus wasting personal and financial efforts, which has already occurred in Colombia (V.H. Gonzalez, personal obs.). Additionally, nest movement might promote the spread of parasites and pathogens, and might alter the genetic structure of both wild and managed populations (e.g., Byatt et al., 2016; Chapman et al., 2018). However, information on the distribution ranges of Colombia’s stingless bees is limited.
As an attempt to understand the distribution of Colombia’s most valued pollinators, as well as to forecast the effects of human-induced global warming, here we assess the spatial distribution of nine species of stingless bees that are relevant in meliponiculture and conduct analyses under present and future climate scenarios projected to the year 2050. Two of these species, namely Melipona eburnea Friese and Meliponafavosa (Fabricius), are also threatened and are considered as vulnerable species to extinction (Nates-Parra, 2007). We discuss the implications of our results to guide on-going conservation efforts as well as the promotion of sustainable productive alternatives in the country.
Material and methodsStudy areaBordered by the Caribbean Sea to the north and the Pacific Ocean to the west, Colombia is one of the hotspots of biodiversity owing to its structural complexity, altitudinal gradient, and location in northern South America. Despite being about one-seventh of the area of Brazil and comprising less than 1% of the Earth’s landmass, Colombia hosts approximately 10% of the Earth’s biodiversity (Rangel-Ch and Aguilar, 1995; Myers et al., 2000; Orme et al., 2005). The Andes transverse the country from the southwest to northeast and create five distinct natural regions (Amazon, Andean, Caribbean, Orinoquia, and Pacific), each characterized by a number of biomes and ecosystems. The Andean is the most species-rich region, but it is also the most densely populated. This region consists of three mountain ranges or cordilleras (Oriental, Central, and Occidental) that create multiple isolated inter-Andean valleys, each with large gradients of climatic conditions. The Andean region holds lowland and highland xeric areas, dry and rain forests, and montane forests and grasslands. The Amazon is the second richest biogeographic region of the country and holds about 10% of the Amazon rainforest. The Chocó biogeographic area is a narrow strip of humid forest along the Pacific coast, and it is considered one of the world’s biodiversity hotspots due to the high levels of endemism of animals and plants (Myers et al., 2000). Xeric and subxerophytic vegetation types dominate the Caribbean region whereas deltaic savannas are predominant in the Orinoquia region. Both regions are extremely understudied and lack of biological inventories for most taxonomic groups (e.g., Rangel-Ch, 2012; Sánchez-Cuervo et al., 2012; Arbeláez-Cortés, 2013).
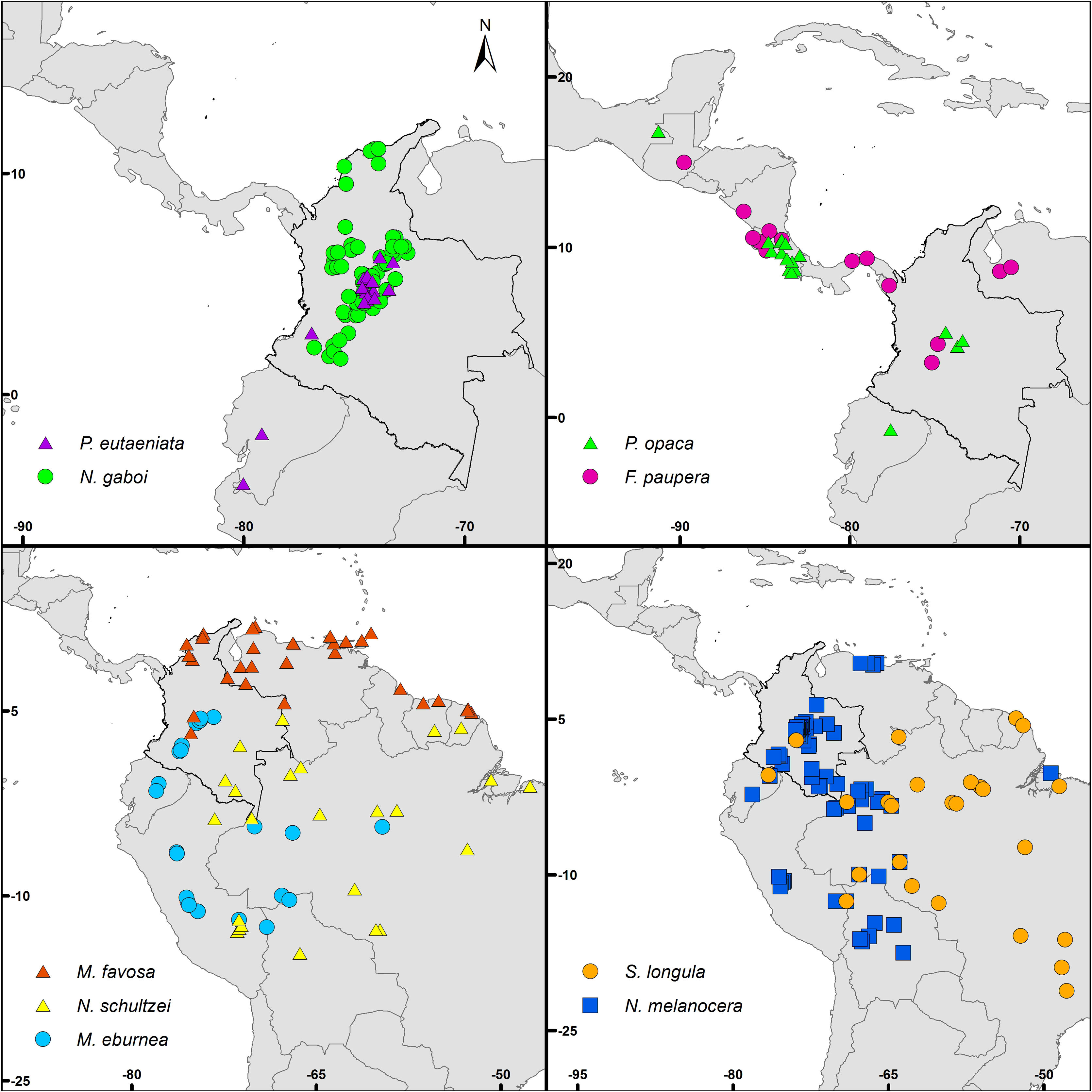
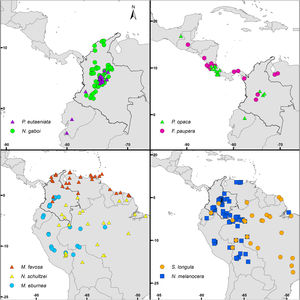
Bee species and occurrence dataAs a guide in the selection of taxa for our analyses, we used the list of stingless bee species used in beekeeping in Colombia by Nates-Parra and Rosso-Londoño (2013). First, we chose species that most people commonly used, as indicated by the high number of hives kept by beekeepers recorded by these authors. Second, we chose species from different genera to increase the taxonomic and geographic representation in our study. Third, we chose taxa with a stable taxonomy. For example, more than half of the managed hives recorded by Nates-Parra and Rosso-Londoño (2013) are from Tetragonisca angustula Latreille, a species that ranges from southern Mexico to Brazil and is composed of several cryptic, undescribed species. The ‘true’ T. angustula might be restricted to Brazil, as it was described from a worker collected in that country and whose whereabouts are unknown (Camargo and Pedro, 2007). Thus, we did not include this species in our analyses. Our last criterion in the selection of taxa was the availability of species-occurrence data. Despite the academic, social, and economic interest on stingless bees, the bee fauna of Colombia remains largely unexplored, taxonomic revisions are few, and data for many specimens of most species in Colombian collections are not digitized and available in databases. We assessed data availability by searching for the occurrence of each of the species listed by Nates-Parra and Rosso-Londoño (2013) in GBIF (www.gbif.org), SpeciesLink (http://splink.cria.org.br/), and SIB Colombia (https://sibcolombia.net/). Based on these criteria above, we selected the following nine species: Frieseomelitta paupera (Provancher), Melipona eburnea Friese, M. favosa (Fabricius), Nannotrigona gaboi Jaramillo et al., N. melanocera (Schwarz), N. schultzei (Friese), Paratrigona eutaeniata Camargo & Moure, P. opaca Cockerell, and Scaura longula Lepeletier. Because no species of Frieseomelitta Ihering was indicated by Nates-Parra and Rosso-Londoño (2013), we chose F. paupera, one of the most common species of the genus in the country. Another frequently used species in Colombia is Nannotrigona mellaria (Smith). However, Jaramillo et al. (2019) revised the species of this genus and found that most specimens in Colombian collections standing under that name belong to a new species, which they described as Nannotrigona gaboi Jaramillo et al. Thus, we included N. gaboi and excluded N. mellaria from our analyses. Except for N. gaboi, which is presently known only from Colombia, all other target species occur through Central and/or South America (Fig. 1).
Data pre-processing and calibration areaWe obtained species-occurrence data from GBIF and SpeciesLink, which we complemented from Jaramillo et al. (2019) for species of Nannotrigona, and Nates-Parra et al. (1999); Gonzalez and Vélez (2007), and Fernández et al. (2010) for species of Paratrigona. Such species-occurrence data might have come from specimens collected at flight, at flowers or from either wild colonies or managed hives. Stingless bees are also often captured while collecting nesting materials (mud, feces, resins, etc.). However, such a detailed information is often absent from specimen labels. Because the specific origins of the specimens are often uncertain, we used all species-occurrence data independently of the specimens’ provenance. The senior author checked all occurrence records to prevent recognizable errors in georeferencing and taxonomy.
We performed further processes of data cleaning that included the removal of occurrences with no coordinates, exclusion of records with 0, 0 coordinates, and duplicate removal. All data cleaning steps were performed in R 3.5.1 (R Core Team, 2018) following Cobos et al. (2018). Finally, to reduce problems derived from spatial autocorrelation, we spatially thinned the data (spatial rarefaction) at a distance of 10 km using the spThin package (Aiello-Lammens et al., 2015) in R. Considering the density of available records, resolution of layers, and environmental heterogeneity in mountainous areas, such as in the Andes, the selected distance avoids excluding environmental conditions in highly heterogeneous areas and helps to reduce sampling bias. Final sets of occurrences were randomly split 50% for training and 50% for testing models (Supplementary Data Table S1).
As environmental data, we used 15 of the so-called “bioclimatic” variables obtained from the WorldClim database version 1.4 at 2.5′ resolution (available at www.worldclim.org). Such variables are derived from the interpolation of average monthly temperature and rainfall data (Hijmans et al., 2005). We removed four variables combining information of temperature and precipitation (BIO8, BIO9, BIO18 and BIO19) due to known spatial artefacts (Escobar et al., 2014). We performed principal component analyses with the remaining variables to reduce dimensionality and prevent for multicollinearity in our analyses. We prepared four sets of predictors using the sixth first raster principal components (PCs), including distinct number of PCs per set (Supplementary Data, Table S2). All these sets were directly included in the process of model calibration, as this has been suggested as an option for optimal selection of variables for ecological niche modeling (Cobos et al., 2019c).
Calibration area is an important element to consider in ecological niche modeling, as it represents a geographic space that has been accessible to the species and is relevant for model calibration (Barve et al., 2011). Here we defined these areas as the zones included in a buffer of 200 km from the occurrence points. This approach was used to avoid including areas that could be inhabited by the species but do not contain records only because of sampling bias (Peterson, 2014). We also considered that the distance selected represents a good estimation of long-term dispersal potential for the species of interest.
Ecological niche modelingFor model calibration, we tested a total of 315 Maxent (v 3.4.1; Phillips et al., 2006) candidate models per species. Each calibration process with distinct settings resulted from combinations of 3 sets of environmental variables, 7 feature classes (all combinations of linear = l, quadratic = q, product = p), and 8 regularization multipliers (0.1–1 at intervals of 0.3, and 2–5 at intervals of 1). Feature classes control the way Maxent treats and uses the variables; for instance, only linear responses of suitability to the variable are produced if linear features are used, whereas convex or concave responses are obtained if combinations of quadratic responses are used. Distinct values of the regularization multiplier help to explore distinct degrees in which the model is smoothed, which is the way suitability is fit to climatic values that are similar to those where the species occur (Simões et al., 2020). Candidate models were evaluated based on statistical significance (partial ROC; Peterson et al., 2008), predictive ability (omission rates, E = 5%; Anderson et al., 2003), and complexity (Akaike information criterion corrected for small sample sizes, AICc; Warren and Seifert, 2011). We chose the best parameterizations among the significant modes that had the lowest omission rates (below 5% when possible) and delta AICc values lower than 2, in that order.
For all species, we created final models using the complete set of occurrences and parameterizations selected during model calibration. We performed 10 replicates by bootstrap, and projected the models to Colombia in current and future climate scenarios. We allowed for extrapolation in our model, differently for each case, by considering the response curves found per each species and the way Maxent performs projections depending on extrapolation features selected (Merow et al., 2013). When the response curves were or tended to be bell shaped, we allowed for free extrapolation (i.e., suitability in conditions outside limits of calibration areas were predicted following the trend of the response). When the curves where truncated (responses with increasing suitability towards the end of calibration conditions), we allowed for extrapolation with clamping (i.e., suitability outside the limits of calibration conditions will be always equal to the value found at these limits). For future scenarios, we used variables at the same resolution than current predictors, and the Representative Concentration Pathways (RCPs) 4.5 and 8.5, which represent a scenario of medium low and high Greenhouse Gas (GHG) emissions (IPCC, 2013). We used three General Circulation Models (GCMs) for each scenario to represent climatic conditions (see Supplementary Data, Table S3 for details on GCMs used). Different climate research centers create GCMs using distinct sets of data and parameter settings; therefore, they can contribute significantly to variation in projection results (Diniz-Filho et al., 2009; Ramírez-Gil et al., 2019). A total of six projections were done for future scenarios, one for each combination of RCP and GCM. We transformed future layers using the rotations found for the PCs created with current layers to make transferences safe. We conducted all calibration exercises, as well as finals models and their transfers, using the kuenm R package (Cobos et al., 2019a) which uses Maxent as the modeling algorithm.
Post-modeling analysesWe calculated medians of all replicates of final models to summarize the results (across all parameterizations when more than one best setting was selected). We defined suitable areas as those having suitability values above a threshold equivalent to a 5% omission percentage in the areas of calibration. For each future scenario (RCP 4.5, and 8.5), we identified changes in the suitable areas between current and future projections and represented the agreement of changes of suitable areas (stable, gain, loss) among the three GCMs used (Cobos et al., 2019b).
To detect areas of strict extrapolation (i.e. areas with future climatic conditions non-analogous to current ones), we used the mobility-oriented parity metric (MOP; Owens et al., 2013). This method evaluates levels of similarity between calibration and projection areas and identifies areas of strict extrapolation when similarity is zero. We conducted all post-modeling analyses using the kuenm R package.
ResultsSelected parameters settings for final models varied among species (Supplementary Data, Table S4). Feature classes including quadratic responses were the most common among the selected response types. Regularization multipliers used in selected parameters ranged from 0.1 to 3.0. For four of the nine species minimum omission rates were right above 0.05 (the maximum expected omission rate), with values of 0.06 or 0.07; for one species the minimum omission rate was 0.18 (Supplementary Data, Table S4). Extrapolation types used for model projections, chosen after examination of predictor response curves, were as follows: free extrapolation for F. paupera, M. eburnea, N. gaboi, N. melanocera, N. schultzei, and P. eutaeniata; and extrapolation with clamping for M. favosa, P. opaca, and S. longula.
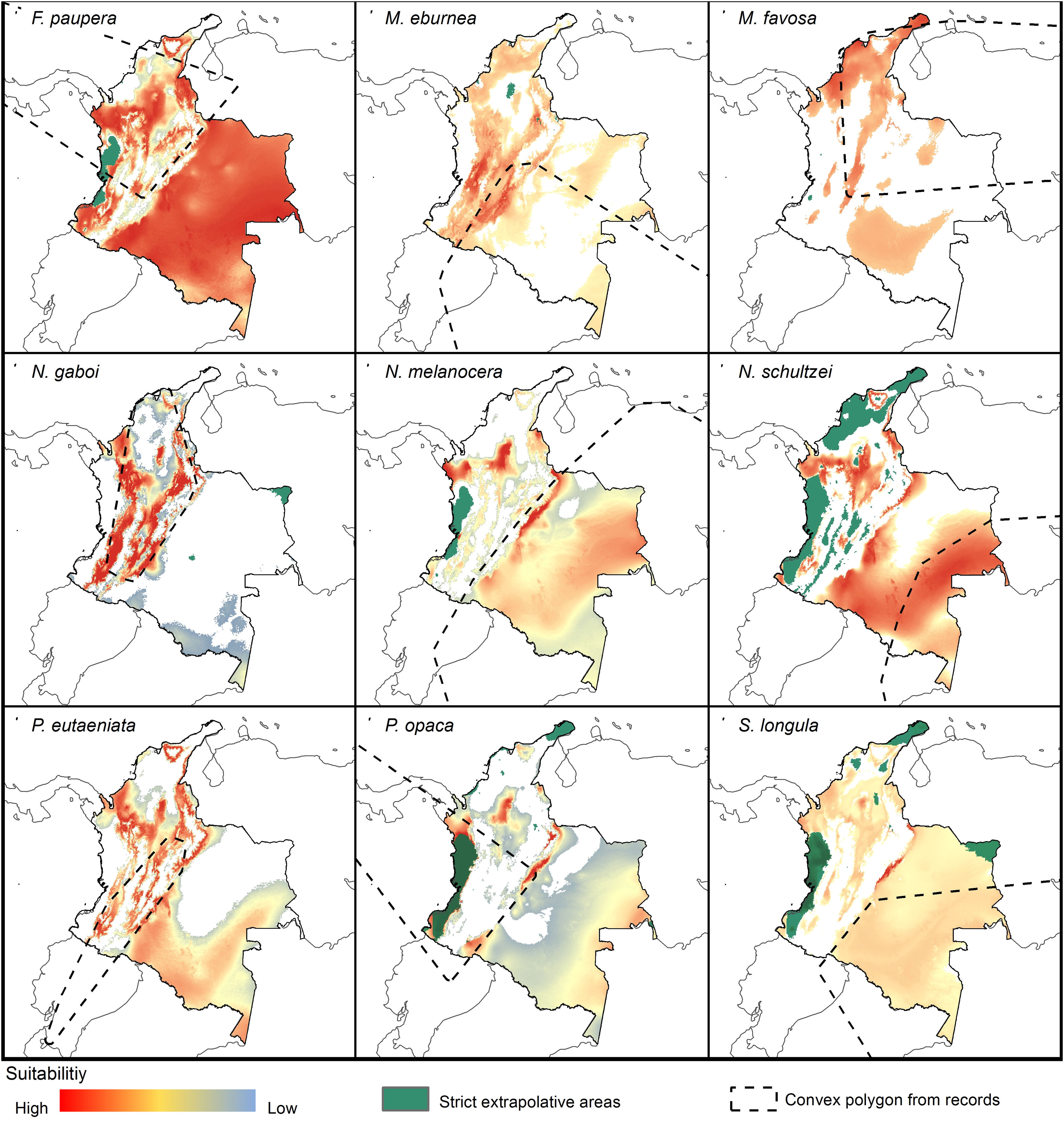
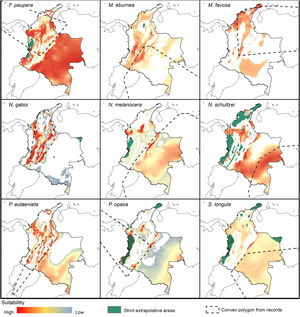
Potential distribution modelsThe patterns of potential distribution varied significantly among the studied stingless bee species. For species recorded from low to mid-elevations in the Andean region (F. paupera, M. eburnea, N. gaboi, P. eutaeniata, and P. opaca), the models predicted high suitability of habitats either across most lowland areas of Colombia (F. paupera, Fig. 2), certain areas (P. opaca) or most areas of the Andean region (M. eburnea, N. gaboi, and P. eutaeniata). For species with occurrence records in the Amazon and Orinoquia regions (N. melanocera, N. schultzei, and S. longula), the models predicted high suitability in some areas of those regions as well as lowland habitats in northwestern Colombia. For M. favosa, high suitability is predicted across lowland areas of the Caribbean regions, as well as some areas along the Magdalena and Cauca’s River valleys, Amazon and Orinoquia regions.
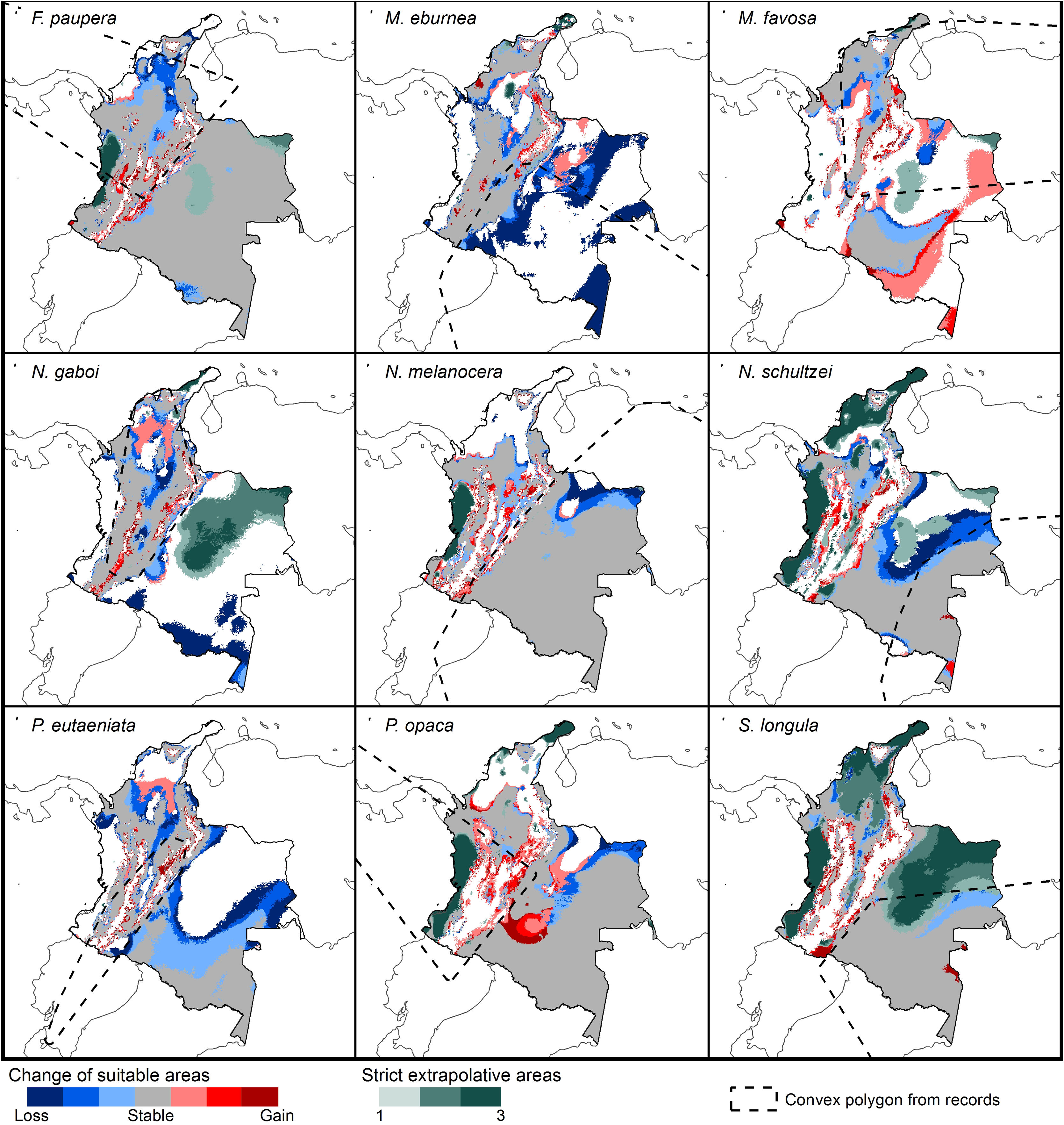
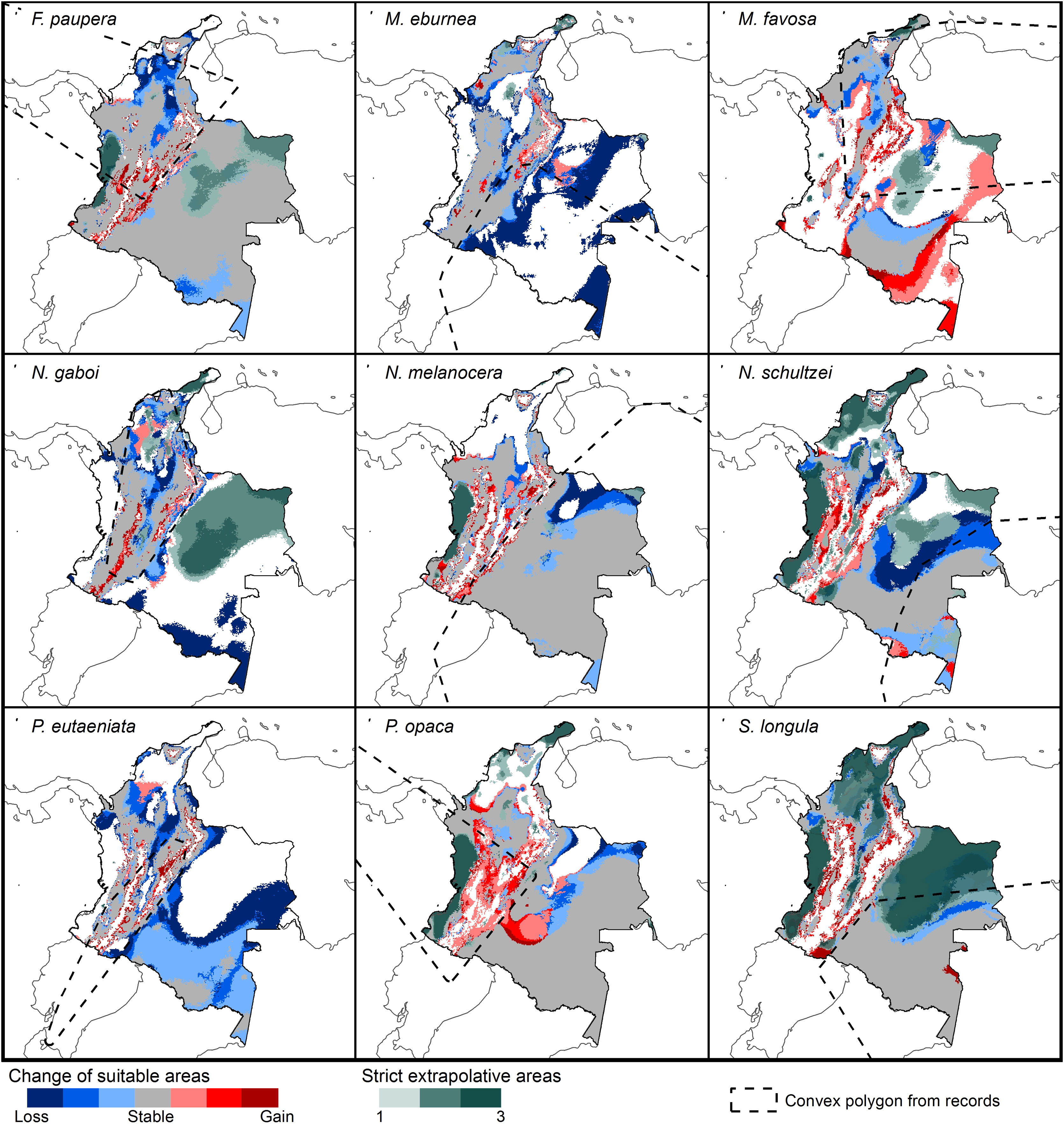
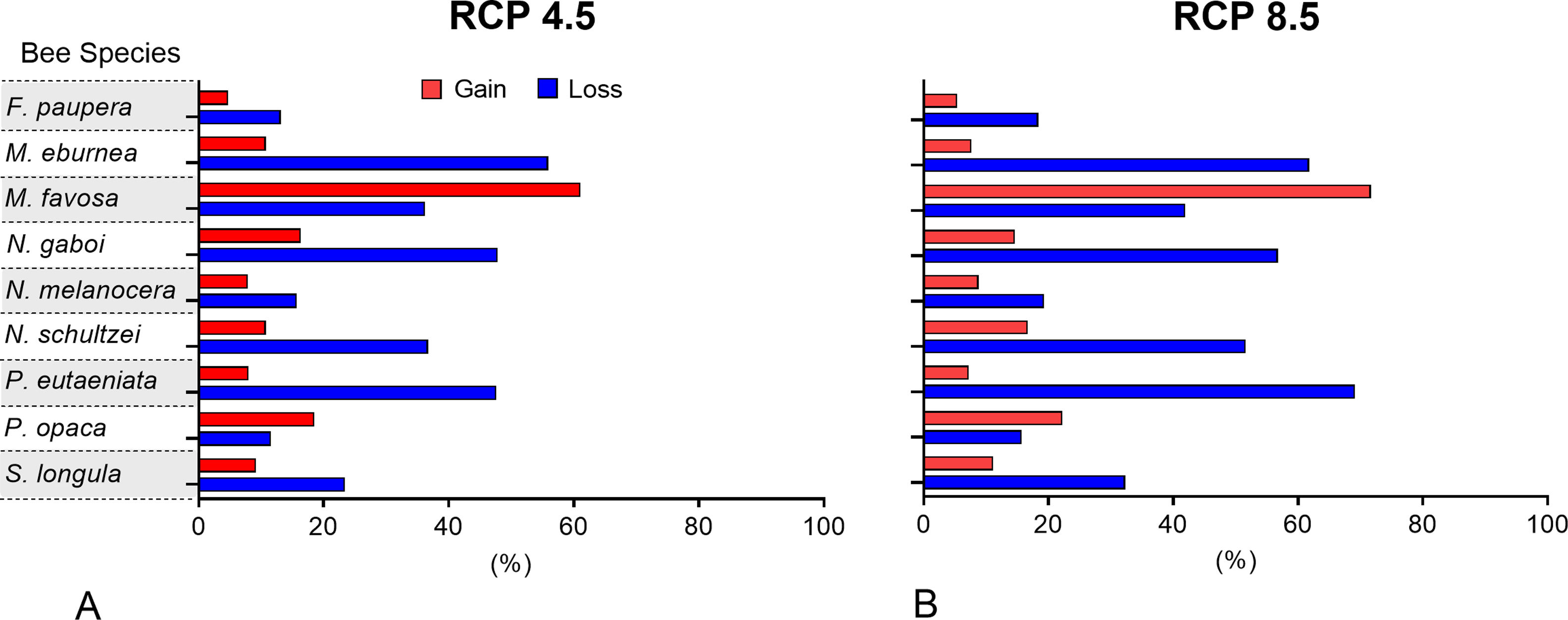
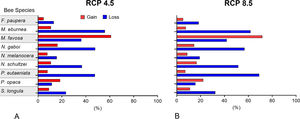
Changes of potential distribution under climate change scenariosThe possible impacts of climate change on the species distributions (either gain or loss) varied among taxa, with most species (seven of nine) losing more suitable areas than gaining (Figs. 3–5, Supplementary Data, Table S5). Under both RCP 4.5 and RCP 8.5, the models predicted more gains than losses of suitable areas for M. favosa and P. opaca whereas for the remaining species they predicted relatively medium (23.4–36.3%) to high losses (>50.0%) within their areas of occurrence. In particular, M. eburnea, N. gaboi, and P. eutaeniata will experience the greatest losses (47.6–55.9%) under RCP 4.5, which increases up to 20% under RCP 8.5.
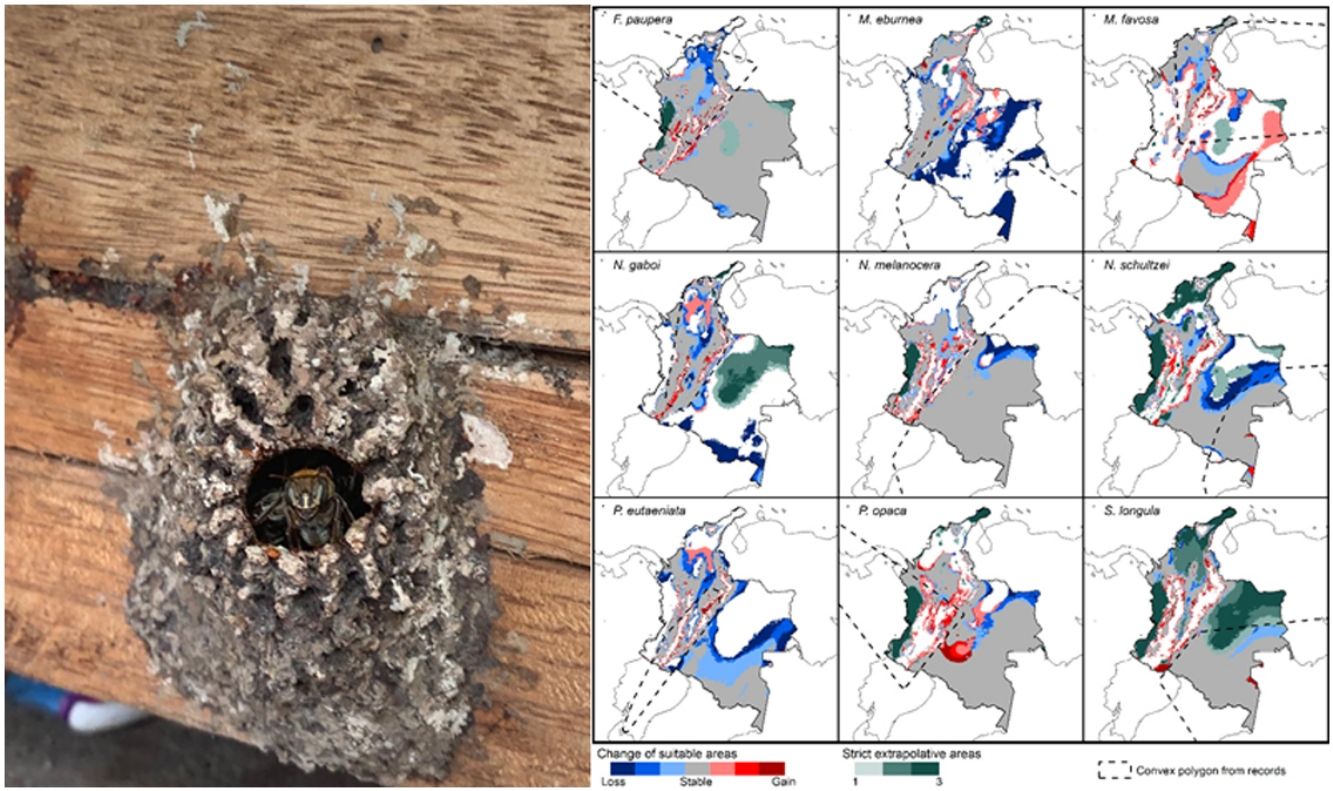
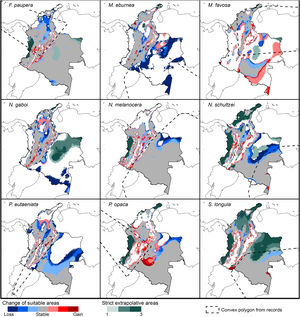
Potential changes in suitable areas in Colombia for the studied species considering RCP 4.5. Changes in suitable areas represent the agreement of predictions among GCMs; for gain and loss, darker colors indicate greater agreement. Areas of strict extrapolation are shown in green colors, color level indicates agreement of these areas among GCMs. Broken lines enclose areas with occurrence data. Genus names: F = Frieseomelitta, M = Melipona, N = Nannotrigona, P = Paratrigona, S = Scaura (For interpretation of the references to color in the figure legend, the reader is referred to the web version of this article).
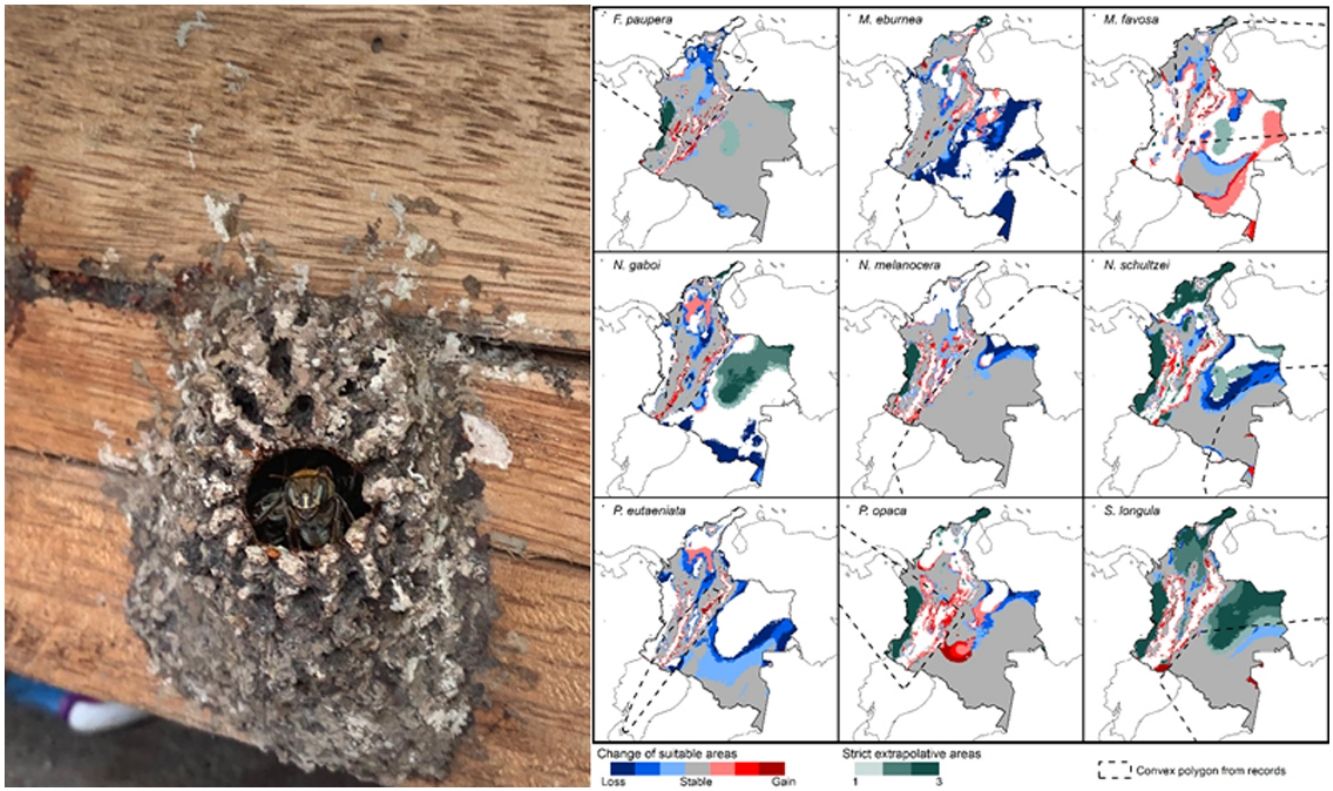
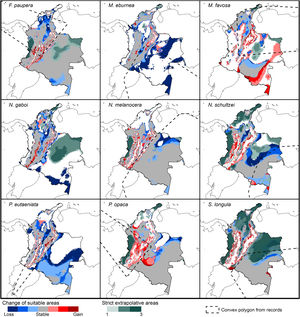
Potential changes in suitable areas in Colombia for the studied species considering RCP 8.5. Changes in suitable areas represent the agreement of predictions among GCMs; for gain and loss, darker colors indicate greater agreement. Areas of strict extrapolation are shown in green colors, color level indicates agreement of these areas among GCMs. Broken lines enclose areas with occurrence data. Genus names: F = Frieseomelitta, M = Melipona, N = Nannotrigona, P = Paratrigona, S = Scaura (For interpretation of the references to color in the figure legend, the reader is referred to the web version of this article).
Several species, namely M. eburnea, M. favosa, N. schultzei, and S. longula, gained suitable areas but these were outside of the known distributional range. For example, M. favosa, a species that primarily occurs along the dry forests of the Caribbean region, gained large areas (61.1–71.7%) in the Amazon and Orinoquia regions. Similarly, N. schultzei and S. longula, species from the Amazon and Orinoquia regions, gained at least 10% of suitable areas along both sides of the three mountain ranges of the Andes, as well as the Sierra Nevada de Santa Marta in the Caribbean.
DiscussionCurrent and potential distribution modelsHerein we presented the known and potential distributions of nine species of stingless bees commonly used in Colombia’s meliponiculture. Our results show that most species exhibit restricted distributions to particular ecosystems or areas within the continental natural regions of the country, even if they are widely distributed in the neotropical region. For example, M. eburnea occurs through Bolivia, Peru, Ecuador, and Brazil (Fig. 1, Camargo and Pedro, 2007). However, this species appears to be restricted to the Andean region of Colombia, although records from the Brazilian Amazon exist. Such restricted distributions within Colombia indicate that these species have distinct and specialized ecological niches and that, among the diversity of ecosystems of Colombia, some are more suitable than others. The predicted distribution for most species supports this idea, but it also shows high suitability in areas outside their known distribution range (Fig. 2).
Particularly interesting are those species with occurrence records in the Amazon and Orinoquia regions (N. melanocera, N. schultzei, and S. longula), for which our models predicted high suitability in lowland habitats in northwestern Colombia, on the other side of the Cordillera Oriental. This Cordillera is the longest and widest of three mountain ranges that traverse Colombia from southwest to northeast, and it intersects with dry Caribbean coastal plains on its northern tip (e.g., Rangel-Ch and Aguilar, 1995). Considering that the Andes represents a major barrier for the distribution and dispersal of many organisms, the presence of these taxa in northwestern Colombia seems unlikely. However, some taxa that appeared to be restricted to the Amazon or Orinoquia regions have recently been recorded from lowland northwestern Colombia (Department of Antioquia). For example, Melipona titania Gribodo is thought to be endemic to the western Amazon (Camargo and Pedro, 2008), but it has been recently found from mid-elevations on the eastern slopes of the Central Cordillera in Antioquia (D. Guevara & V.H. Gonzalez, unpublished observations). Likewise, species from other bee taxa that were also presumably restricted to the western Amazon, such as the South American leaf-cutter bee Megachile subgenus Zonomegachile, have recently been found in this area (Gonzalez et al., 2018b).
Given the antiquity of stingless bees and that major diversifications in the New World occurred about 30–40 Mya (Rasmussen and Cameron, 2010), the presence of these Amazonian taxa in inter-Andean ecosystems in Colombia suggests that they were present there before the uplifting of the Oriental Cordillera less than 14 Mya ago (Gregory-Wodzicki, 2000). Although recent cross-Andean dispersal is possible for some taxa, as reported for orchid bees (Dick et al., 2004), this seems unlikely for stingless bees because they are not capable of long-distance flights and new colonies are highly dependent on the mother nest (e.g., Roubik, 2006). These areas in northwestern Colombia are poorly sampled and future surveys will test the predictions by our models. Finally, it is likely that the apparently restricted distribution of the species considered in this work might just be a sampling bias. Most collecting effort in Colombia has been focused on the Andean region, particularly in the central Andes near Bogotá, where major universities and research centers are located (Gonzalez and Engel, 2004; Arbeláez-Cortés, 2013). Thus, our results are useful to plan further sampling efforts in Colombia.
Potential distribution under future climate scenariosOur models predict that seven of the nine stingless bee species would experience reduction in their climatically suitable areas within their areas of occurrence. In particular, M. eburnea, N. gaboi, and P. eutaeniata will have the greatest suitable area losses (Fig. 5). Some species, such as N. schultzei and S. longula, will also gain suitable areas in higher elevations (Figs. 3 and 4). Thus, our data agree with other studies showing that, under future climate scenarios, the suitable area for some species will be reduced while for others it might move upwards in elevation to compensate for the increase in temperature (e.g., Feehan et al., 2009; Prieto-Torres et al., 2020). Local losses of pollinators not only could disrupt an entire pollinator network (e.g., Brosi and Briggs, 2013), but could also have significant economic and social consequences, particularly for species involved in crop pollination (Giannini et al., 2020). For example, Colombia is one the main producers of coffee in the world, a crop that benefits from pollination by stingless bees (Imbach et al., 2017). Some of the species modeled here are common in coffee plantations in Colombia, such as P. eutaeniata and P. opaca, which farmers often referred to as the “coffee bee” because it is a common visitor of this crop (Giraldo et al., 2011; V.H. Gonzalez, pers. obser.). Models under future climatic scenarios predict that suitable areas for coffee farming in South America will experience either a significant reduction in size along with a decline of their pollinators or a relatively small increase in coffee-suitable areas but with a reduction in pollinator richness (Imbach et al., 2017). Because small farmers are the main producers of coffee in Colombia, such reduction in suitable areas will likely have a significant impact on agriculture, and therefore, in their food security or livelihoods.
Although information is limited, few palynological studies indicate that some of the species modeled in this work (M. eburnea, M. favosa, and P. opaca) might also be important pollinators of native plants in Colombia (Giraldo et al., 2011). For example, in certain regions M. eburnea relies heavily on pollen of Myrcia D.C. (Myrtaceae), a plant genus abundant in the Andean region and relevant for plant succession (Obregon and Nates-Parra, 2014). Thus, the expected reduction of suitable areas under climate change scenarios will also affect the reproduction of native plants.
Only a few studies have addressed the possible impact of climate change on the spatial distribution of stingless bees, and the results are not encouraging. While some species will gain suitable habitats (Giannini et al., 2020) or experience relatively small (up to 35%) habitat reductions (Giannini et al., 2012, 2017), others will have similar or even higher loss of occurrence area to the predictions by our models. For example, Melipona quadrifascita Lepeletier, an endemic species to the Brazilian Atlantic Forest, will experience up to 63% of reduction in its occurrence area (Marchioro et al., 2020) while other species from the eastern Amazon of Brazil are predicted to potentially lose more than 80% of their occurrence area (Giannini et al., 2020).
Variation in stingless bees’ physiology, as well as in their ability to thermo- and hydroregulate, might explain the differences in their current and predicted distribution ranges under future climate scenarios. Numerous studies demonstrate that thermal limits (hot and cold tolerances) determined species’ fundamental niche and thus have a strong influence on the species potential distribution (Angilletta, 2009; Sunday et al., 2011). Although most studies emphasize the role of temperature (e.g., Becker et al., 2018), desiccation tolerance appears to be equally important (Bujan et al., 2016; Burdine and McCluney, 2019). For example, while some species of stingless bees can regulate brood temperature, others generally thermoconform (Torres et al., 2007, 2009; Halcroft et al., 2013; da Silva et al., 2017). In some cases, regulation of humidity appears to be more relevant than regulation of temperature to maintain colony health (Solarte et al., 2015; Ayton et al., 2016). Both aspects of species’ physiology, temperature and humidity tolerances, are also critical to predict their responses to changes in land use and climate change (Deutsch et al., 2008; Hamblin et al., 2017). However, these functional traits remain poorly studied in bees (Burdine and McCluney, 2019; Gonzalez et al., 2020).
Implications for conservation and sustainable useOur results have significant implications for the future of conservation and sustainable use of stingless bees in Colombia. We showed that species have restricted distribution ranges as well as suitable areas in the country (Fig. 2), results that support conclusions from previous taxonomic studies (Jaramillo et al., 2019; Guevara et al., 2020). From a practical standpoint, this means that long-distance relocation of nests, an increasingly common practice in meliponiculture, needs to be avoided, even into areas where our models predicted high suitability but where the species has not yet been collected. For example, N. melanocera is currently known from locations east of the Andes (Fig. 1) but our models predicted high suitability in the Departments of Choco and Antioquia, west of the Andes (Fig. 2). Long-distance relocation of nests may be problematic for several reasons: First, introductions of species to a new habitat might disrupt pollinator networks, promote the spread of parasites and pathogens, or alter the genetic structure of wild and managed populations (e.g., Byatt et al., 2016; Chapman et al., 2018). Second, populations of some species might be adapted to local conditions of temperature and humidity, as documented in ants (Baudier and O’Donnell, 2020), and thus the new conditions might lead to low rates of colony establishment or total loss. Third, we still have a limited understanding of their physiology, taxonomy, and distribution, thus preventing us from making accurate predictions of their distribution ranges. Finally, some of the key biological interactions occurring in the bees’ native range can dramatically change in areas of introduction; thus, the effects of such introductions into new areas need to be carefully considered.
Unfortunately, environmental laws that regulate the use and relocation of wild colonies in Colombia do not exist, except in the Amazon region. In 2017, CorpoAmazonia (www.corpoamazonia.gov.co), a governmental autonomous regional corporation that has jurisdiction over three departments (Amazonas, Caquetá, and Putumayo), granted license to a program on stingless bees that promoted Colombia’s transition to peace. While restricting the extraction of colonies from wild nests as well as relocation of nests, it also allowed certified beekeepers to begin with five colonies that they could captured only using trap-nests, a method developed by Brazilian beekeepers and the only legal method in the country (Oliveira et al., 2013). Such legislation was expanded in 2018 to accommodate the growing interest of stingless bees in the area, maintaining restrictions in the acquisition and relocation of nests but allowing new beekeepers to obtain up to 10 colonies per species (or per common name) either from other beekeepers or from trap-nests. The CDA (www.cda.gov.co), the other regional corporation that has jurisdiction over the remaining three departments of the Amazon region (Guainía, Guaviare, and Vaupés), adopted these laws in 2019. The legislation for the Amazon region represents a step forward in developing effective conservation policies in Colombia, but it needs to be implemented rapidly in other areas of the country, particularly in the Andean region. Most beekeepers are in the Andes (Nates-Parra and Rosso-Londoño, 2013), a region that also contains most crops and a higher richness of stingless bees (Gonzalez and Engel, 2004) than the Colombian Amazon.
Developing and implementing an effective policy for the growing activity of stingless bee keeping in Colombia is challenging because of the high number of species (>100 spp.), difficulty to identify them, and lack of information regarding their distribution. One solution is to promote the use of particular species or a set of species that are easy to identify and capture using trap-nests. We could select species by natural regions or ecosystems while restricting the use of other species until more information is available. For example, M. favosa and some species of Nannotrigona are good candidates for meliponiculture in northern Colombia. However, trap-nests do not capture these species as easily as T. angustula, the main species used by beekeepers in Colombia, as well as in other countries of the Americas (Quezada-Euán et al., 2018). Unfortunately, the taxonomy of T. angustula is troubling, as several cryptic, undescribed species are under this name (Camargo and Pedro, 2007). Doubtless, resolving the taxonomy of T. angustula is a priority because cryptic species tend to have small populations and distribution ranges, and thus they are a priority for conservation (Bickford et al., 2007).
Our results also represent the first step in forecasting the effects of human-induced global warming on pollinators in Colombia, and thus they highlight the need to begin developing climate adaptation strategies. Some of these strategies might include programs that help beekeepers to shift to other species in areas where our models predicted a reduction in their potential distribution range for bees. This would be particularly important for beekeepers relying on M. eburnea, a species vulnerable to extinction (Nates-Parra, 2007) that our models predicted to be vulnerable to a drastic reduction in its suitable areas under future climate scenarios. Unfortunately, climate change is not the only driver that will affect Colombian pollinators. Although pollinator losses due to deforestation, agricultural intensification, and rampant pesticide use have not yet been documented in Colombia, circumstantial evidence suggests this is already happening. For example, due to the loss of insect pollinators, ex-combatants in Caquetá have abandoned passion fruit crops (Passiflora edulis Sims), a highly dependent pollinator crop that government agencies promoted as an alternative to illegal crops. Many farmers in Huila now rely entirely on hand pollination, an unsustainable practice that was rare a decade ago (Calle et al., 2010; V.H. Gonzalez, per. obs.). Farmers in other departments have followed this practice as well. A law that safeguards pollinators and their habitats, as well as promotes the sustainable use of pollinators has been under consideration by the Colombian Congress. Last year, Congress turned it down because of disagreements in the law’s restrictions on pesticides. Sadly, the loss of passion fruit pollinators might represent the canary in the mine shaft for Colombia’s pollinator-dependent food crops.
Author contributionsVHG and MEC conceived and designed the study. VHG, JJ, and RO compiled the database and MEC performed the ecological niche models. All authors contributed in the interpretation of results. VHG and MEC wrote the paper.
Data availability statementThe authors confirm that the data supporting the findings of this study are available within the article’s supplemental material.
FundingThis work was supported by the University of Kansas Center of Latin American and the Caribbean Studies and National Science Foundation’s REU program (DBI 1560389).
Competing interests statementThe authors declare no conflict of interest
We are indebted to Amy R. Comfort, Catalina Gutierrez, Mariano Lucia, John Barthell, and anonymous reviewers for comments and suggestions that improved this manuscript.