Miscanthus sinensis-dominated semi-natural grassland is one component of a typical Satoyama landscape. M. sinensis most notably forms ring patches as a result of human management, which includes the removal of aboveground stems by burning. In this study, I hypothesized that M. sinensis aids the coexistence of several plant species under managed conditions because of its notable ring patches. To test this hypothesis I monitored the richness of plant species inside and outside M. sinensis ring patches for 5 years, which included one non-managed year, and compared richness between managed and non-managed years. Results showed that species richness was higher inside than outside patches in all cases, but that this effect was more prominent in managed years than in the non-managed year. Consequently, human management is promoting the coexistence of plant species in M. sinensis-dominated semi-natural grassland. Human management will likely play an important role in conserving plant species diversity in semi-natural grasslands by changing relationships among plants.
Plants that co-occur can interact both positively and negatively (Callaway, 1994; Brooker et al., 2008; Kikvidze et al., 2009). Positive interactions can increase species survival and diversity when the presence of one species modifies the environment so as to reduce the frequency of some disturbance or stress, allowing less stress-tolerant species to survive (Hacker and Gaines, 1997; Cavieres and Badano, 2009). However, these plant-to-plant interactions can alternate between positive and negative effects, when plants change their growth forms or patterns (Armas and Pugnaire, 2005; Ervin, 2005; Miriti, 2006; Osawa, 2011; Estape et al., 2013). For example, Ervin (2005) showed that only collapsed culms on Juncus effusus L. contributed to neighboring plants in wetland. Armas and Pugnaire (2005) showed that such plant interactions could alter with life stage or environmental variability.
Human management activities such as controlled burning and mowing may alter the growth forms or patterns of some plants. Semi-natural grasslands, which are maintained by continuous human management, are decreasing in several regions (White et al., 2000; Kitazawa and Ohsawa, 2002; Kawano et al., 2009; Koyanagi et al., 2009; Koyanagi and Furukawa, 2013). In Japan, semi-natural grasslands dominated by Miscanthus sinensis are a component of the typical Satoyama landscape, which has long been developed and maintained by continuous human management (Yasuda and Programme, 2001). M. sinensis is a clonal perennial plant that forms ring patches, a common behavior among clones of tussock grasses (Kobayashi and Yokoi, 2003). Notably, ring patches form under human management activities such as burning and mowing (Osawa, 2011;Fig. 1A).
One study found that plant species richness was higher inside than outside M. sinensis ring patches in semi-natural grassland dominated by M. sinensis (Osawa, 2011). The present study suggests that human management alters interactions between M. sinensis and other plant species, which can range from negative or neutral to positive through the changing forms of M. sinensis ring patches because ring patches are notable under management (Fig. 1A and B). In other words, M. sinensis ring patches might facilitate the establishment of coexisting plants under management. Thus, I hypothesized that human management enhances the coexistence of M. sinensis and other species in M. sinensis-dominated semi-natural grasslands. Should this hypothesis be supported, it would provide new evidence in support of traditional grassland management that not only maintains the semi-natural grassland itself but also biodiversity conservation.
In this study, I examined the hypothesis that human management, i.e., the removal of aboveground M. sinensis by burning in early spring, mediates plant coexistence between M. sinensis and other plant species. To test this, I monitored plant species richness both inside and outside of M. sinensis ring patches generated by 5 years of annual burning, which included one non-managed year (see Materials and methods section). I predicted that plant species richness would be higher in managed years than in the non-managed year, especially inside M. sinensis regimes because of the notable ring patches formed in this human-managed semi-natural grassland. Based on the results, I discuss the significance of burning management in semi-natural grassland from the perspective of biodiversity conservation.
Materials and methodsStudy areaThis study was conducted at Sengokuhara in Hakone, Kanagawa Prefecture, Japan (35°13.3′N, 139°2.5′E). The climate is warm-temperate with a mean annual precipitation of 3228mm and a mean annual temperature of 16.1°C (Osawa and Inohara, 2008). There are approximately 13-ha of M. sinensis-dominated semi-natural grassland on the slope of Mt. Daigatake.
This semi-natural grassland has been maintained by annual burning every March since 1989 (Osawa, 2007). Although this area was maintained as M. sinensis-dominated semi-natural grassland for the use of grasses as building material historically, the management work was stopped in the 1950s because of decreasing material needs. As a result, several shrubs grew in the grassland before 1989 (Toyama, 1990). In 1989, burning management was reintroduced to restore the historical M. sinensis-dominated landscape (Toyama, 1990). Currently, burning is conducted by a local fire company annually.
In 2011, the annual burning practices were not conducted because of a disruption following the Great East Japan Earthquake of 11 March 2011. The local fire company that conducts the management left that spring to take part in activities in earthquake-affected areas.
Management proceduresBefore burning the grassland, an approximately 10-m-wide fire belt is cut using large lawnmowers. All areas other than the fire belt are burnt. Immediately after burning, the surface of the ground is covered by ash. There are very few intact (natural) grassland areas in the area. Thus, most years, the effect of management could not be evaluated. In this study, I focused only on burning as a management practice because cutting was conducted in 2011 to produce the fire belt, even though no burning was undertaken.
Species richnessI estimated species richness in the M. sinensis grassland in late April, approximately 1 month after final burning in 2009, 2012, 2013, and 2014. Burning was conducted on 30 March 2009, 15 March 2012, 12 March 2013, and 24 March 2014. I also investigated species richness in the M. sinensis grassland area that did not experience burning in late April 2011. Fifty patches of M. sinensis, which were surrounded by open bare ground (i.e., isolated from other patches), were picked randomly in each year, and the sizes of patch rings along the minor and major axes were measured each year. I could not fix patches because there is a ban on setting markings such as colored tags in the area (Fuji-Hakone-Izu National Park Plan: http://www.env.go.jp/park/fujihakone/intro/ accessed 01.09.14). The average patch size in the burning years was 3728.22±1964.11cm2 (average±SD) in 2009, 2263.00±1027.93cm2 in 2012, 4562.26±2343.09cm2 in 2013, and 4055.50±1653.84cm2 in 2014. The average patch size in the year without burning (i.e., 2011) was 2911.50±1145.30cm2.
The insides of rings were defined as quadrats inside patches. Plots of the same size were established immediately to the right of patch rings, and these were defined as quadrats outside patches. All plant species found inside and outside patch quadrats were identified. This sampling design allowed me to test for differences in plant richness with and without burning management and M. sinensis patches that might support plant richness.
I assessed the life-forms of all investigated plants to evaluate differences in their response to management. Plant life-forms were categorized as annual, perennial, or woody, following the method of Satake et al. (1981, 1982a,b).
Data analysisI used generalized linear mixed models (GLMMs) with a Poisson distribution (log-link), and model selection based on Akaike's information criteria (AIC; Burnham and Anderson, 2002) to examine the effects of year (2009, 2012, 2013, and 2014 were managed years, while 2011 was not a managed year), M. sinensis ring patches (inside versus outside patches), and patch sizes for the total number of species, including the number of annuals, perennials, and woody species. Although I had data for one non-managed year only, I could apply such inferential statistics because my hypothesis and data, i.e., managed and non-managed, were clear and had well-defined categories (Oksanen, 2001). A model with a lower AIC value is more plausible because it has higher explanatory power and fewer explanatory variables (Burnham and Anderson, 2002). To select the model, I constructed all of the candidate models, including the null model, and used the lowest one as the best model. I incorporated patch identity into the models as a random term to account for differences among patches of non-measured environmental factors such as soil type, nutrient content, and water content. I attached a unique patch identity for 5 years of data because the patches were not the same along the years. All analyses were conducted using R ver. 2.14.2 (R Core Development Team, 2012).
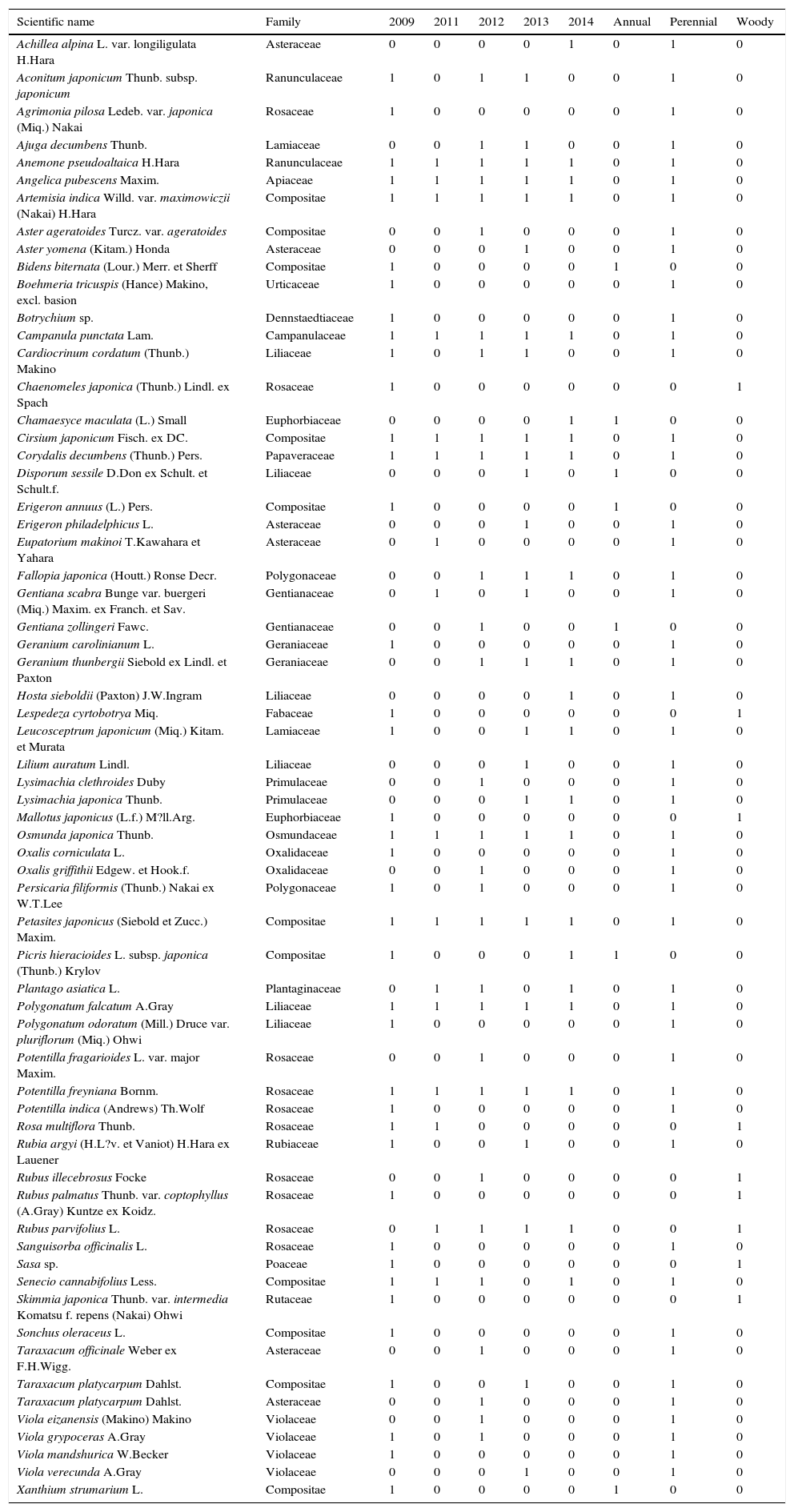
ResultsIn total, 64 plant species from 24 families were identified over the 5 years (Table 1). Sixteen species were identified in 2011 (non-managed year), whereas 39, 29, 26, and 21 species were identified in 2009, 2012, 2013, and 2014 (managed years), respectively, excluding un-identified species (Table 1). Ten species were identified in all 5 years, two species were found in 4 years, and 37 species were found in only 1 year (Table 1). Eupatorium makinoi T. Kawahara et Yahara was found only in the non-managed year (Table 1).
List and life forms of plants species occurring in the study areas.
| Scientific name | Family | 2009 | 2011 | 2012 | 2013 | 2014 | Annual | Perennial | Woody |
|---|---|---|---|---|---|---|---|---|---|
| Achillea alpina L. var. longiligulata H.Hara | Asteraceae | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Aconitum japonicum Thunb. subsp. japonicum | Ranunculaceae | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Agrimonia pilosa Ledeb. var. japonica (Miq.) Nakai | Rosaceae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Ajuga decumbens Thunb. | Lamiaceae | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Anemone pseudoaltaica H.Hara | Ranunculaceae | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Angelica pubescens Maxim. | Apiaceae | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Artemisia indica Willd. var. maximowiczii (Nakai) H.Hara | Compositae | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Aster ageratoides Turcz. var. ageratoides | Compositae | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Aster yomena (Kitam.) Honda | Asteraceae | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Bidens biternata (Lour.) Merr. et Sherff | Compositae | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Boehmeria tricuspis (Hance) Makino, excl. basion | Urticaceae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Botrychium sp. | Dennstaedtiaceae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Campanula punctata Lam. | Campanulaceae | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Cardiocrinum cordatum (Thunb.) Makino | Liliaceae | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Chaenomeles japonica (Thunb.) Lindl. ex Spach | Rosaceae | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Chamaesyce maculata (L.) Small | Euphorbiaceae | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Cirsium japonicum Fisch. ex DC. | Compositae | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Corydalis decumbens (Thunb.) Pers. | Papaveraceae | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Disporum sessile D.Don ex Schult. et Schult.f. | Liliaceae | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Erigeron annuus (L.) Pers. | Compositae | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Erigeron philadelphicus L. | Asteraceae | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Eupatorium makinoi T.Kawahara et Yahara | Asteraceae | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Fallopia japonica (Houtt.) Ronse Decr. | Polygonaceae | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| Gentiana scabra Bunge var. buergeri (Miq.) Maxim. ex Franch. et Sav. | Gentianaceae | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Gentiana zollingeri Fawc. | Gentianaceae | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Geranium carolinianum L. | Geraniaceae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Geranium thunbergii Siebold ex Lindl. et Paxton | Geraniaceae | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| Hosta sieboldii (Paxton) J.W.Ingram | Liliaceae | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Lespedeza cyrtobotrya Miq. | Fabaceae | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Leucosceptrum japonicum (Miq.) Kitam. et Murata | Lamiaceae | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Lilium auratum Lindl. | Liliaceae | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Lysimachia clethroides Duby | Primulaceae | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Lysimachia japonica Thunb. | Primulaceae | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Mallotus japonicus (L.f.) M?ll.Arg. | Euphorbiaceae | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Osmunda japonica Thunb. | Osmundaceae | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Oxalis corniculata L. | Oxalidaceae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Oxalis griffithii Edgew. et Hook.f. | Oxalidaceae | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Persicaria filiformis (Thunb.) Nakai ex W.T.Lee | Polygonaceae | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Petasites japonicus (Siebold et Zucc.) Maxim. | Compositae | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Picris hieracioides L. subsp. japonica (Thunb.) Krylov | Compositae | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Plantago asiatica L. | Plantaginaceae | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| Polygonatum falcatum A.Gray | Liliaceae | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Polygonatum odoratum (Mill.) Druce var. pluriflorum (Miq.) Ohwi | Liliaceae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Potentilla fragarioides L. var. major Maxim. | Rosaceae | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Potentilla freyniana Bornm. | Rosaceae | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Potentilla indica (Andrews) Th.Wolf | Rosaceae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Rosa multiflora Thunb. | Rosaceae | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Rubia argyi (H.L?v. et Vaniot) H.Hara ex Lauener | Rubiaceae | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Rubus illecebrosus Focke | Rosaceae | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Rubus palmatus Thunb. var. coptophyllus (A.Gray) Kuntze ex Koidz. | Rosaceae | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Rubus parvifolius L. | Rosaceae | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| Sanguisorba officinalis L. | Rosaceae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Sasa sp. | Poaceae | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Senecio cannabifolius Less. | Compositae | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| Skimmia japonica Thunb. var. intermedia Komatsu f. repens (Nakai) Ohwi | Rutaceae | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Sonchus oleraceus L. | Compositae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Taraxacum officinale Weber ex F.H.Wigg. | Asteraceae | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Taraxacum platycarpum Dahlst. | Compositae | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Taraxacum platycarpum Dahlst. | Asteraceae | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Viola eizanensis (Makino) Makino | Violaceae | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Viola grypoceras A.Gray | Violaceae | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Viola mandshurica W.Becker | Violaceae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Viola verecunda A.Gray | Violaceae | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Xanthium strumarium L. | Compositae | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
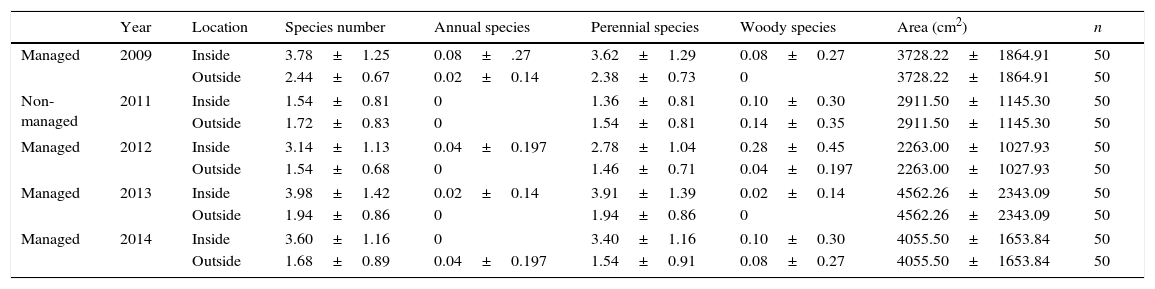
The average number of species in each quadrat is shown in Table 2. The number of annual species could not be determined in 2011. In all managed years (2009, 2012, 2013, and 2014), average species numbers were larger inside than outside the patches (Table 2).
Summary of the field observation in the study area. Mean with S.D. were presented for numerical variables. Species numbers excepted Miscanthus sinensis.
| Year | Location | Species number | Annual species | Perennial species | Woody species | Area (cm2) | n | |
|---|---|---|---|---|---|---|---|---|
| Managed | 2009 | Inside | 3.78±1.25 | 0.08±.27 | 3.62±1.29 | 0.08±0.27 | 3728.22±1864.91 | 50 |
| Outside | 2.44±0.67 | 0.02±0.14 | 2.38±0.73 | 0 | 3728.22±1864.91 | 50 | ||
| Non-managed | 2011 | Inside | 1.54±0.81 | 0 | 1.36±0.81 | 0.10±0.30 | 2911.50±1145.30 | 50 |
| Outside | 1.72±0.83 | 0 | 1.54±0.81 | 0.14±0.35 | 2911.50±1145.30 | 50 | ||
| Managed | 2012 | Inside | 3.14±1.13 | 0.04±0.197 | 2.78±1.04 | 0.28±0.45 | 2263.00±1027.93 | 50 |
| Outside | 1.54±0.68 | 0 | 1.46±0.71 | 0.04±0.197 | 2263.00±1027.93 | 50 | ||
| Managed | 2013 | Inside | 3.98±1.42 | 0.02±0.14 | 3.91±1.39 | 0.02±0.14 | 4562.26±2343.09 | 50 |
| Outside | 1.94±0.86 | 0 | 1.94±0.86 | 0 | 4562.26±2343.09 | 50 | ||
| Managed | 2014 | Inside | 3.60±1.16 | 0 | 3.40±1.16 | 0.10±0.30 | 4055.50±1653.84 | 50 |
| Outside | 1.68±0.89 | 0.04±0.197 | 1.54±0.91 | 0.08±0.27 | 4055.50±1653.84 | 50 | ||
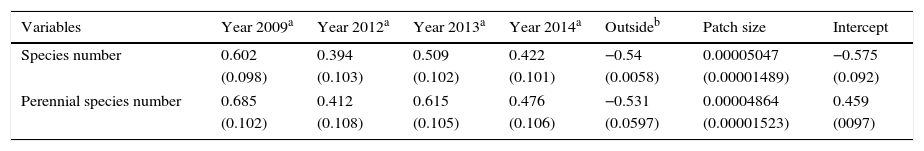
The numbers of annual and woody species were not analyzed using a GLMM due to small numbers (Table 2). The results of model selection from GLMM results showed that full models that included all explanatory variables were optimal, i.e., had the lowest AIC. The AIC values of the best models for both all species and perennial species were lower than those of the second best models by >2 (the AIC values of the best and second best models were 236.5 and 245.4 for all species, and 265.1 and 272.8 for perennial species). The GLMM results of the best models are shown in Table 3. GLMM analyses indicated that both total species number and perennial species number were higher in managed years than in the non-managed year (Table 3). The total number of species and the number of perennial species were both lower outside than inside the patches (Table 3). In addition, both total species number and perennial species number increased with increasing patch size (Table 3).
GLMM tests for effects of year (treatment), patch and circle area on plant species richness and each life forms. Values are means and standard errors (SE; in parentheses). Full model AIC is the lowest of all candidate models.
| Variables | Year 2009a | Year 2012a | Year 2013a | Year 2014a | Outsideb | Patch size | Intercept |
|---|---|---|---|---|---|---|---|
| Species number | 0.602 | 0.394 | 0.509 | 0.422 | −0.54 | 0.00005047 | −0.575 |
| (0.098) | (0.103) | (0.102) | (0.101) | (0.0058) | (0.00001489) | (0.092) | |
| Perennial species number | 0.685 | 0.412 | 0.615 | 0.476 | −0.531 | 0.00004864 | 0.459 |
| (0.102) | (0.108) | (0.105) | (0.106) | (0.0597) | (0.00001523) | (0097) | |
Species richness was higher in all managed years (2009, 2012, 2013, and 2014) than in the non-managed year (2011), and was also higher inside the patches than outside. These results suggested that both burning, which removes aboveground M. sinensis in the early spring, and the presence of M. sinensis itself, contribute to plant species richness in M. sinensis-dominated semi-natural grasslands. My prediction was supported.
Both total species number and perennial species number were higher inside than outside the patches. These results suggest that M. sinensis ring patches contribute to species richness in both managed and non-managed years, similar to results reported by Osawa (2011), who also showed that M. sinensis ring patches contributed to plant species richness under burning management. However, I also showed that M. sinensis ring patches can contribute to plant species richness without burning management. Increasing abiotic stress often shifts the balance of plant interactions from competition to facilitation in what is known as the stress gradient hypothesis or SGH (Choler et al., 2001; Callaway et al., 2002). One possible explanation is that a constant state of both competition and facilitation exists between M. sinensis and other species, but the relative importance of the two states could change according to the management regime. Unfortunately, I could not evaluate the effects of interactions between management and M. sinensis patches because only one non-managed year occurred due to exceptional circumstances. Another possible explanation is that changing the strength of the environmental filter could selectively allow plant colonization and persistence in a community (Keddy, 1992; Grime, 2006; Cornwell and Ackerly, 2009). For example, clear-cutting in grassland may relax light restrictions (decreasing the strength of the shade filter) or increase ambient temperature (increasing the strength of the temperature filter) (Braithwaite and Mallik, 2012). In my case, the largest ring patch differences between managed and non-managed years were seen in aboveground stems, which could influence micro-environmental conditions such as light and temperature. Future studies should determine the functions of M. sinensis ring patches in more detail.
Although both total species number and perennial species number were higher in the managed years than in the non-managed year, coefficient values differed among managed years. Specifically, before the non-managed year in 2009, coefficient values were highest, and just after the non-managed year, in 2012, I found the lowest coefficient values for both total species and perennial species numbers. These results suggest that once management ceases, the depressed number of species would not recover for at least 1 year. In short, once the plant community experiences relatively severe conditions, i.e., environmental filters, the effects of those conditions are likely to continue for some years. Only 10 perennial plants were found in 2012 [e.g., Viola eizanensis (Makino) Makino, Plantago asiatica L., Potentilla fragarioides L. var. major Maxim.]. The cessation of human management may result in strong sorting of species. Koyama and Tsuyuzaki (2012) indicated that relationships among coexisting species could collapse in irregular years when severe environmental conditions are experienced, but whether the collapse occurs is dependent on species-specific traits (Koyama and Tsuyuzaki, 2012). Although I could only observe one irregular year, my results suggest that relationships between coexisting M. sinensis and other species collapsed.
Patch size was a positive influence on both types of species richness. Previous studies have suggested that large patches display high productivity and diversity of early successional plant communities (Phillips and Shure, 1990; Osawa, 2011). Large patches of M. sinensis may generate high plant species richness due to similar mechanisms.
ConclusionShifts in plant interactions may contribute to species coexistence and may help to explain the occurrence of species-rich communities (Kikvidze et al., 2009). This study showed that the burning of M. sinensis-dominated semi-natural grasslands contributed to the maintenance of plant species richness. The M. sinensis-dominated grassland itself is maintained by human management (Osawa, 2011), and M. sinensis form ring patches under management. Therefore, human management is the most important factor maintaining plant species richness in M. sinensis-dominated semi-natural grasslands. Additionally, results suggest that once management ceases, the resulting reduction in species richness will not be easily reversed. Thus, management activities should continue in M. sinensis-dominated semi-natural grasslands, as it is important in maintaining plant species richness.
Conflicts of interestThe author declares no conflicts of interest.
I would like to thank N. Kozuma for his plant identification and Dr. A. Koyama for commenting on an early version of this manuscript. Also I would like to thank national park volunteers in Hakone. Two reviewers gave me several useful comments and suggestions.