Patch size is considered a major driver of species diversity in fragmented landscapes. Yet, assemblages of forest-dependent species, such as tropical arboreal mammals, can also depend on vegetation characteristics within the patch, i.e. patch quality. To test this, we assessed the influence of patch size and quality (measured through six attributes of vegetation structure) on arboreal mammals in 20 forest patches in the Lacandona rainforest – a biodiversity hotspot in the Mesoamerican Biological Corridor. We placed camera traps in 100 trees and registered arboreal mammals for one year. We used generalized linear models with a multimodel averaging approach and a distance-based redundancy analysis to identify the relative importance of patch size and quality on arboreal mammal diversity and composition. Species diversity was mainly and positively influenced by tree basal area – a vegetation attribute indicative of older and better-preserved forests – while species composition was driven by both patch size and quality. Patch size was negatively related to the abundance of kinkajous and Deppei’s squirrels, likely due to a higher density (and detectability) of individuals in small patches. The abundance of kinkajous and Deppei’s squirrels was lower in patches with higher tree density – an attribute typically related to forest disturbance. Therefore, to effectively preserve this highly endangered and ecologically relevant group of mammals, both patch size and quality should be considered, paying special attention to the conservation of large trees.
Land-use change has led to the rapid conversion of continuous forest into highly deforested and fragmented landscapes, especially in the tropics (Taubert et al., 2018). Such a loss of the world’s tropical forests is considered to be one of the major threats to global biodiversity (Newbold et al., 2016) since at least two thirds of the Earth’s terrestrial species inhabit the tropics (Barlow et al., 2018). Both forest loss and fragmentation decrease mean patch size in human-modified landscapes (Fahrig, 2003), and there is evidence that vegetation structure can be altered in smaller forest patches, with cascading effects on associated biodiversity (Arroyo-Rodríguez and Mandujano, 2006; Laurance et al., 2006; Santos et al., 2008; Tabarelli et al., 2008). However, as most studies in fragmented landscapes focus on assessing the effect of forest patch size on biodiversity (Fahrig, 2013), and usually use a relatively small number of patches (e.g. Arroyo-Rodríguez et al., 2013), our understanding of species’ responses to both forest patch size and vegetation structure in forest patches remains very poor.
Vegetation structure can play an essential role in the maintenance of tropical biodiversity. The overwhelming majority of biodiversity in the tropics is vertically distributed in strata that provide different resources (Kays and Allison, 2001; Whitworth et al., 2016a). Trees, especially the largest ones, are of particular importance, providing niches, shelter, and food for a plethora of organisms (Lindenmayer and Laurance, 2016; Pinho et al., 2020). Similarly, epiphytes in the canopy are important sources of nectar, fruits, invertebrates, nesting materials, and water for arboreal organisms, and have been used as indicators of forest integrity (Benzig, 2004; Krömer et al., 2014). Lianas are also of great importance, promoting canopy connectivity and valuable fallback foods to forest wildlife (Dunn et al., 2012), although the proliferation of lianas in disturbed forests can reduce growth, fecundity and survival of their host trees (Schnitzer and Bongers, 2002; Toledo-Aceves, 2015).
Vegetation structure can be particularly important for forest-dependent species, such as arboreal mammals (i.e. species with morphological adaptations for climbing that spend most of their lifetime on trees). Arboreal mammals constitute a large proportion of tropical mammal diversity, and depend directly on vegetation structure (Kays and Allison, 2001). Arboreal mammals may be more susceptible to forest disturbance than their terrestrial counterpart (Whitworth et al., 2019). This could have important implications for forest conservation since these animals are involved in crucial ecological processes such as pollination (e.g. Ganesh and Devy, 2000), seed dispersal (e.g. Andresen et al., 2018), herbivory (e.g. Chapman et al., 2013), and pest control in the upper rainforest strata (Kays and Allison, 2001; Estrada et al., 2017). Despite their importance, several groups of arboreal mammals are highly threatened – e.g. primates (Estrada et al., 2017), marsupials (Burbridge et al., 1996), sloths (Superina et al., 2010) – and others lack enough data to fully assess their conservation status (e.g. rodents: IUCN Red List version, 2019). Therefore, population declines and/or extirpation of these taxa could have cascading effects, altering biotic communities and ecosystem functioning (e.g. Terborgh et al., 2006; Uriarte et al., 2011).
Understanding the responses of arboreal mammals to changes in vegetation structure in fragmented forests is crucial for the adequate management and conservation of forest patches in anthropogenic tropical landscapes. Most studies that have evaluated the importance of vegetation structure on arboreal mammals have focused on particular species (e.g. howler monkeys: Arroyo-Rodríguez et al., 2007; but see Whitworth et al., 2019). This is not surprising due to the difficulties involved in accessing the canopy to make accurate surveys of these mammals, and due to their often cryptic and nocturnal habits (Kays and Allison, 2001; Lowman, 2009). For instance, in the anthropogenic landscape of the Lacandona rainforest – a biodiversity hotspot within the Mesoamerican Biological Corridor which has experienced increasing deforestation (Carabias et al., 2015) – the few studies on arboreal mammals have been limited to primates (e.g. Galán-Acedo et al., 2018), leaving an information gap regarding the responses of all other arboreal mammals to land-use change. Such information is critical in the case of locally threatened and data-deficient species (SEMARNAT, 2010), such as pigmy silky anteaters (Cyclopes didactylus), tayras (Eira barbara), northern tamanduas (Tamandua mexicana), and Mexican hairy dwarf porcupines (Coendou mexicanus).
Here we assessed how vegetation attributes influence the diversity and composition of arboreal mammal assemblages in forest patches of varying size. We expected mammal diversity to be positively influenced by vegetation attributes that are related to forest maturity and resource availability (tree basal area, epiphyte cover, canopy cover, tree connectivity), and negatively influenced by attributes related to edge effects and forest disturbance (tree density, liana basal area). The information we gathered sheds light upon arboreal mammal resilience to changes in vegetation structure in fragmented habitats and can help guide management decisions along the Mesoamerican Biological Corridor and other fragmented tropical forests.
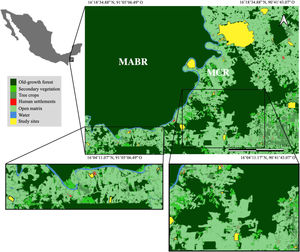
Materials and methodsStudy areaThe Lacandona rainforest, Chiapas, Mexico (91˚6′42.8″–90˚41′8.7″W; 16˚19′17.1″–16˚2′49.3 N; 100 to 700 m a.s.l.) has a warm (mean annual temperature 24−26 °C) and humid climate (mean annual precipitation ranges from 2500 to 3500 mm). The original vegetation is tall evergreen rainforest (Carabias et al., 2015). The Lacantún River separates a large protected forest tract on the western side of the study area, the Montes Azules Biosphere Reserve, from the Marqués de Comillas region on the eastern side, a heavily impacted area with approximately 50% of remaining forest cover (203,999 ha; Arce-Peña et al., 2019) dominated by cattle-ranches, annual crops and oil palm plantations. The study was conducted in 20 forest patches in the Marqués de Comillas region. Patches ranged in size from 5 to 1500 ha and were separated from each other by a distance of at least 2.5 km, measured from their geographical centers (Fig. 1).
Location of the 20 forest patches (in yellow) studied in the Marqués de Comillas Region (MCR) separated from the Montes Azules Biosphere Reserve (MABR) by the Lacantún River (shown in blue), Chiapas, Mexico. Tree crops include, but are not limited to, oil palm, rubber and cacao plantations. Open matrix includes, but is not limited to, annual crops such as corn, bean, chili, and cattle pastures.
As suggested by Fahrig (2013), sampling was not proportional to patch size; we rather standardized sample size across different-sized patches to avoid potential confounding effects related to the so-called ‘sample-area effect’; i.e. the fact that larger sample areas can hold more individuals and species (reviewed by Fahrig, 2013). In particular, we established five 10 × 50 m plots, separated by at least 30 m, in the geographical center of each patch, avoiding forest edges and gaps to avoid potential confounding effects associated with edges or gaps. In these plots, we measured the diameter at breast height (DBH, 130 cm above the ground) of all trees with DBH ≥ 10 cm, and the diameter at waist height (DWH, 90 cm) of all liana ramets with DWH ≥ 2 cm. At the beginning (0 m), middle (25 m), and end (50 m) of each plot, we took hemispherical photographs with a wide-eye lens (Apexel 198° Fisheye Lens) to calculate canopy cover using the program Gap Light Analyzer (Frazer et al., 1999).
In each plot, we selected a tree with suitable climbing conditions (branches ≥ 20 cm wide, preferably hard wood species, whose architecture allowed to install a camera trap facing other main branches) and established a single-rope climbing system (20 patches × 5 plots × 1 focal tree = 100 focal trees). On each focal tree, we visually estimated the percentage of vascular epiphyte cover in four 1 × 0.2 m plots: three located on main branches (≥ 20 cm wide) on the section most proximal to the trunk (i.e. section 3 according to Gradstein et al., 2003), and one plot on the superior section of the trunk (i.e. section 2b, Gradstein et al., 2003). We excluded trees with exfoliating bark since they prevent epiphyte establishment. To estimate the degree of connectivity among trees through lianas, we counted the number of trees connected by lianas to the focal tree. Of the five focal trees per patch, four reached the canopy (mean ± SD = 21.8 ± 6.2 m, range = 10.2–36.6 m; Table S1, Supporting information) and one the midstory (9.1 ± 4.7 m, 3.4–19.6 m; Table S1). This allowed us to better represent the vertical distribution of epiphytes and mammals.
Sampling of arboreal mammalsWe used one camera trap (Bushnell Trophy Cam HD Aggressor Low Glow©) per patch. Arboreal camera trapping can overcome some of the difficulties associated with studying arboreal animals, providing new opportunities for canopy research (Whitworth et al., 2016b). Cameras were placed at varying heights depending on the characteristics of the focal tree (camera height of canopy and midstory trees was 15 ± 4.3 m and 2 ± 0.6 m, respectively; Table S1). Cameras were continuously active from May 2018 to May 2019, and were serviced once a month (change of batteries, downloading of pictures, replacement of malfunctioning cameras). We changed the location of cameras, rotating them once a month among the five focal trees in each patch, except from October to December when they remained on the same focal tree. Total sampling effort was 7387 camera trap nights (mean per patch = 369 ± 11.6 nights), with 6233 active camera trap nights (mean per patch = 311.7 ± 19.9 nights; Table S2). To increase the probability of photo-capturing arboreal mammals inhabiting the forest patches, we used baits in the midstory trees (tuna fish, peanut butter with oatmeal and a banana). As revealed by photographs, bait was consumed the first or second night after placement. Since we did not provide more bait while the camera was active on that tree and no camera malfunctioned on the period the trees contained bait, all sites had the same bait sampling effort. We did not bait canopy trees since placing the bait on the branch that was being recorded required to install a second rope-climbing system, which in most cases was non-viable (e.g. the architecture of the tree did not allow it) or compromised climber’s safety (e.g. the far end of the branch cannot support as much weight as the branch collar). We processed all photographs with the program Digikam© and extracted photograph metadata with the package ‘camtrampR’ (Niedballa et al., 2016). We identified each mammal species based on Reid (2009) field guide. Except for the Mexican hairy dwarf porcupine (Coendou mexicanus) and squirrels, all other rodents were excluded from the analyses due to imprecision in identification from photographs.
Predictor variablesWe defined several vegetation structural attributes as potential predictors of arboreal mammal communities. Tree density (i.e. average tree stalks per patch: number of trees/2500 m2) was as a proxy for stand maturity since stem density decreases with stand maturity, while pioneer trees proliferate in edge-dominated habitats and secondary forests (Laurance et al., 2006; Dupuy et al., 2012). We used total tree basal area (i.e. sum of all trees’ basal area in the 5 plots per patch, expressed in m2/ha) as a proxy of tree biomass, which is also positively associated with stand age (Dupuy et al., 2012; Poorter et al., 2016). We also used total liana basal area (i.e. sum of all lianas’ basal area in the 5 plots per patch, expressed in m2/ha) as a proxy of liana biomass, which is also positively associated with stand maturity, canopy connectivity, and resource provision (Dewalt et al., 2000; Schnitzer and Bongers, 2002). We also measured epiphyte cover (i.e. average percentage of epiphyte cover of the five focal trees per patch), because it can be considered a proxy of canopy water and provision of insects (Benzig, 2004; Van Stan and Pypker, 2015), and canopy cover (i.e. inverse of the percentage of open sky calculated from hemispherical photographs) as a proxy of light incidence and tree connectivity (Frazer et al., 1999; Malcolm, 2004). Finally, we also measured tree connectivity by lianas (i.e. number of trees connected by lianas to the focal tree) as a proxy of alternative connectivity routes (Schnitzer and Bongers, 2002). None of these variables presented multicollinearity problems since all showed Variance Inflation Factors lower than 4 (Neter et al., 1996).
Response variablesWe analyzed the response of arboreal mammal diversity and species composition (dissimilarities in species abundances across sites using Bray distance). We considered photo captures as independent events when there was at least a 24 h interval between captures of the same species. We used the sum of independent photo-capture events for a species as a proxy of the abundance of that species. We then evaluated sample completeness within each site using the estimator of sample coverage from the ‘entropart’ package (Marcon and Hérault, 2015). All sites showed a sample completeness > 0.92 (mean = 0.98, max. = 1), thus indicating that our sampling effort was adequate, and that diversity values were not biased by differences in completeness among sites; thus, rarefaction or extrapolation procedures were not necessary (Chao and Jost, 2012). We estimated species diversity with the exponential of Shannon entropy (1D) using the ‘entropart’ package. This index weighs species abundances without disproportionately favoring either rare or dominant species, and is therefore interpreted as the number of common (or typical) species in the assemblage (Jost, 2006). We did not assess species richness (°D) because it gives disproportionate weight to rare species (Jost, 2006), which could lead to spurious conclusions associated with the somewhat random distribution of rare (and difficult to detect) species.
Data analysesAll predictor variables were standardized to zero mean and unit variance using the ‘vegan’ package for R version 3.6.0 (Oksanen et al., 2019), and patch size was log transformed. To assess the relative influence of patch size and quality (i.e. six vegetation attributes) on species diversity (1D) we used a multimodel inference approach using generalized linear models with Gaussian distribution (Burnham and Anderson, 2002). Given the relatively large number of predictor variables relative to our sample size (n = 20), we focused on additive effects only. In total, we constructed 99 models, which were built by combining a maximum of four predictors, plus the respective null model (which includes the intercept only). We calculated the Akaike’s information criterion corrected for small sample size (AICc) for each model, and then estimated the sum of Akaike’s weights (∑wi) for each predictor, considering all candidate models in which it appeared. This sum is widely used as a proxy of relative importance (i.e. the probability that such variable is included in the best model if the data were collected again under identical circumstances; Burnham and Anderson, 2002). We summed wi of ranked models until Σwi ≥ 0.95, which represents a set of models for which we have 95% confidence that the set contains the model that best approximates the true model (Burnham and Anderson, 2002). To be more conservative, a given predictor was considered important for explaining the response variable if the following two criteria were met: (i) it showed a comparatively high Σwi; and (ii) the model-averaged parameter estimate was higher than its unconditional variance (i.e. it did not include zero). These analyses were done with the ‘glmulti’ package for R (Calcagno and de Mazancourt, 2010).
To assess how patch size and quality related to the composition of the arboreal mammal assemblages, we performed a distance-based redundancy analysis (dbRDA, Borcard et al., 2018) on a Bray-Curtis distance matrix derived from the site × species abundance matrix. Following Garmendia et al. (2013), we excluded from the analysis those species that did not appear in more than half of the sites –i.e. tayras (Eira barbara), margays (Leopardus wiedii), coatis (Nasua narica), northern raccoons (Procyon lotor)– to avoid obtaining spurious results (i.e. high probability of Type II statistical error). We tested the entire model and each of its terms, using permutational ANOVA tests with 999 permutations. We used an automatic stepwise backward model selection to identify significant predictors including vegetation attributes and patch size. These analyses were done with the ‘vegan’ package for R (Oksanen et al., 2019).
ResultsWe obtained 1672 independent photo-captures of 15 species (Table S2; Fig. S1, Supporting information). The most frequently recorded species were Deppei’s squirrels (Sciurus deppei), kinkajous (Potos flavus), and black howler monkeys (Alouatta pigra), together representing 49.5% of all records (Fig. S1). Rarely recorded species were margays (Leopardus wiedii), northern raccoons (Procyon lotor) and tayras (Eira barbara), together representing 0.9% of the records. On average, species were present in 13 out of 20 sites; 11 of the 15 species were present in more than half of the sites (Fig. S1 and Table S2).
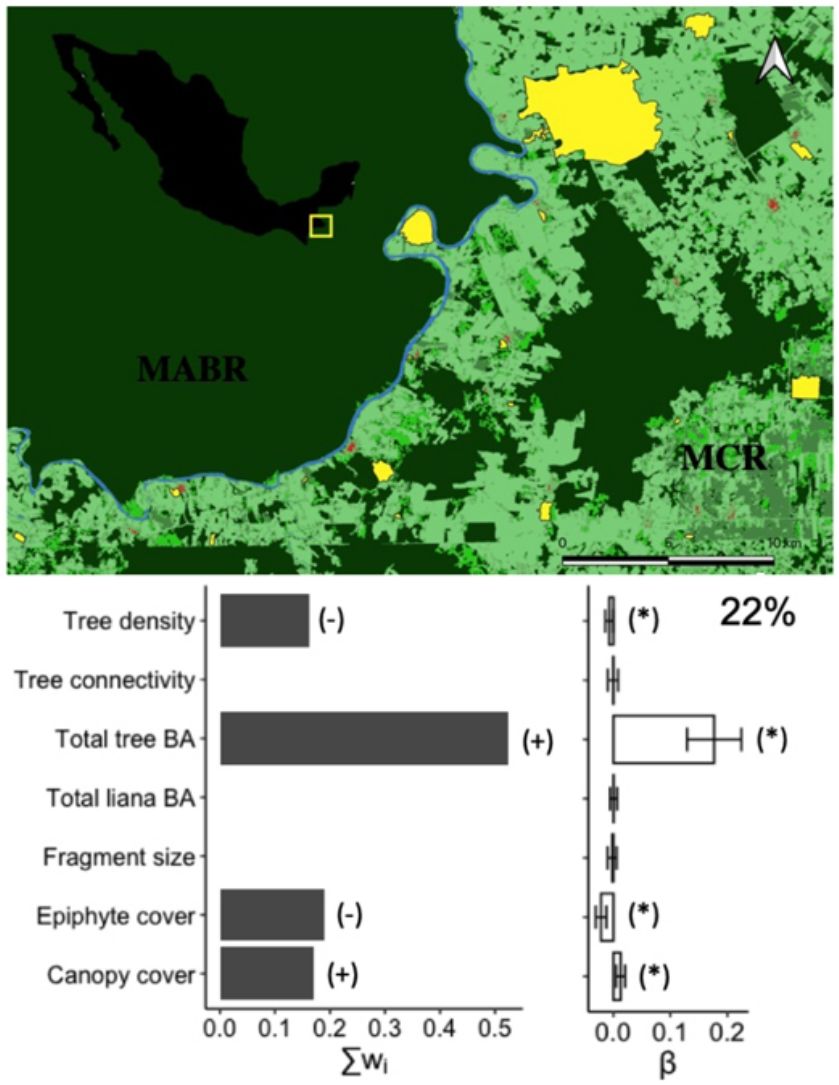
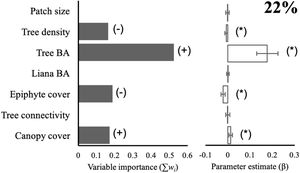
Species diversityThe full model (including all vegetation attributes and patch size) explained 22% of deviance, suggesting that most variables are weakly related to the diversity of arboreal mammals. Yet, total tree basal area was a relatively important predictor (Σwi = 0.52) that was positively related to the diversity of arboreal mammals (Fig. 2). In fact, this was the only predictor included in the best model (Table 1), although the null model also showed high empirical support (ΔAICc = 1.02; Table 1). Epiphyte cover (Σwi = 0.19), canopy cover (Σwi = 0.17), and tree density (Σwi = 0.16) were comparatively less important predictors (Fig. 2). Tree connectivity, total liana basal area, and patch size were not related to mammal diversity; they all presented lower averaged parameter estimates than their unconditional variance (i.e. parameter estimates did not differ from zero; Fig. 2).
Effect of patch size and different vegetation attributes on the diversity (exponential of Shannon entropy) of arboreal mammals in the Lacandona rainforest, Mexico. BA = basal area. The importance of each predictor (panel on the left side) is shown by the sum of Akaike weights (∑wi) of all the models in which each predictor was included, where the direction of the effect (+/-) is indicated. We do not show the importance (∑wi) of those predictors for which the unconditional variance was higher than the averaged parameter estimates (see panel on the right side), as the parameter estimates (β) in these cases include zero, which is interpreted as an inaccurate and non-significant effect. Parameter estimates with asterisks (*) indicate the opposite. The percentage of deviance explained by the complete model is also indicated.
The ten best models assessing the response of arboreal mammal diversity (exponential of Shannon entropy, 1D) to seven predictor variables in the Lacandona rainforest, Mexico. Models showed in decreasing order of empirical support (i.e. from lowest to highest Akaike Information Criterion values, AICc). The Akaike weights (wi) of each model are also indicated.
| Model | AICc | Δ AICc | wi |
|---|---|---|---|
| 1D ∼ 1 + total tree basal area | 51.81 | 0 | 0.17 |
| 1D ∼ 1 | 52.43 | 1.02 | 0.13 |
| 1D ∼ 1 + total tree basal area + epiphyte cover | 54.47 | 2.66 | 0.05 |
| 1D ∼ 1 + tree connectivity + total tree basal area | 54.72 | 2.91 | 0.04 |
| 1D ∼ 1 + total tree basal area + patch size | 54.79 | 2.98 | 0.04 |
| 1D ∼ 1 + canopy cover | 54.83 | 3.02 | 0.04 |
| 1D ∼ 1 + total tree basal area + tree density | 54.96 | 3.15 | 0.04 |
| 1D ∼ 1 + total tree basal area + canopy cover | 54.97 | 3.16 | 0.04 |
| 1D ∼ 1 + total tree basal area + total liana basal area | 54.97 | 3.16 | 0.04 |
| 1D ∼ 1 + epiphyte cover | 54.99 | 3.18 | 0.03 |
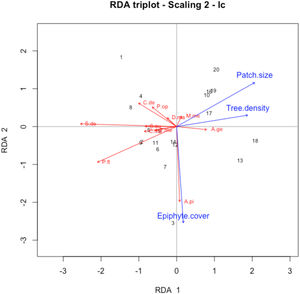
Differences in species composition across sites were significantly related to both patch size and quality (dbRDA analysis; F = 1.39; p = 0.017; Fig. 3). Patch size (F = 2.04; p = 0.015) was negatively related to the abundance of kinkajous and Deppei’s squirrels. Tree density (F = 2.17; p = 0.013) also showed a significant effect on species composition, being negatively related to the abundance of kinkajous and Deppei’s squirrels. Epiphyte cover had a marginally significant effect (F = 1.61; p = 0.059), being positively related to the abundance of black howler monkeys.
Distance-based redundancy analysis (dbRDA) triplot showing the relationship between vegetation attributes and patch size (blue arrows) and abundance of eleven mammal species (red arrows) in the Lacandona rainforest, Mexico. Only predictors with associated P-value < 0.10 are shown. Sample sites are indicated by numbers ordered from smallest to largest patch size. A.pi = Alouatta pigra; A.ge = Ateles geoffroyi; C.de = Caluromys derbianus; C.me = Coendou mexicanus; D.ma = Didelphis marsupialis; M.me = Marmosa mexicana; P.op = Philander opossum; P.fl = Potos flavus; S.au = Sciurus aureogaster; S.de = Sciurus deppei; T.me = Tamandua mexicana.
Our study identifies, for the first time, the relative influence of both patch size and quality on arboreal mammals in the Lacandona rainforest, Mexico. While total tree basal area was the best predictor of species diversity, both patch size and quality drove the composition of mammal assemblages. These results highlight the importance of considering both types of predictors for understanding the distribution and abundance of animal communities in fragmented forests, and thus guiding effective conservation plans.
Our findings indicate that, compared to other vegetation attributes, total tree basal area is a relatively important predictor of mammal diversity. This attribute is positively correlated with forest maturity (Dupuy et al., 2012; Poorter et al., 2016). In fact, in the studied patches, total tree basal area is positively correlated with measures of tree size, such as tree basal area (r = 0.9), tree height (r = 0.7), and canopy width (r = 0.7), as well as liana basal area (r = 0.6; Table S3) – attributes which are associated with old-growth forests (Lindenmayer and Laurence, 2016). Other studies have also found that total tree basal area drives forest patch occupancy by arboreal mammals (e.g. howler monkeys, Arroyo-Rodríguez et al., 2007; spider monkeys, Urquiza-Haas et al., 2009), likely because it can determine the availability of resources (e.g. food, shelter, support) for arboreal mammals (Chapman et al., 1992; Pinho et al., 2020), and lead to the relaxation of interspecific competition, potentially promoting species coexistence (Asensio et al., 2007). Therefore, maintaining old-growth forests seems to be particularly important to preserve arboreal mammal diversity in human-modified landscapes.
Contrary to our expectations, mammal diversity is weakly related to other vegetation attributes, such as epiphyte cover, tree density, and liana basal area. This finding may reflect the plasticity and resilience of arboreal mammals to vegetation changes. In fact, although they are all forest-dependent species, the species of this study are able to use a diversity of vegetation attributes and adjust their diet and behavior to changes in vegetation structure. For example, only two of the registered species (spider monkeys and margays) are classified as old-growth forest specialists by the IUCN, while the rest are described as second-growth tolerant or habitat generalists (Table S2). The ability of black howler monkeys, spider monkeys, coatis, gray squirrels, and common opossums to use and even inhabit the anthropogenic matrix is well documented (Arroyo-Rodríguez et al., 2017; Galán-Acedo et al., 2019; Koprowski et al., 2017; Romero-Balderas et al., 2006; Vaughan and Foster-Hawkins, 1999). Therefore, as discussed above, tree basal area or a proxy of it (e.g. abundance/density of large trees) seems to be the best single indicator of forest patch quality for this group of mammals, at least in the Lacandona rainforest, Mexico.
In contrast with mammal diversity, the ordination of the sites based on species composition was best and significantly explained by patch size and tree density. This analysis reveals novel insights on species’ responses to patch characteristics in fragmented tropical forests. However, it is important to note that the ordination results are mainly correlated with the abundance patterns of three mammal species: black howler monkeys, kinkajous, and Deppei’s squirrels. The other species included in the analysis (spider monkeys, woolly opossums, gray four-eyed opossums, Northern tamanduas, Mexican hairy dwarf porcupines, common opossums, Mexican mouse opossums, gray squirrels) showed weak correlations with the vegetation attributes or patch size, and hence, will not be further discussed.
Contrary to our expectations, patch size was negatively, not positively, associated with the abundance of individuals, particularly with that of kinkajous and Deppei’s squirrels. A possible explanation is that the study patches may be experiencing a crowding effect – an increase in species density due to recent forest loss in the surrounding landscape (Grez et al., 2004; Vallejos et al., 2020). This would result in individuals revisiting the study trees more often in smaller patches than they would in larger patches since smaller patches offer less area for foraging. If this is so, we would expect a relaxation of the crowding effect in upcoming years as the extinction debt is settled. However, further studies are needed to determine the mechanism responsible for this negative association between patch size and species abundance.
Our results also suggest that most registered mammal species could prefer less disturbed forest patches. In particular, tree density showed a negative association with most species, particularly with Deppei’s squirrels and kinkajous. Since tree density decreases with stand maturity (Dupuy et al., 2012), while pioneer tree species proliferate in edge-dominated habitats and secondary forests (Laurance et al., 2006), our findings suggest that, as argued above, arboreal mammals are associated with mature forests.
Conservation implicationsOur study reveals the important effect of both patch size and quality on arboreal mammals in the fragmented tropical forests. This information is essential to efficiently use conservation resources, promoting the maintenance of biodiversity in human-modified tropical landscapes. In particular, our findings are indicative of the importance of protecting mature forests and preventing the loss of large trees. In fact, we highlight the importance of preventing the replacement of forest stands with large old trees by faster-growing stands of younger trees. Large trees not only benefit biodiversity, they also play key roles in ecosystem functions, such as carbon storage, soil retention, and water infiltration (Lindenmayer and Laurence, 2016; Pinho et al., 2020; Melito et al., 2021). This is why Sist et al. (2014) recommend that large trees above a set diameter should be excluded from logging. Monitoring population dynamics of tree species that reach large size is also necessary to ensure sufficient recruitment of new cohorts and maintain viable populations. Enrichment planting with such species could be implemented and while the outcomes of this approach require a long period of time, we must ensure the replacement of disappearing old trees. Forest management interventions, such as stand thinning and vine cutting, to promote growth and development of selected trees could also be applied. These interventions create less dense forests, which, according to our findings, could have a positive impact on some of the studied species.
Finally, in agreement with recent reviews on the value of forest patches for biodiversity conservation (Arroyo-Rodríguez et al., 2020; Fahrig, 2020), our study also indicates that small forest patches can host a high abundance and diversity of arboreal mammals. However, current environmental legislations and land management policies do not offer enough protection or incentives to safeguard small forest patches. For instance, in Mexico, the Payment for Ecosystem Services program defines a minimum area of 100 ha as a criterion of eligibility (CONAFOR, 2020), thus excluding most remaining forest patches from protection. Note that most forest patches in Mexico and the world are very small (< 20 ha; Taubert et al., 2018), and thus, limiting this conservation program to larger patches can leave smaller patches open to destruction in the coming years. Policy programs to support the maintenance and sustainability of small forest patches are urgently required to enhance benefits for landowners and provide alternative land uses that are compatible with biodiversity conservation.
FundingThis research was supported by Rufford Small Grants (23706-1), SEP-CONACyT (Project 2015-253946) and IdeaWild. S.C.-V. obtained a graduate scholarship from CONACyT. This paper constitutes a partial fulfillment of the PhD program of the Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México.
Author contributionsSCV and VAR developed the idea of the study, with support from EA and TTA. SCV collected the data and analyzed it with significant guidance from VAR, FMA, GAP and SM. All authors made substantial contributions to the intellectual content, interpretation and editing of the manuscript.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We especially thank Audón Jamangapé, Adolfo Jamangapé and Marta Aguilar for their invaluable field assistance and accommodation in the Marqués de Comillas Region. We also thank the landowners for allowing us to collect data on their properties.