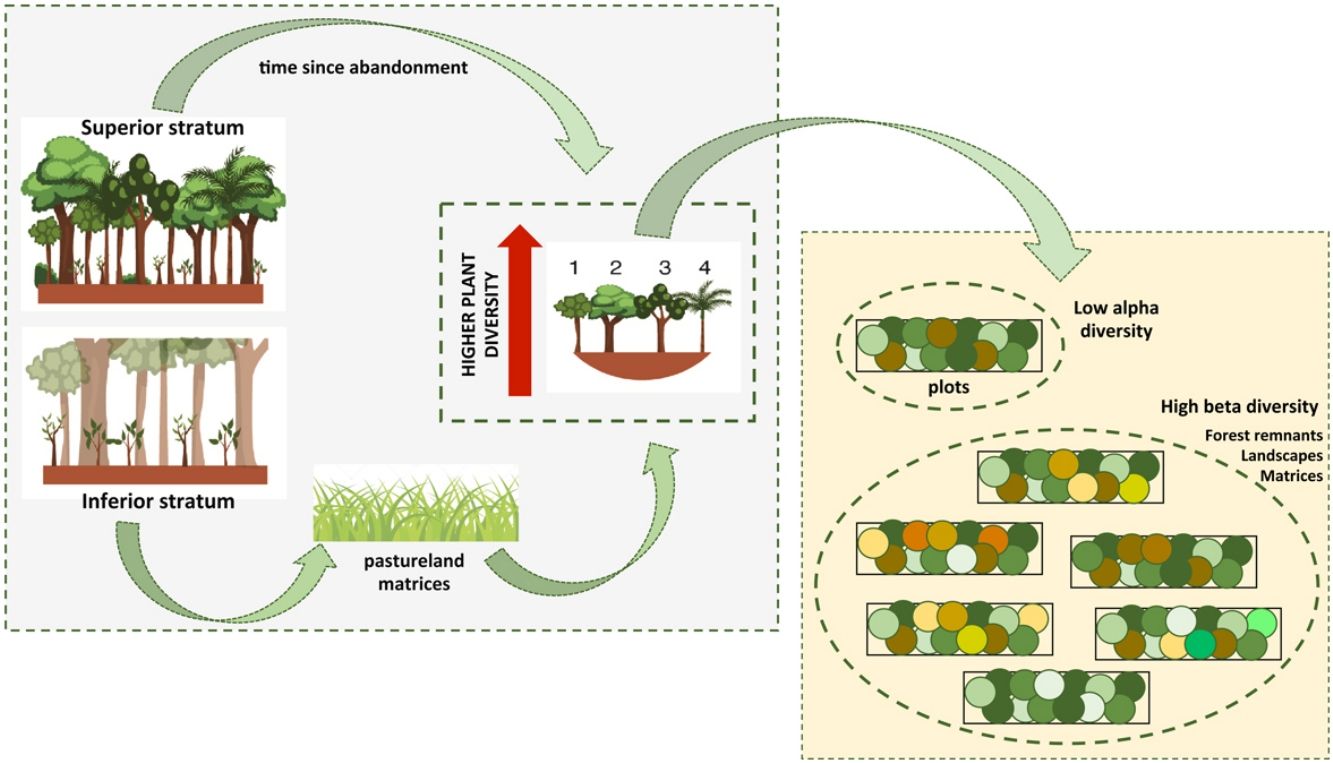
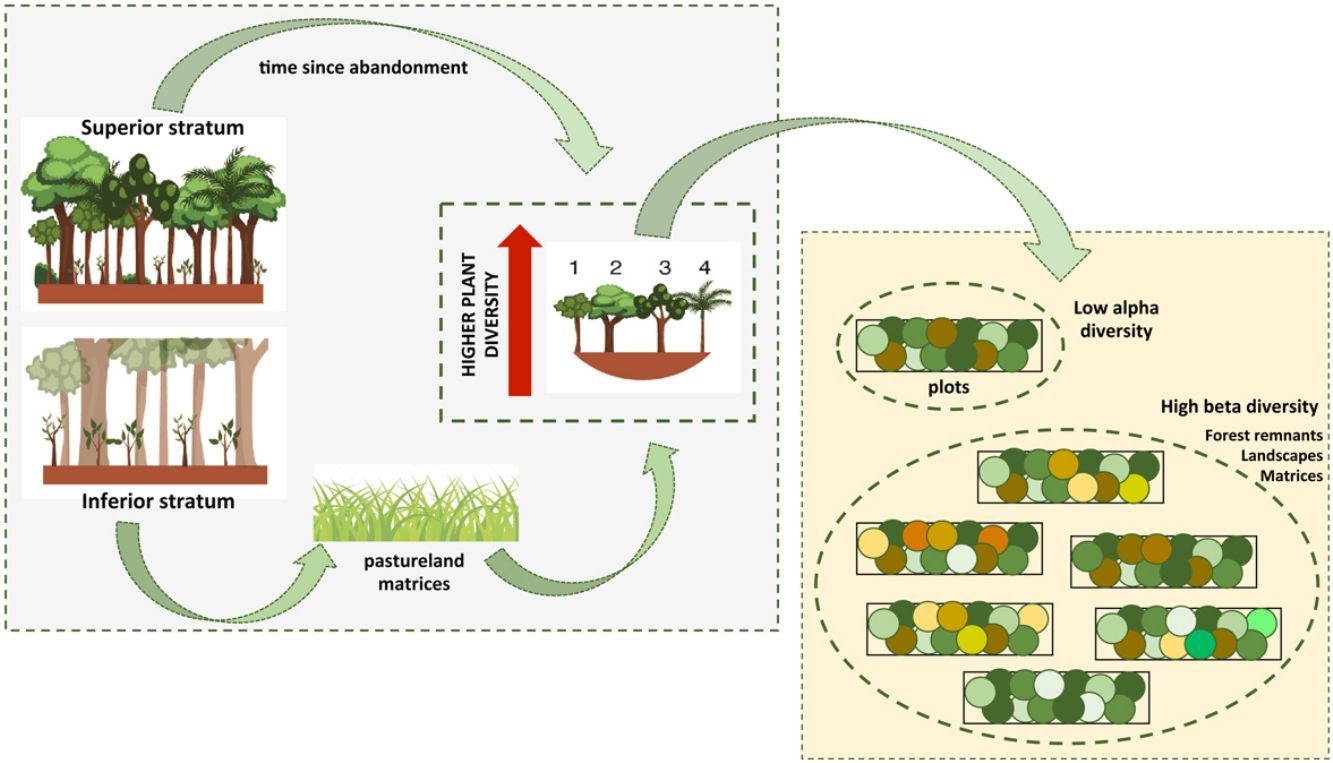
Different types of matrices can perform different disturbance regimes over remnant forests, which can ultimately affect plant diversity. To address these issues, we studied 60 plots in forest remnants embedded within sugarcane plantations and pasturelands in the Atlantic Forest of Southeastern Brazil. Our objectives were to evaluate general patterns of plant diversity and to assess the influence of landscape structure and dynamics on plant richness and diversity. We recorded 297 species in two strata. (DBH superior stratum ≥ 4.8 cm > inferior stratum). Overall, diversity of the inferior stratum was higher in pastureland forest remnants than in sugarcane matrices. In the superior stratum, time since abandonment represented a greater influence. Considering the partition of diversity, more than 40% of total diversity was due to the alpha component alone in all cases, but it was significantly lower than expected. Beta components among forest remnants, landscapes and matrices were higher than expected for both strata, whether together or separately. Habitat heterogeneity showed a higher contribution to the total floristic diversity, but among remnants this contribution was even more significant. Forest remnants in pasturelands had a more dynamic land use history and parameters of landscape dynamics were more related to plant diversity. For remnants in the sugarcane matrices, species richness and composition were explained by landscape structure. Our results highlight that there is an important plant community diffused in the forest remnants of human-modified landscapes. Therefore, conserving the majority of remnants and restoring degraded ones must be a key conservation strategy.
Most of the remaining forests in the tropical region is restricted to steep areas, hindered by human occupation, agriculture, and represented by small isolated remnants (Metzger et al., 2009, Ribeiro et al., 2009), which are highly disturbed (Ribeiro et al., 2009) and embedded in agricultural landscapes (Melo et al., 2013). Typically, such landscapes are an agricultural mosaic, containing forest fragments with different degradation histories and drivers, that usually include (i) old-growth forest remnants that have never experienced clear-cutting, but are still degraded by anthropogenic disturbances, (ii) secondary forests that are regenerating after a clear-cut, fire or the abandonment of croplands and pasturelands (Gardner et al., 2009; Molin et al., 2017), and (iii) secondary forests under recurrent events of disturbance (Molin et al., 2018; Reid et al., 2018).
Recent studies have reported the importance of such secondary forests to conserve biodiversity, supply ecosystem services, provide stepping stones, corridors, fauna and flora refuges, and other key conservation elements (Arroyo-Rodríguez et al., 2009; Brancalion et al., 2012; Chazdon et al., 2009; Viani et al., 2015). Nonetheless, different recurrent disturbances play important roles in forest succession when defining the plant community (Arroyo-Rodríguez et al., 2015). Disturbances such as wind, fire, temperature increases and exotic species invasion are important components of many ecosystems, and variations in the disturbance regime can affect an ecosystem's structure and functioning (Hobbs and Huenneke, 1992). The effects of disturbances can be expressed at different levels. Within fragments, environmental (soil conditions and light availability) and ecological filtering (demographic stochasticity, seed dispersal limitations, competition, recruitment, etc.) can alter the species composition and the spatial patterns of plant regeneration (Wright, 2010), by selecting species and defining future biodiversity (Arroyo-Rodríguez et al., 2015; Wills et al., 2006; Wright, 2010). For established woody plants, time since disturbance can play an important role on defining species composition, considering time-lag response that can lead to an extinction debt (Tilman et al., 1994). Such effects on both strata can lead to decreasing local diversity or to biotic homogenization at the local level (Püttker et al., 2015). From a regional perspective, disturbances have been observed to increase the compositional and functional differentiation among forest remnants, especially in highly deforested landscapes with long land use histories (Arroyo-Rodríguez et al., 2015). On the other hand, tropical forests under severe landscape disturbances can also be susceptible to floristic convergence (i.e., species homogenization) (Lôbo et al., 2011) driven by the proliferation and dominance of some fast-growing plant species, such as pioneers and vines (Arroyo-Rodríguez et al., 2015; Liebsch et al., 2008; Lôbo et al., 2011; Melo et al., 2007). Both floristic differentiation and homogenization are dependent on the landscape configuration and spatial scale of analysis (Arroyo-Rodríguez et al., 2013).
Landscape characteristics (e.g., amount of vegetation and matrix context) are known to heavily influence population and metapopulation dynamics (Püttker et al., 2011; Tscharntke and Brandl, 2004). Fragmentation, habitat loss or the integration of both mechanisms has led to several changes in landscape structure and configuration (Fahrig, 2003), driving plant and animal populations to reductions or even to extinctions at the landscape scale in tropical forests (Benchimol et al., 2017; Lima and Mariano-Neto, 2014; Morante-Filho et al., 2015). Species diversity in several tropical plant communities has been strongly and positively related to forest cover and connectivity.
The degree of change in species diversity along with a range of different scales (e.g., samples, habitats or communities) is measured by beta-diversity (β), since it provides a direct link between biodiversity at local (α diversity) and regional scales (γ diversity) (Magurran, 1988). In the Atlantic Forest, some studies have addressed patterns of beta diversity in native vegetation (Carneiro et al., 2016; Machado et al., 2016; Sfair et al., 2016; Bergamin et al., 2017; Benchimol et al., 2017), which remains a crucial field in understanding the patterns related to plant community assembly and its maintenance in highly degraded landscapes. The landscape-divergence hypothesis, postulated by Laurance et al. (2007), states that differential human disturbances among forest remnants, such as the different degrees of reduction of local richness (patches) after habitat loss, can lead to differential trajectories of vegetation succession. Those disturbances may increase the compositional and functional differentiation of communities in fragmented landscapes, leading to high beta diversity between patches in a landscape (Benchimol et al., 2017; Sfair et al., 2016). Along with understanding communities' structures along landscapes, it is also important to understand the historical transformations that habitat patches and biodiversity have been submitted to (Molin et al., 2017). This is particularly important in dynamic landscapes, where deforestation, regeneration, harvesting, grazing, urbanization, and other actions have shaped the distribution of these patches throughout the landscape, playing an important role in selecting species (Arroyo-Rodríguez et al., 2015).
Even with the increase in the number of studies about the Brazilian Atlantic Forest, little is still known about its biodiversity. Less than 1% of the remnant area has been sampled, and most of the current knowledge about this domain comes from private lands and areas outside of forest reserves (Lima et al., 2015). In addition, our understanding of the biological dynamics of such human-modified ecosystems (Arroyo-Rodríguez et al., 2013) has been inadequately studied. Therefore, the combination of beta-diversity analyses with different landscape contexts can inform us about the fundamental mechanisms shaping the remaining diversity. Given such importance, this approach must be investigated in the most varied landscapes possible.
Here, we investigated several ecological processes interacting collectively at multiple landscape contexts and scales of analysis. To discuss these issues, we assessed the floristic composition and diversity of forest remnants embedded in human-modified landscapes which are highly fragmented with a dynamic history of occupation. Our specific goals were (1) to evaluate the general patterns of α-plant diversity of the superior and inferior strata among forest remnants in different periods of regeneration, and among sugarcane and pastureland matrices; (2) to analyze the β-plant diversity patterns at different scales (among forest remnants, among landscapes, and among agricultural matrices); and (3) to assess the influence of landscape structure and dynamics (present and past events) on plant species diversity. Considering the studied forest remnants are embedded in different matrices, isolated from each other, and have been experiencing different disturbance histories over time, we would expect a plant community differentiation among forest remnants, among landscapes and among agricultural matrices resulting in higher β-plant diversity. In contrast, we would expect a lower α-plant diversity within forest remnants (among plots) as a result of the limited seed dispersal inter-forests imposed by the landscape structure.
Material and methodsStudy siteThe study areas are situated in the countryside of São Paulo state, Brazil, along the Corumbataí River basin. It is located between the latitudes 22°04′46″S and 22°41′28″S, and longitudes 47°26′23″ W and 47°56′15″W, and covers an area of approximately 1700 km2 (Cassiano et al., 2013). Eight municipalities either totally or partially comprise this basin which contains approximately 650,000 inhabitants. Most of the area is flat relief, disrupted only by the basaltic cuestas, which still maintain a considerable portion of native vegetation. The basin is located in the transition of the Atlantic Forest to the Brazilian Savanna (Cerrado) and its vegetation is composed mostly of seasonal deciduous and semi-deciduous forests (Garcia et al., 2006).
The replacement of the forests by agricultural uses goes back to the early 19th century, and nowadays, the major agricultural land uses are sugarcane plantations and pasturelands. However, this region has a very dynamic history with cycles of different crops, mainly sugarcane, coffee, silviculture, and on a smaller scale, row crops and fruticulture (Garcia et al., 2006). Together with increasing urbanization, these dynamic cycles of high-tech agriculture and recurrent impacts on forest remnants (such as regular fires and pesticides) have influenced the distribution of native vegetation along the basin. In 2001, Valente and Vettorazzi (2005) concluded that 90% of forest remnants were smaller than 5 ha and less than 1% were larger than 85 ha. Ferraz et al. (2014) however, observed an increase in forest cover of 8–15% in sugarcane landscapes and 10% in pasturelands. This expansion mostly occurred in proximity to previously existing forest remnants, resulting in the current remnants being highly heterogeneous. Although the authors observed that forest regeneration had overcome forest loss, old-growth forests are being replaced by young secondary forests representing an overall decrease in habitat quality.
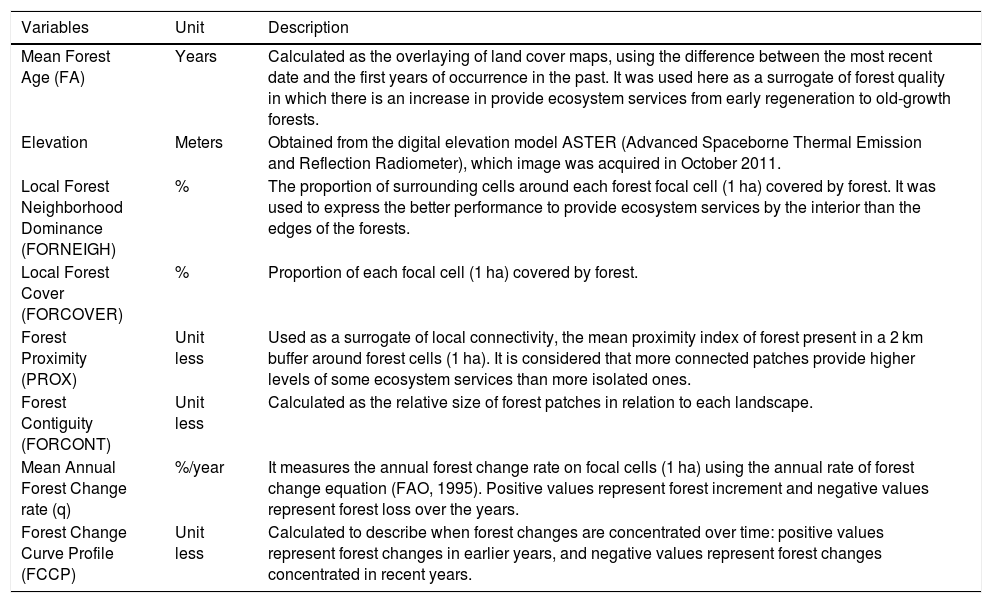
Landscape selection, structure, and dynamic metricsIn 2010, six landscapes were selected to be representative of the predominant agricultural matrices of the Corumbataí River basin: three landscapes in the sugarcane matrix and three in the pastureland matrix. The landscapes are 4 km x 4 km in size, and in 2008, presented at least 10% of native vegetation cover and at least 70% of agricultural matrix from the satellite image available when the selection was performed. Photographic interpretation of the images was used and the following land use classes were considered: sugarcane plantations, pastureland, native vegetation cover, orange plantations, eucalyptus plantations, urban areas, and others. One can see further details of the landscape selection in Ferraz et al. (2014). In order to describe the structure of each landscape, we have employed six variables: Mean Forest Age (in years), Local Forest Neighborhood Dominance (in %), Local Forest Cover (%), Elevation (in meters), Forest Proximity (unitless), and Forest Contiguity (unitless). To assess the landscape dynamic, aerial photographs from 1962, 1978 and 1995, and satellite images from 2008 (used as a reference for mapping) were also photo-interpreted and classified using the same land use classes. Land use transitions of each landscape were analyzed to calculate the following metrics: Mean Annual Forest Change Rate and Forest Change Curve Profile. All variables are described in Table 1 and follow the methods proposed by Ferraz et al. (2014).
Variables of forest remnants and landscape structure and dynamic parameters selected in the Corumbataí River Basin, Southeastern, Brazil.
| Variables | Unit | Description |
|---|---|---|
| Mean Forest Age (FA) | Years | Calculated as the overlaying of land cover maps, using the difference between the most recent date and the first years of occurrence in the past. It was used here as a surrogate of forest quality in which there is an increase in provide ecosystem services from early regeneration to old-growth forests. |
| Elevation | Meters | Obtained from the digital elevation model ASTER (Advanced Spaceborne Thermal Emission and Reflection Radiometer), which image was acquired in October 2011. |
| Local Forest Neighborhood Dominance (FORNEIGH) | % | The proportion of surrounding cells around each forest focal cell (1 ha) covered by forest. It was used to express the better performance to provide ecosystem services by the interior than the edges of the forests. |
| Local Forest Cover (FORCOVER) | % | Proportion of each focal cell (1 ha) covered by forest. |
| Forest Proximity (PROX) | Unit less | Used as a surrogate of local connectivity, the mean proximity index of forest present in a 2 km buffer around forest cells (1 ha). It is considered that more connected patches provide higher levels of some ecosystem services than more isolated ones. |
| Forest Contiguity (FORCONT) | Unit less | Calculated as the relative size of forest patches in relation to each landscape. |
| Mean Annual Forest Change rate (q) | %/year | It measures the annual forest change rate on focal cells (1 ha) using the annual rate of forest change equation (FAO, 1995). Positive values represent forest increment and negative values represent forest loss over the years. |
| Forest Change Curve Profile (FCCP) | Unit less | Calculated to describe when forest changes are concentrated over time: positive values represent forest changes in earlier years, and negative values represent forest changes concentrated in recent years. |
Collinearity describes a situation where two or more predictor variables in a statistical model are linearly related (Dormann et al., 2013). We performed a pairwise Spearman correlation among all variables in a preliminary analysis and no correlation coefficients were greater than 0.70. Based on this, we decided to keep all variables in the analyses.
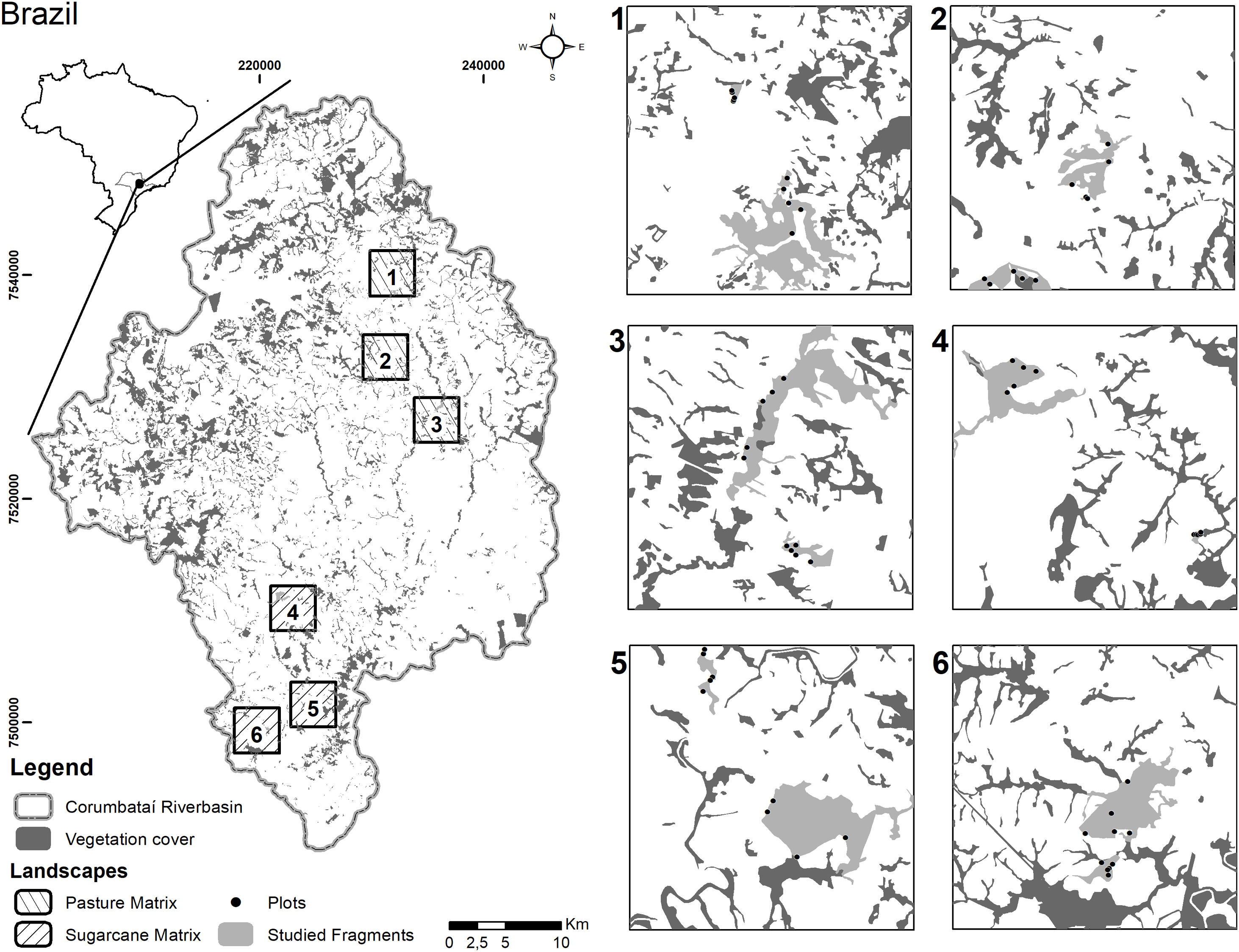
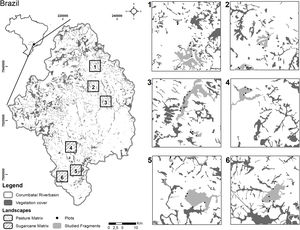
Vegetation sampling and biotic dataIn 2011, we conducted floristic assessments in 12 Seasonal Semi-deciduous forest remnants of the Corumbataí River basin, two located in each landscape; therefore, six remnants embedded in sugarcane matrices and six in pastureland matrices. We considered forest remnants to be all-natural forest habitats in the landscapes, regardless of their stage of succession. In two forest remnants in each of the six selected landscapes, individuals of shrub and tree species were sampled (Fig. 1). In each remnant, five plots of 30 m × 8 m were established to record the individuals of the superior stratum. Individuals of the inferior stratum were sampled in one sub-plot of 30 m × 2 m. In total, we sampled 1.44 ha for the superior stratum and 0.36 ha for the inferior stratum. All plots were established in the edge-interior direction at least 3 m from the edge to avoid large canopy gaps, grasses, and vines. In total, 60 plots were sampled in the Corumbataí River basin, 30 plots in forest remnants embedded in the sugarcane matrix and 30 plots in forest remnants embedded in pasturelands. The distance among sampled remnants varied from 0.2 to 2.5 km in sugarcane landscapes and 0.6–1.2 km in pasturelands. The size and shape of the forest remnants in the landscapes were very restrictive and did not allow us to increase the distances between the sampled plots, but the spatial autocorrelation is an important issue in spatial data at different scales in community ecology (Diniz-Filho et al., 2012). Thus, before statistical tests, for each response variable, we checked the spatial dependence with Moran's I test through 999 permutations at a 5% significant level. We considered the geographical coordinates of the plots using the binary coding scheme in the neighbor's parameters. This analysis is available in "spdep" package (Bivand and Wong, 2018) in R software (R Core Team, 2016).
Distribution of the landscapes dominated by pastureland (1–3) and sugarcane (4–6) in the Corumbataí River basin (Southeastern Brazil), with the corresponding land use classes, with a highlight to the two forest remnants and plots sampled in each landscape. Percentage of forest cover in each landscape: 1 = 19,9%; 2 = 14,7%; 3 = 21,4%; 4 = 15,9%; 5 = 12,1%; 6 = 24,0%.
We sampled two forest layers in each plot, all individuals with a diameter at breast height (DBH) ≥ 4.8 cm were considered superior stratum (including arborescent palms) and all individuals with a DBH < 4.8 cm and at least 30 cm high were considered inferior stratum. All sampled individuals were identified at species level by comparison to the ESA Herbarium (Escola Superior de Agricultura “Luiz de Queiroz”, Universidade de São Paulo) collection and expert consultation. We excluded exotic species and other life forms (vines and herbs, mainly), individuals that we could not classify at least at the family level, and dead tree individuals from the data.
All species were classified into three functional groups (Supplementary material - Table 1), based on literature data and expert consultation. For seed dispersal syndrome, species were classified as Autochoric, Zoochoric, Anemochoric, and Unclassified (sensu Van der Pijl, 1982). For successional groups, we classified species as pioneer, non-pioneer of canopy (which includes species that reaches the canopy and sub-canopy), understory, and unclassified (which includes species lacking appropriate information for classification and also typical species from other physiognomies) (adapted from Gandolfi, 2000; Whitmore, 1989). For dispersal syndrome, some species were classified into two classes, mainly due to primary and secondary syndromes. Finally, for life-form classifications, we used undershrub, shrub, treelet, tree, palm, and unclassified, and some species were also classified into two or three groups due to phenotypic variation in the study region. These mixed categories were included to avoid missing information, to allow the inclusion of contrasting classification of species in the literature, and to consider the phenotypic variation of species. We also classified species according to their threatened status, following the Brazilian National Red List (Portaria MMA 443, 2014).
Data analysesOverall plant diversity (floristic α-diversity among matrices and ages of restoration)We chose a diversity index insensitive to the sample size to evaluate the general patterns of α-plant diversity in forest remnants in human-modified landscapes (Objective 1). Since the abundance of individuals of the superior and inferior strata varied considerably among plots, we used the Fisher alpha diversity index (Fisher et al., 1943) to describe biodiversity among plots, forest remnants, landscapes, and also to compare diversity with landscape structure and dynamic metrics (see next section). This index is insensitive to sample size (Magurran, 1988) and it has been previously used for ecological studies in fragmented landscapes (Benchimol and Peres, 2015; Laurance et al., 2001). Low values of Fisher's alpha indicate that few species are added as the sampling size increases, which means there is a higher dominance by common species. When the alpha value is high, rare species are more abundant, and increasing the sampling size adds more species (Magurran, 1988). Thus, to freely compare and describe plant diversity, we performed the Fisher alpha diversity index for all 60 plots considering superior and inferior strata together and separately.
To assess whether superior and inferior strata were correlated to each other, we performed the Mantel test considering the Euclidean distance after a Hellinger transformation, which is appropriated to reduce the importance of large abundances (Borcard et al., 2011). We tested the significance of the correlations through 999 random permutations (Legendre and Legendre, 2012). If the two distance matrices are correlated, this means that the results found for the superior stratum would be expected to be the same as those found for the inferior stratum. On the other hand, if the strata are not correlated to each other, this means the ecological processes may act differently on each separate stratum.
Considering that most forest remnants in the landscapes experienced forest suppression and/or regeneration, we assessed differences in floristic diversity among groups of regeneration. We assembled the plots into four groups according to their time of regeneration (time since abandonment from previous anthropic uses), based on aerial photographs and satellite images used to calculate metrics of landscape dynamic: (1) 20 plots with at least 30 years of regeneration (i.e., plots that were already forests in 1978, and for analyses purposes, we included nine plots in this group that were already classified as forests in 1962); (2) 17 plots with at least 13 years of regeneration (i.e., plots that were first classified as forests in 1995); (3) 17 plots with at least eight years of regeneration (i.e., plots that were first classified as forests in 2000); and finally, (4) five plots that are Eucalyptus plantations that have been abandoned and now present an understory of native species regeneration, but still cannot be considered forests. Subsequently, we only used 59 plots in the analysis, since one plot was excluded due to missing landscape data from Ferraz et al. (2014). To test the difference in diversity and total richness between the type of matrix and years, we used Fisher’s alpha diversity index and the total number of spec. All these response variables were not spatially autocorrelated (Supplementary Material - Table 2), providing the independence assumption for statistical analyses per plot, analyzing superior and inferior strata together and separately. We constructed boxplots to visualize the differences between diversities of superior and inferior strata among different ages of regeneration and among agricultural matrices (pastureland and sugarcane). We performed a two-way ANOVA to test the differences in Fisher’s diversity and total richness between the type of matrix and years of regeneration, considering a significance level of 5%.
Partition of diversityTo analyze hierarchical patterns of diversity at different scales (Objective 2), we performed a partition of diversity to determine which scales of our sampling contributes more to the total diversity. In other words, we investigated whether the total diversity is an outcome of differentiation among the matrices, the landscapes, the forest remnants or the plots themselves. To accomplish this, we analyzed our data according to sampling design in four distinct categories: (A) 59 plots, (B) 59 plots arranged in 12 forest remnants, (C) 12 forest remnants arranged in six landscapes, and (D) six landscapes arranged in two matrices. Thus, we performed an Additive Partitioning of diversity using two metrics: (I) the Shannon Wiener index, a diversity metric that accounts for both abundance and evenness of the species present in the community (Fisher’s alpha diversity index was not available in the R package that was used to perform this analysis), and (II) the species richness, that corresponds to the species recorded in the community. Available in the “vegan” package (Oksanen et al., 2016) in R software (R Core Team, 2016), this analysis decomposes gamma (γ) diversity into alpha (α) and beta (β) components that are expressed in the same units (Crist et al., 2003). Thus, the total species diversity and the total richness (γ) found for the entire region is partitioned into the average diversity and richness that occur for each plot (α) and among plots (β), so the contributions of α and β to total diversity can be compared across the hierarchical sampling design (Crist et al., 2003). Thus, we tested whether the gamma diversity and richness (γ) are randomly decomposed into alpha (each plot) and among three levels of beta diversity: β1 – among forest remnants; β2 – among landscapes; and β3 – among matrices. We randomized 999 times to generate a distribution of expected values for each diversity component and richness according to the null model. Null models are typically indicated for analyzing different components of diversity by comparing the observed similarity in community composition to that which is expected by a random assembly (Chase et al., 2011). This approach is especially recommended when changes in beta diversity are simultaneous with differences in α and/or γ diversity, as in the case of habitat loss (Püttker et al., 2015). Specifically, p < 0.05 indicates that alpha and beta diversity or richness is higher or lower than expected according to the null hypothesis, which means that there are differences in diversity or richness between plots (αplot), among forest remnants (βrem), among landscapes (βlan) or among matrices (βmat). Bar charts were plotted with the proportion of contribution to gamma diversity and richness of each component observed against the expected. This analysis was performed on all species together and separately in both the superior and inferior strata.
Influence of landscape structure and dynamic metrics on species distributionWe applied a Canonical Redundancy Analysis (RDA; Rao, 1964) to assess the influence of landscape structure and dynamics on plant richness and diversity in order to understand the role of present and past events on current plant assembly (Objective 3). An RDA is a constrained ordination method that preserves the Euclidean distances among sites in the full-dimensional space. This analysis was performed at the plot level, and we used 58 plots as two were excluded due to a lack of landscape information. We considered only species recorded in more than one plot in the analysis to diminish noise created by rare species, which could mask patterns (Ter Braak, 1995). We analyzed a matrix of 197 species, since 100 of the 297 original species were recorded at one site only, against the 58 plots considering species of the two strata together. For the superior stratum species, we analyzed 99 of the original 161 species, and for inferior stratum, 160 of the original 238 species were analyzed. For longer gradients or landscapes, as in our case study, many species are replaced by others along the gradient, which generates many zeros in the species data matrix (Legendre and Gallagher, 2001) even after rare species are excluded. To minimize this issue, we performed a resemblance matrix among sites using Hellinger’s transformation of species data (Legendre and Gallagher, 2001), which resulted in a matrix of distance that is appropriate for the analysis of community composition (Legendre and Legendre, 1998). This noise reduction in the analysis also reduces the effects of differences in sample size among superior and inferior strata when analyzed together. In the environmental matrix used as a constraining matrix for the RDA, we used the landscape structure, dynamic metrics, and elevation for each plot obtained from the digital elevation model ASTER (Advanced Spaceborne Thermal Emission and Reflection Radiometer), whose image was acquired in October 2011. Since the environmental matrix has different units, we also performed a standardization on the environmental matrix by scaling each datum to zero mean and unit variance. Both transformations were realized using the deconstand function in R. There were no significant autocorrelations among environmental variables after the Spearman test, considering α = 0.05. We then applied a Monte Carlo test with 999 random permutations to estimate the significance of the relationship between the floristic data set and the environmental variables, assuming H0 as a non-linear relationship between the matrices. To perform these analyses, we used the “vegan” package (Oksanen et al., 2016) in R software (R Core Team, 2016).
ResultsOverall plant diversity (floristic alpha diversity among matrices and ages of restoration)In total, we sampled 7686 individuals classified into 297 species; of which 161 species and 1803 individuals were sampled in the superior stratum and 5883 individuals from 238 species were in the inferior stratum. Among the individuals from the superior stratum, six species were not confirmed, and two were only classified until the genus level. Among species from the inferior stratum, two were not confirmed, eight were only classified until the genus level, and nine were only classified to the family level.
Fabaceae and Meliaceae were the most abundant families, each with more than 1000 individuals sampled. The most abundant species in the inferior stratum were the shrubs Hybanthus atropurpureus (A.St.-Hil.) Taub. and Acnistus arborescens (L.) Schltdl., and in the superior stratum, the trees Trichilia clausseni C.DC., Eugenia florida DC. and Cupania vernalis Cambess. According to the Brazilian National Red List (Portaria MMA 443, 2014), six of the species sampled are classified as “nearly threatened” (Aspidosperma polyneuron Müll.Arg.; Balfourodendron riedelianum (Engl.) Engl.; Handroanthus impetiginosus (Mart. ex DC.) Mattos; Ocotea puberula (Rich.) Nees; Xylopia aromatica (Lam.) Mart.), two are considered vulnerable (Cedrela fissilis Vell.; Cedrela odorata L.), and one is threatened (Cariniana legalis (Mart.) Kuntze) and all were sampled with less than five individuals.
From more than half the species (171), ten or less individuals were sampled in all plots, and from 51 species, only one individual was sampled, most classified as non-pioneer of canopy trees. Most species were classified from shrub to tree (84%) and mainly classified as non-pioneer of canopy (39%), understory (24%), and pioneer (17%). Almost 65% of species were classified as zoochoric (including a few species also classified as autochoric and anemochoric) and 21% were classified as anemochoric (including a few species also classified as autochoric).
According to the Mantel test, the similarity among plots was correlated between the superior and inferior stratum (r = 0.38, p = 0.001). In other words, the similarity in species composition found among plots for superior stratum species was related in 38% to that found for plants recorded in the inferior stratum. This correlation may indicate that the plant composition of the inferior stratum is partially constituted of regenerating species from the superior stratum. Alternatively, the drivers that act to assemble plant species communities may act equally in both strata.
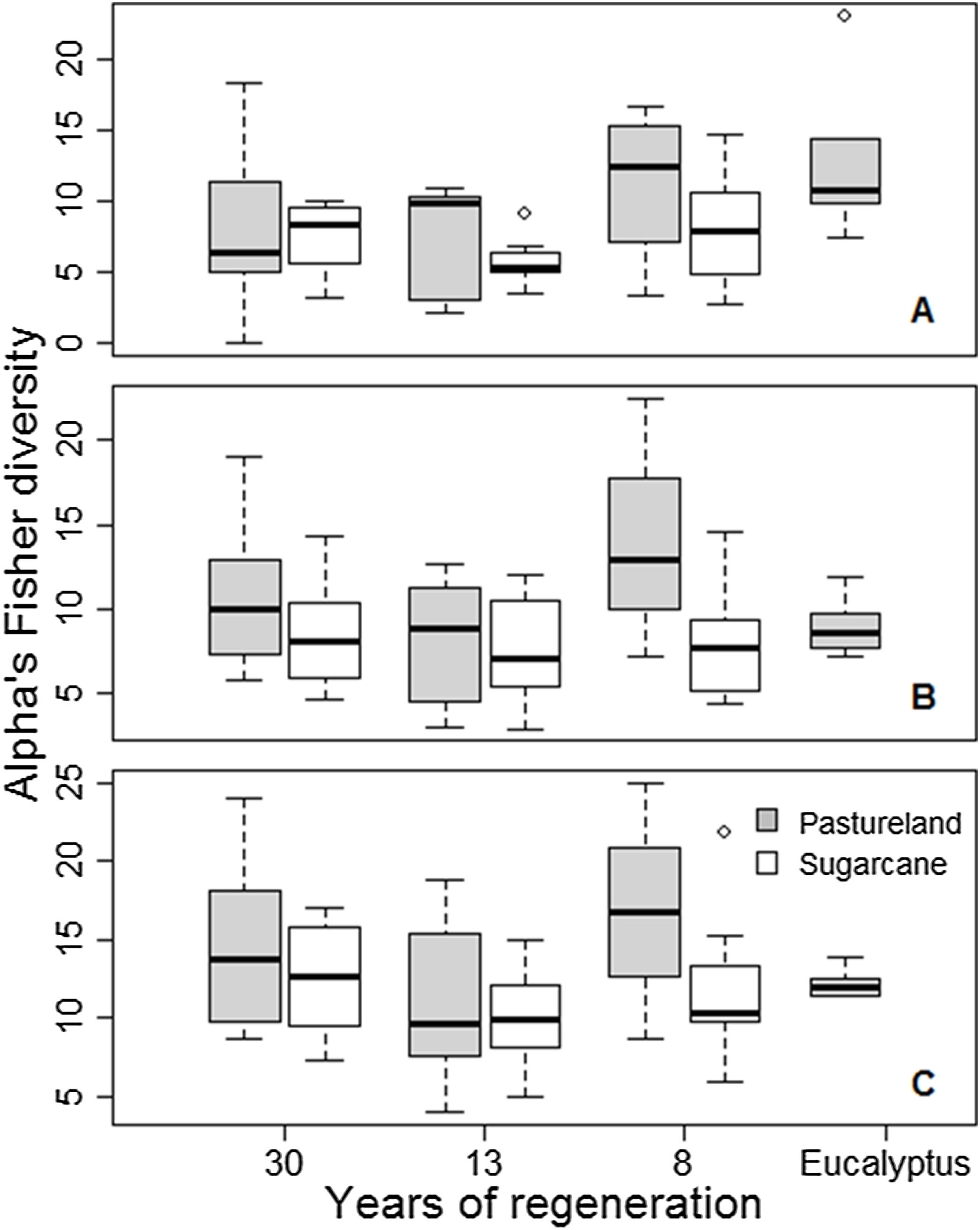
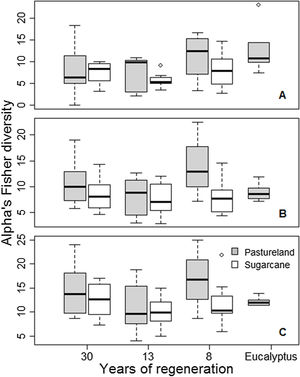
Regarding the agricultural matrix, pastureland was richer and more diverse (247 species; Fisher alpha = 480.7) than sugarcane (192 species; Fisher alpha = 365.9). In the landscape, the inferior stratum was more diverse than the superior (Fisher alpha = 488.1 and 407.4, respectively), and species density was 111 and 661 species/ha, respectively. At the landscape level, the total density found was 1252 superior stratum ind.ha−1 and 16 341 inferior stratum ind. ha-1. At the plot level, the diversity for superior stratum (Fisher alpha) ranged from 2.23 to 23.15 (8.02 on average). For the inferior stratum, diversity ranged from 2.83 to 22.42 (9.10 on average; Fig. 2). Considering only the superior stratum, we found a difference in diversity between years of regeneration (F = 2.855, p = 0.046), but not for the matrix (F = 3.636, p = 0.062) or their interaction (F = 0.464, p = 0.631). For the inferior stratum, diversity varied among agricultural matrices (F = 6.116, p = 0.0168), but not for the years of regeneration (F = 1.137, p = 0.343) or their interaction (F = 2.037, p = 0.141). Finally, for the superior and inferior strata together, the same results were found: diversity varies between pastureland and sugarcane (F = 4.251, p = 0.044), but no difference was found considering the years of regeneration (F = 1.451, p = 0.238) or interaction between the two variables (F = 0.882, p = 0.42). For species richness, there was no difference in any analyses. Overall, the diversity is higher for forests in the pastureland matrix than in the sugarcane matrix (except for superior stratum), higher in eight years of regeneration than in 30 years, and lower in 13 years of regeneration.
Plant diversity among agricultural matrices (pasturelands and sugarcane), years of regeneration (time since abandonment), and abandoned Eucalyptus with native species’ understory, on 60 sampling plots on the Corumbataí River basin, Brazil. A: superior stratum; B: inferior stratum; and C: both strata together.
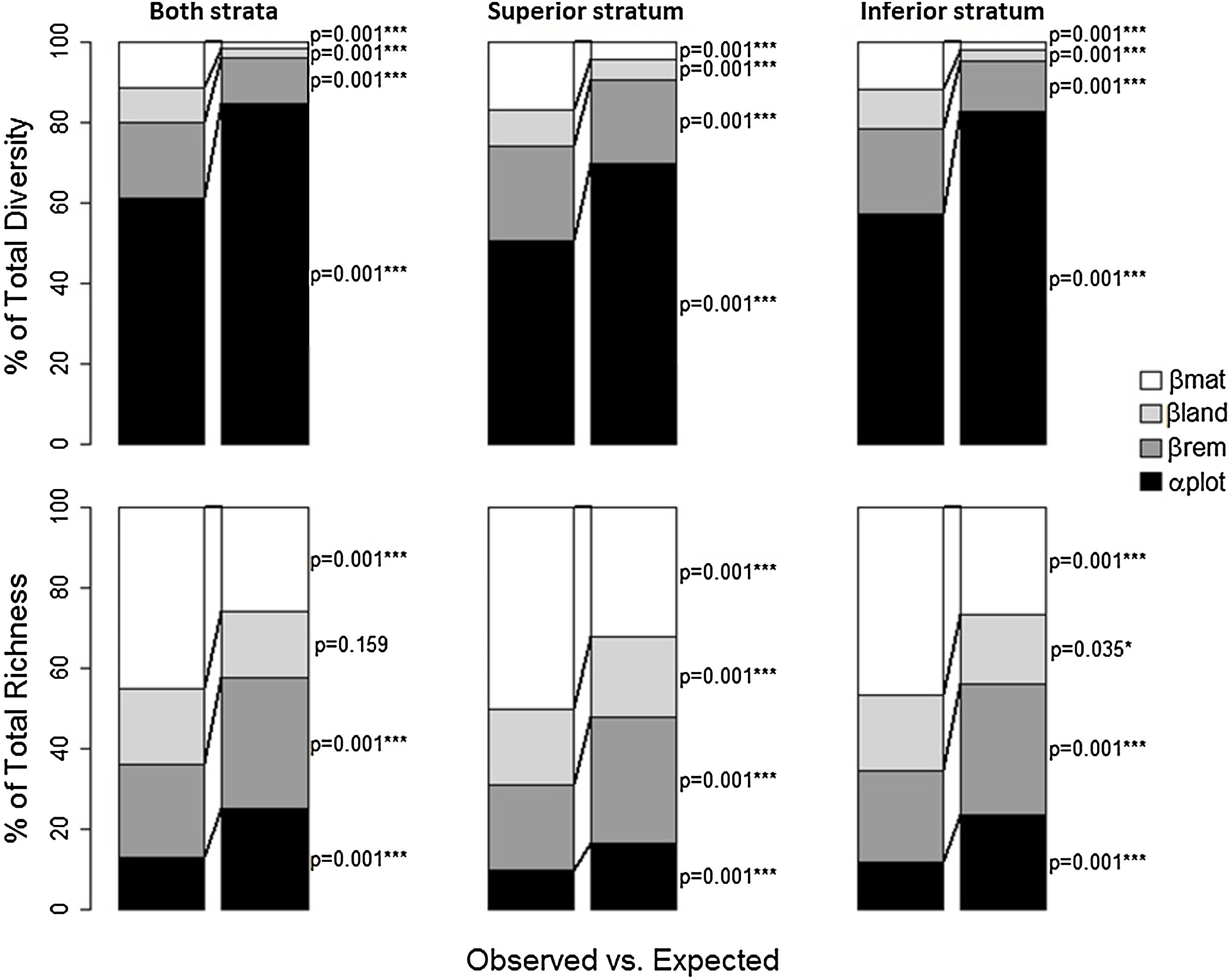
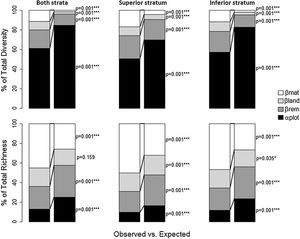
The total diversity and richness differed significantly from the null model for all alpha and beta components, considering the two strata together or separately (Fig. 3 and Supplementary Material - Table 3). More than 40% of total diversity was due to the alpha component alone in all cases (for both strata together, it was close to 60%), but it was significantly lower than expected. Beta components among forest remnants (βrem), among landscapes (βlan) and matrices (βmat) were higher than expected for superior and inferior strata, together or separately. However, βrem was particularly more important for total diversity, comprising 18% for both strata together, 23% for the superior stratum, and 21% for the inferior stratum. Although the portion of total diversity at the local component (plot level) was high, this component was lower than expected according to the null models in all cases. Thus, the differences among forest remnants, among landscapes, and among matrices are more important (significantly greater than expected) for total diversity than the α component. For total species richness, less than 40% was due to α and βrem together in all cases, as both were significantly lower than expected, which contrasted with total diversity. On the other hand, βlan and βmat were higher than expected, and together they were responsible for more than 60% of the total species richness of the superior and inferior strata (together or separately). According to the null models, the differences among the matrices contributed more to total richness than the other components.
Additive partition of the total plant species diversity (Shannon index) and the total species richness among 59 plots on the Corumbataí River basin, Brazil. Values are expressed as the percent of the total diversity and total richness explained by each hierarchical level of sampling: αplot — Alpha diversity (plot level); βrem — Beta diversity among forest remnants; βlan — Beta diversity among landscapes; and βmat — Beta diversity among sugarcane and pastureland matrices. The observed partitions are compared to the expected values obtained through randomization.
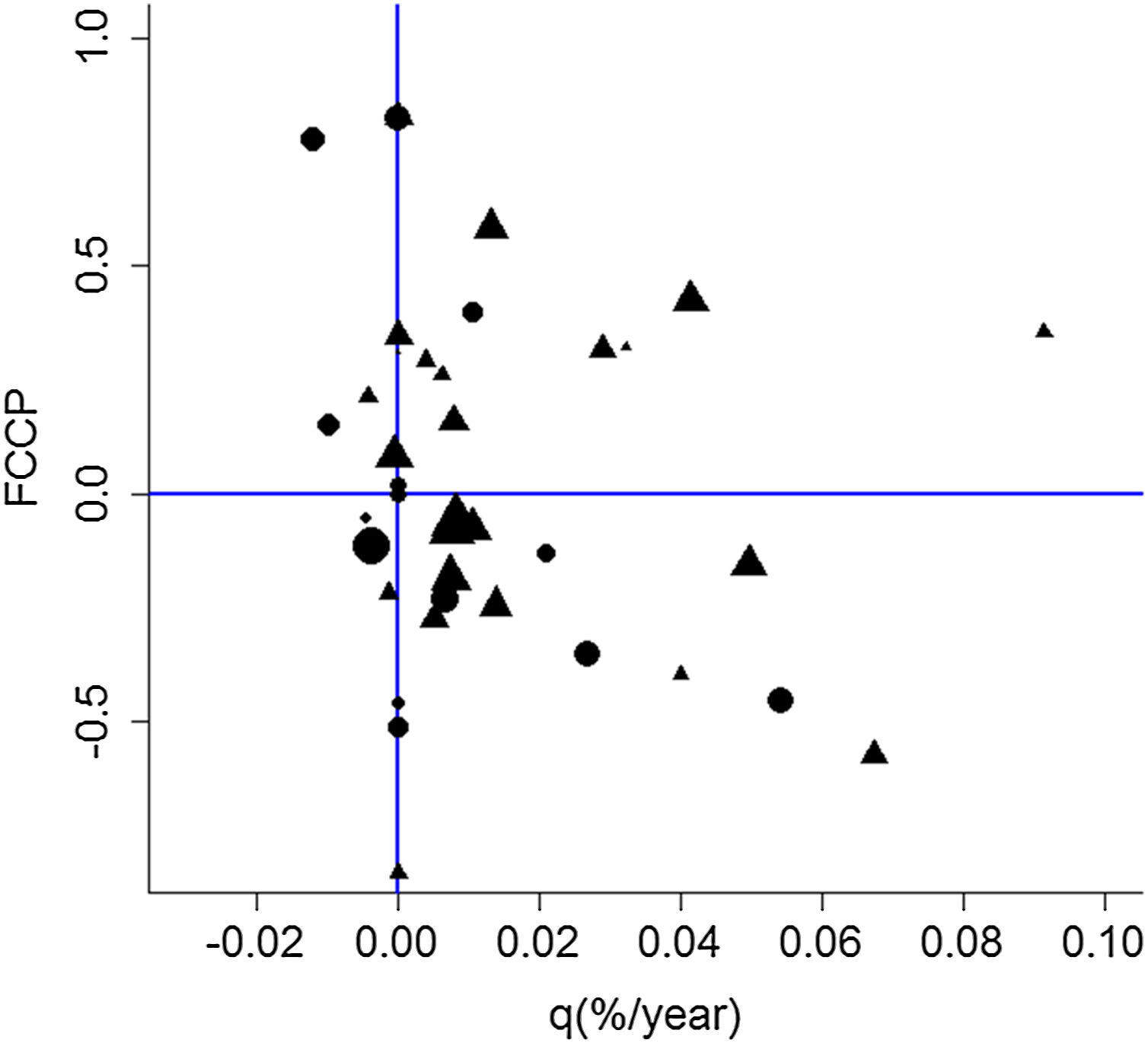
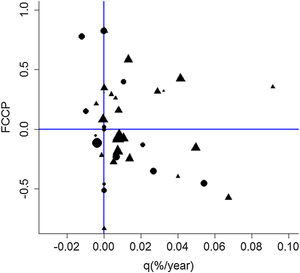
Overall, forest increment under the natural regeneration process has been superior to forest loss in plots for both matrices, indicated by the forest change rate (q%). Although this increment was generally higher for pastureland (Fig. 4), sugarcane plots were predominantly less dynamic over time since some plots were kept without forest suppression or natural regeneration. Processes of deforestation and natural regeneration of the forests were slightly more frequently observed in recent years for the sugarcane matrix indicated by the negative values of FCCP (Fig. 4).
Distribution of sampling plots according to Forest Change Profile Curve (FCCP) and Annual Forest Change rate (q). Circles represent plots embedded in sugarcane landscapes, and triangles represent plots embedded in pasturelands, and their size is related to the diversity index. Adapted from Ferraz et al. (2014).
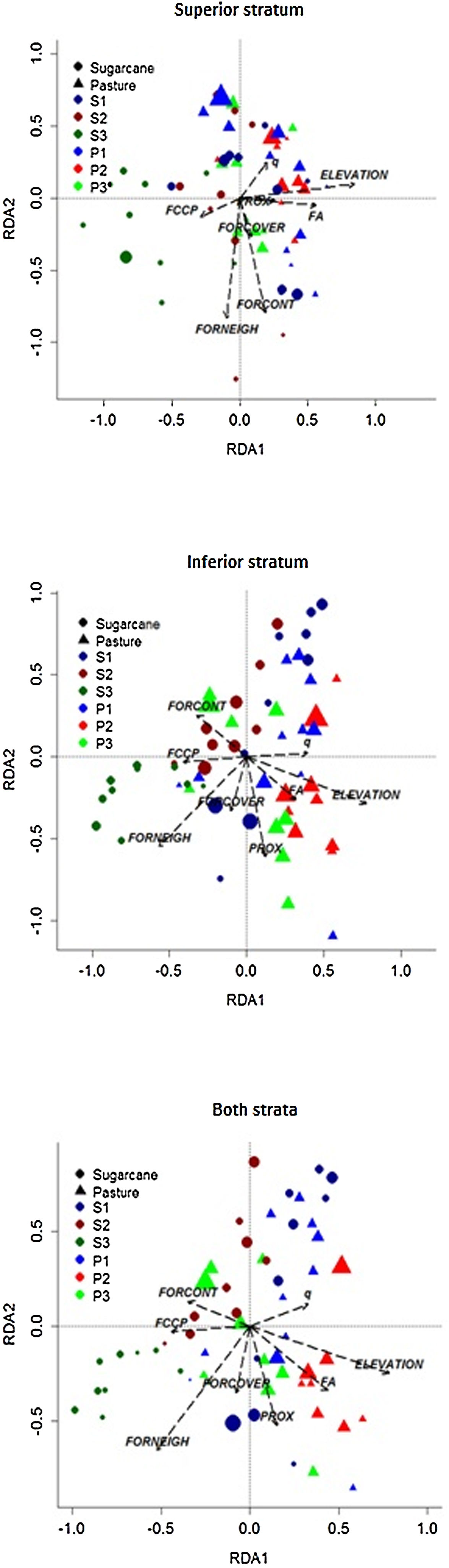
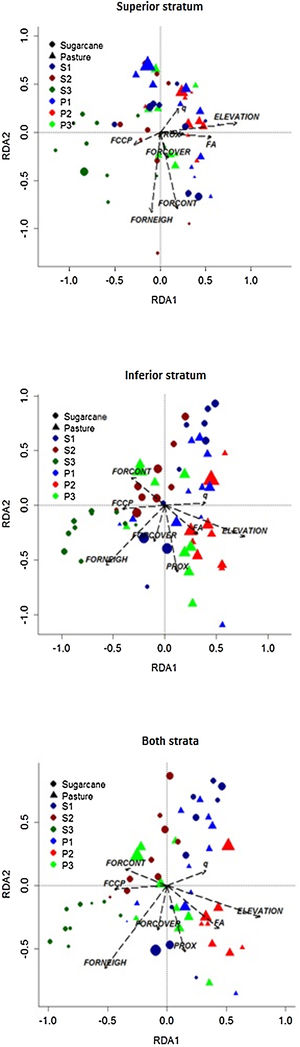
Considering all species together in the superior and inferior strata (Fig. 5), the total inertia was 0.785, the inertia of the constrained axes was 0.148, and the R2 was 18.84. In other words, this value is the proportion of total variance in plant composition explained by landscape variables. Also, the relationship between the floristic data set and the landscape variables was significant (F = 1.422, p = 0.001). Plots were grouped mostly among landscapes in a pattern that plots embedded in pastureland matrices, center to right in ordination, and were positively influenced by Elevation, Mean Forest Age (FA), and Mean Annual Forest Change rate (q). Adversely, plots embedded in sugarcane matrices were positively influenced by Local Forest Neighborhood Dominance (FORNEIGH), Forest Change Curve Profile (FCCP), and Forest Contiguity (FORCONT). Despite this general pattern, few plots of S1 and P3 landscapes were mixed among all of the plots.
Relationship between landscape structure and dynamic metrics on plant species composition, in 58 sampling plots on the Corumbataí River basin, Brazil. FA: Mean Forest Age; FCCP: Forest Change Curve Profile; FORCOVER: Local Forest Cover; FORCONT: Forest Contiguity; FORNEIGH: Local Forest Neighborhood Dominance; PROX: Forest Proximity; q: Mean Annual Forest Change rate. S1, S2, S3: Studied landscapes in sugarcane matrix; P1, P2, P3: Studied landscapes in pasturelands.
We found similar results for the superior and inferior stratum analyzed separately with those found for both strata together (Fig. 5). For superior stratum species, the total inertia was 0.838 and for constrained axes it was 0.159 with R² = 18.57. These values for the inferior stratum were 0.805, 0.147, and 18.30, respectively. Also, the relationship between the floristic data set and the landscape variables was significant for both strata (F = 1.398 and 1.373, respectively, p = 0.001).
DiscussionPrevious studies have shown, and our results support, that there is an important plant diversity and richness that remains in the agricultural and deforested landscapes with recurrent impacts on biodiversity (Magnago et al., 2014; Machado et al., 2016; Farah et al., 2017). Plant diversity differed among agricultural matrices, probably as a response to the different disturbance regimes of pasturelands and sugarcane fields. Forest remnants in pasturelands were overall more dynamic, where deforestation and regeneration occurred more frequently over the past few years (Ferraz et al., 2014), and therefore, the highest habitat heterogeneity could also explain the highest diversity (Ricklefs, 1977). The inferior stratum was richer and more diverse than the superior, due to the presence of plants from different growth forms on the understory (shrubs and small trees). Furthermore, in the inferior stratum, diversity varied among agricultural matrices, being higher in the pastureland matrix. Thus, the matrix is likely to be important to forest dynamics. For example, the matrix will often act as a selective filter for species’ movement across the landscape. The type of vegetation, height, or management (e.g., use of herbicides, pesticides, fire and fencing to restrict livestock transit) in the matrix will determine the permeability level for movements of individuals (da Silva et al., 2015). Characteristics of the surrounding matrix can strongly influence the plant regeneration patterns in forest remnants (Grau, 2004). The presence of isolated tress and the scattered distribution of forest remnants, common in pasturelands, are proven to facilitate seed dispersal (Chazdon and Guariguata, 2016; Holl et al., 2016; Toh et al., 1999). Our results support that pastureland matrices seem to provide better conditions for plant regeneration in similar forest edges when compared to sugarcane matrices.
Although individuals from superior stratum are present in lower densities when compared to other fragments of Tropical Semi-deciduous Forests (Ivanauskas et al., 1999), this species pool and high individual density per hectare suggests that these forest remnants still present resilience and therefore may be able to persist in the landscape. The high number of animal-dispersed plant species, as adults and regenerating, suggest that seed dispersal processes are still happening in such anthropic landscapes. However, the local population persistence of such tree species is firmly related to the effective conservation of their frugivorous bird communities responsible for complementary functional processes (Rother et al., 2016). The process of defaunation of large-bodied dispersers observed in human-modified landscapes remains one of the main issues to be addressed and limits the potential persistence of large-seeded plant species in the study sites and other tropical regions (Cramer et al., 2007; Dirzo et al., 2014; Melo et al., 2006).
Previous studies have also shown a great influence of agricultural uses on plant diversity, ecological processes such as seed dispersal and recruitment, and ultimately, forest resilience (Herrera et al., 2010; Jakovac et al., 2015). It is important to recognize that our plots were located near forest edges, where these impacts are stronger, and diversity is expected to be low (Murcia, 1995). Our results showed that diversity is low at the plot level, which might be explained by a myriad of factors that influence plant diversity at different scales. At the plot level, there are natural processes such as environmental filtering (soil conditions and light availability) and ecological filtering (demographic stochasticity, seed dispersal limitations, competition, recruitment, etc.) that have a considerable influence on selecting species and defining biodiversity (Arroyo-Rodríguez et al., 2015; Wills et al., 2006). In degraded environments, these processes are strengthened by anthropogenic disturbances, such as edge effects, competition with hyper abundant native species, mainly vines in our case (Schnitzer and Carson, 2010; Tabarelli et al., 2012), and regenerating species selection due to cattle pressure (Griscom et al., 2009). These disturbances have been shown to decrease diversity, thus leading to biotic homogenization at the local level (Püttker et al., 2015). Disturbances at plot level are even more important as our results showed that plots with eight years of regeneration are more diverse than plots with 13 or 30 years of abandonment. Although previous studies show an increase in forest cover in recent years for other tropical regions along the Corumbataí River basin (Ferraz et al., 2014; Molin et al., 2017), this coincides with a decrease in forest quality, because old-growth forests are being replaced by secondary forests in many stages of succession. This result can be explained by two observations. Firstly, there is an urgent need for the conservation and restoration of old-growth forest patches in human-modified landscapes. These patches are irreplaceable in biodiversity conservation (Gibson et al., 2011), particularly in the protection of groups of species sensitive to fragmentation and anthropogenic disturbances, such as large-seeded species (Melo et al., 2007), endemic species (Liebsch et al., 2008) and rare species (Mouillot et al., 2013). The removal of degradation drivers and restoration of these patches could reverse the trajectory of degradation and increase their potential for housing such sensitive groups (Vidal et al., 2016). Secondly, this result reinforces the importance of forest regeneration for conserving biodiversity at the landscape scale (Latawiec et al., 2016). In our study sites, young forest regeneration accounted for the greatest diversity within communities at the plot level. Natural regeneration has also been included as one of the main methods to restore degraded and open areas in large-scale projects, which would increase forest cover in highly fragmented landscapes (Chazdon and Guariguata, 2016). Together with old-growth forests (and not by replacing them), young forest patches and disturbed patches increase habitat cover for native species, increase habitat heterogeneity at the landscape level, and enhance landscape connectivity, which enhances the potential for biodiversity conservation of human-modified landscapes (Tabarelli et al., 2010).
It is interesting to point out that different matrix types may influence the forest fragment edge(Laurance and Yensen, 1991; Mesquita et al., 1999). The higher diversity observed in eight year plots in the pastureland matrices shows that matrix quality clearly affects plant dynamics and dispersal across the landscape. Pasturelands are widely considered to be a hostile mode of land use (Peres et al., 2010). However, sugarcane is often considered even more detrimental due to the high level of degradation caused by management practices, such as burning the adjacent forests, since sugarcane leaf biomass was typically burned before harvesting in the dry season up until recently (Martinelli and Filoso, 2008). This could explain the differences we observed for species diversity in eight year plots compared to 13 and 30 year plots occurring mainly in sugarcane matrices. Betadiversity was higher than expected by chance for total diversity among plots, among time of regeneration and among matrices, and in most cases for species richness (except among plots). This is consistent with previous studies in other tropical regions (Arroyo-Rodríguez et al., 2013; Machado et al., 2016). In our study area, the impermanence of secondary forests together with recurrent human pressure, implies different successional pathways for such plant communities. Each trajectory implies different forest assemblages, which allows the accumulation of a higher number of species at the landscape level (Arroyo-Rodríguez et al., 2015; Püttker et al., 2015). Essentially, as proposed by Tscharntke et al. (2012), the landscape-moderated dissimilarity of local communities (alpha) determines landscape-wide biodiversity (beta) and overrides any negative local effects of habitat fragmentation on biodiversity.
This understanding of biodiversity in fragmented habitats has a substantial influence on management strategies and the conservation role of forests under the distinct age of regeneration in human-modified landscapes. Previous studies have shown, and our results also support, that these disturbed forests have low plant diversity and richness, especially when compared to large tracts of pristine forests (Santo-Silva et al., 2015). On the other hand, all remaining and regenerating forests together still preserve high diversity at the landscape level (i.e., high beta diversity) even when compared to large conservation units (Farah et al., 2017), and not only for plants but also for other biological groups, such as large-bodied mammals (Beca et al., 2017). In other words, in human-modified landscapes, each forest regardless of its size has an immense value to the conservation of overall biodiversity, and the discussion regarding the conservation role of secondary forests should be migrated from the local to the landscape level (Arroyo-Rodríguez et al., 2015, 2009; Bergamin et al., 2017). Our results reinforce the need for secondary forests to be prioritized in future conservation and restoration efforts at the landscape scale to improve their persistence (Reid et al., 2018).
Notwithstanding, it is not often clear what the best strategy is to conserve remnant biodiversity in highly modified landscapes. Previous studies have reported that in landscapes with a low amount of forest patches, increasing connectivity is ineffective, whereas at low connectivity increasing high patch numbers is useless (Johst et al., 2011). Others have found that 30% of habitat cover is the turning point for species occurrence and the persistence of functional groups sensitive to fragmentation (Estavillo et al., 2013; Pardini et al., 2010). Therefore, landscapes below this threshold of 30% should not be targeted as priority areas for restoration, due to the low cost-effectiveness of these projects (Banks-Leite et al., 2014; Tambosi and Metzger, 2013). Nonetheless, each biological group has a different response to fragmentation thresholds and landscape structure, and for plants, regenerating individuals tend to reflect the current quality and configuration of the landscape, while adults are able to remain in the landscape for a very long time following habitat loss (Rigueira et al., 2013).
In the Atlantic Forest, 88% of forest cover is present in landscapes below the theoretical biodiversity thresholds (Banks-Leite et al., 2014), and therefore would be in danger of not being targeted for restoration programs. In addition, landscapes with different anthropic occupations present different distributions of patches and different historical dynamics. In our study sites, the influence of landscape structure and dynamic metrics on plant diversity differed among agricultural matrices, suggesting that, in highly fragmented landscapes, only the percentage of forest cover is not enough to define restoration and conservation strategies. In our study region, sugarcane landscapes present larger and less connected forest remnants, whereas, in pastureland landscapes, forest remnants are mostly connected but usually smaller and linear (Ferraz et al., 2014). Therefore, different strategies should be applied to each landscape to conserve biodiversity. In landscapes with low forest cover and highly dynamic histories, it might be more effective to increase biodiversity persistence by decreasing patch destruction than by increasing patch creation (Johst et al., 2011), so it is crucial that the remaining biodiversity should be maintained through conservation and restoration of current forests. This strategy must be considered to implement new protected areas, therefore increasing the number of conservation units, along with the assistance of financial incentive programs and specific legislation since biodiversity is spread in forest remnants in the landscape (Bergamin et al., 2017). Although nowadays there are legal instruments that can increase landscape connectivity through the restoration of riparian areas, very few require an increase in quality of the actual remaining forests and prevention of within-forest disturbances (Brancalion et al., 2012; Viani et al., 2015; Barlow et al., 2016; Rother et al., 2018).
Final considerationsOur study showed the high potential for biodiversity conservation of secondary forests within private properties in highly modified landscapes. We found plant diversity to be higher than expected in both strata, but local plant communities differed from forests and agricultural matrices. Therefore, to conserve biodiversity in highly deforested landscapes, conservation plans should be enacted at the regional level and not at the forest patch level. Since every forest remnant presents a distinctive group of species that must be conserved and they play a unique role in biodiversity conservation.
Our results underscore the importance of assessing the structure and function of secondary forests, but we still have a limited understanding of how to provide more options for the persistence of populations of threatened and endangered species in agricultural landscapes. It is also important to continue research on how we can restore these forest remnants in order to improve their role in biodiversity conservation.
We thank the students and partners of the Laboratory of Ecological Restoration (LERF/ESALQ/USP) for helping us in fieldwork and discussion of the data. We are indebted to the Brazilian National Research Council (CNPq) for the productivity fellowship (grant #308503/2014-7), to KMPMBF and the research grant for JRSAM (#140825/2013-4). The authors also thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior do Governo do Brasil, #88882.305844/2018-01) for the fellowship grant to DCR. This research was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (Biota Project #2013/50718-5 and #1999/09635-0), FAPESP (#2011/06782-5) and CNPq (#561897/2010-7).