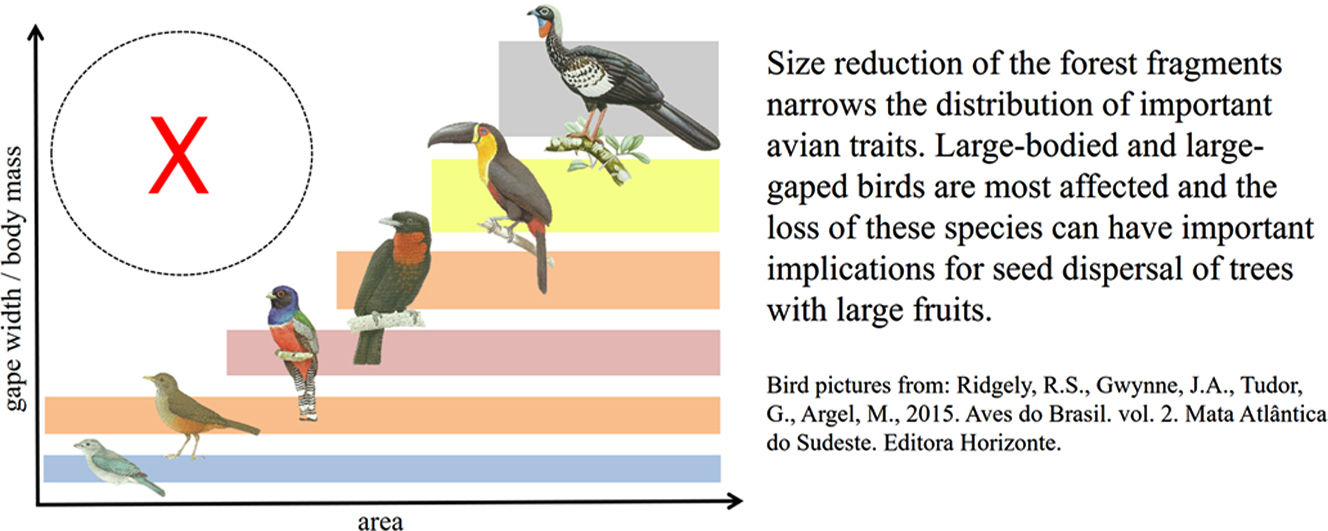
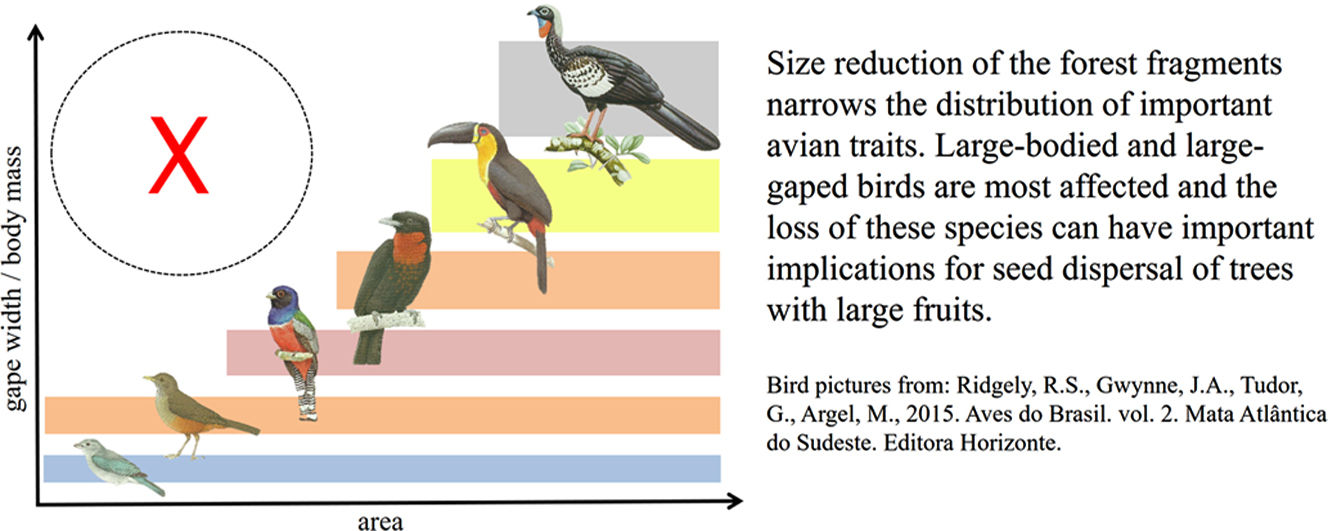
Land-use change influences biodiversity in non-random ways, affecting some species and functional groups more than others, with potential implications for the loss or degradation of important ecological processes, such as seed dispersal. Here we investigate the effect of patch-size reduction on the composition and functional richness (FRic) of avian communities in Atlantic Forest fragments, focusing on morphological traits associated with seed dispersal in frugivorous birds. We found that FRic of three key traits—hand-wing index, body mass and gape width—decreased with patch size reduction, because species with larger values for morphological traits were lost through local extinction. The relative absence of large-gaped and more-dispersive frugivores in small forest fragments has important implications because these species play a pivotal role in seed dispersal, carrying higher seed loads for longer distances, and consuming larger-sized seeds that cannot be dispersed by smaller-gaped frugivores. Our results highlight the importance of preserving large or interconnected habitat patches, and promoting habitat restoration of cleared areas, to ensure that sufficient avian functional diversity is maintained to supply the full range of seed dispersal services required by tropical forests, both currently and in future.
The conversion of tropical forests to anthropogenic land uses is driving the rapid loss and fragmentation of natural habitat into smaller patches, with deleterious consequences for biodiversity (Laurance et al., 2014; Taubert et al., 2018). However, the effects are non-random because species vary widely in their responses to habitat fragmentation, with negative impacts increasing for large, forest-dependent or dispersal limited species (Bregman et al., 2014; Coelho et al., 2016). A combination of area effects and edge effects (Pfeifer et al., 2017), as well as changes in physical conditions of the forest fragments and their surroundings, determine which species are able to survive and colonize those modified areas (Magnago et al., 2015). Because the sensitivity of species to land-use change is influenced by their traits (Burivalova et al., 2015), habitat fragmentation does not merely affect species richness, but also the structure, composition and functional diversity of species assemblages (Uezu and Metzger, 2011; Magioli et al., 2015; Bregman et al., 2016). However, the implications for ecological processes remain poorly understood.
Recent studies have reported a decrease in the functional integrity of bird communities, and the abundance of their constituent species, in tropical forests (De Coster et al., 2015; Coelho et al., 2016). In particular, species with particular ecological and physical traits, such as large body mass and frugivory, are often the most impacted (Galetti et al., 2013; Burivalova et al., 2015). A range of mechanisms may increase local extinction of these traits in small habitat patches, including low population density, large area requirements, and reduced likelihood of recolonization for larger species (Tobias et al., 2013), as well as hunting and interspecific competition for food resources, both of which can significantly increase in forest fragments (Bregman et al., 2015, 2016).
Non-random extinctions mediated by body size and dietary niche have important implications for ecological processes (Burivalova et al., 2015; Bregman et al., 2016). In particular, the loss of frugivorous species from tropical forests is potentially disastrous because more than 90% of woody plant species are dispersed by animals (zoocoric; Jordano, 2016), with birds particularly important, especially when other large vertebrates have been extirpated (Holbrook et al., 2002). Moreover, the long-term resilience of tropical forests, and their ability to regenerate, relies on the continued presence of frugivores that can transfer seeds from remaining forest into adjacent disturbed habitats (Alexandrino et al., 2016; Pizo, 2007). Yet, we still have only a limited understanding of how land-use change effects the structure and functioning of seed-disperser communities in fragmented tropical forests.
In this investigation, we assess how reduction in forest patch size affects the composition of morphological traits and functional richness of frugivorous bird assemblages. We focused on survey data from forest fragments in the Atlantic Forest of Brazil, an area famed for habitat fragmentation and the consequent loss of biodiversity (Brooks et al., 1999). Given that biodiversity tends to decline in association with habitat patch size in tropical forests (Bregman et al., 2014; Magioli et al., 2015; De Coster et al., 2015), we predict that functional richness of frugivore assemblages will decrease in line with patch size. We also explore the association between habitat patch size and the occurrence of key traits associated with seed dispersal—including beak shape and wing shape—both of which can provide insight into the size of seeds consumed by frugivores and the distance seeds are likely to be dispersed (see Bregman et al., 2016; Pigot et al., 2016).
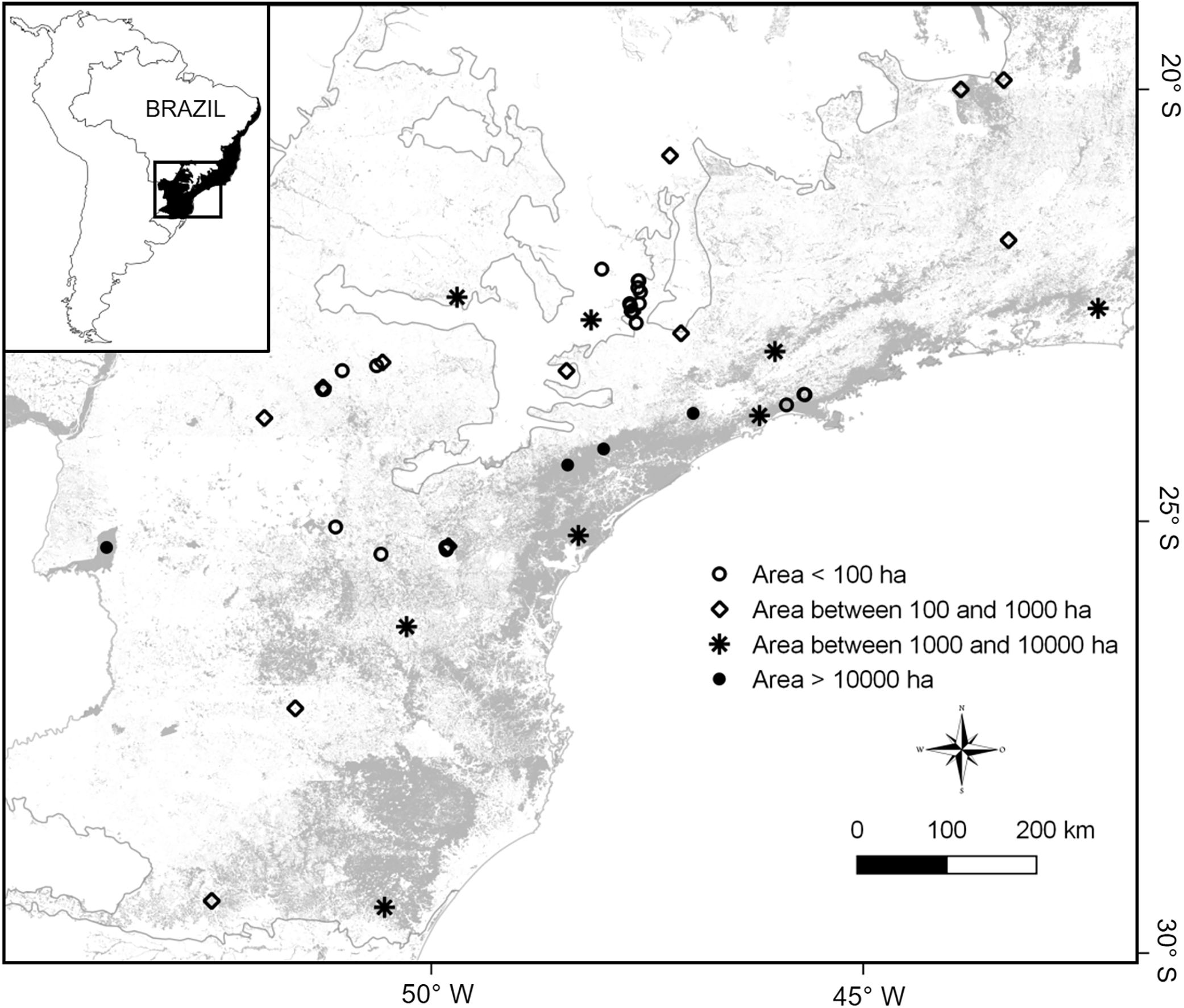
Material and methodsStudy areaWe focused on the southeastern sector of the Brazilian Atlantic Forest, a region that has undergone dramatic human-modification such that only ca. 12% of the original forested habitat remains, much of which is divided into forest fragments smaller than 50ha (Ribeiro et al., 2009). The Atlantic Forest as a whole is a biodiversity hotspot supporting approximately 688 bird species, of which ∼30% are endemic (Goerck, 1997). Many regional bird species have been negatively affected or even extirpated by forest loss and fragmentation (Brooks et al., 1999; Pereira et al., 2014).
Bird assemblage databaseWe created a database of bird assemblages using data collected through field surveys of Atlantic Forest habitat patches between 1990 and 2014. We searched for indexed papers on Web of Science, Google Scholar and SciELO, and also used online search engines to look for relevant gray literature including non-indexed papers, management plans of protected areas, theses, dissertations and monographs. We searched in English using the following keywords (bird* OR avian) AND (Atlantic Forest OR forest OR fragment OR remnant OR community*). We also conducted the same searches translated into Portuguese. Any study restricted to a particular subset of the bird community, whether defined by ecology (e.g. understory species) or taxonomic groups (e.g. Passeriformes), were discarded. Because we are concerned with the impacts of fragmentation on seed dispersal in tropical forests, we restricted our dataset to species that were both frugivorous and forest-dependent. Frugivores were classified as species with >10% of their diet consisting of fruit, berries and seeds (Wilman et al., 2014), since species classified as omnivorous can act as important seed dispersers in modified areas (Pizo, 2007). Seed predators such as Tinamidae and Psittacidae were excluded, following Bregman et al. (2014). Forest-dependent species were classified based on Parker et al. (1996).
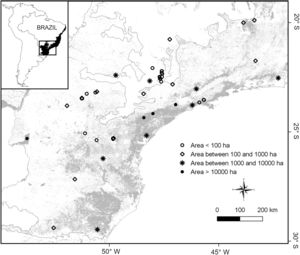
The final dataset contained a total of 33 studies supporting a total complement of 157 frugivorous forest-dependent bird species (see supplementary data 1, supplementary data 2, Table S1). This produced data on frugivore assemblages in 48 areas, covering a wide gradient of latitude (19°28′–29°28′ S), longitude (40°32′–53°47′ W), elevation (30–1059masl), and patch sizes (mean=6169ha [2.59–185,000ha]; Fig. 1). Many of our assemblages are directly related to surveys in habitat fragments whereas other surveys were conducted in forests embedded in a non-habitat agricultural matrix (i.e., sugarcane and pasture) as forest patches. Some of our study sites (indicated in supplementary data 1) lie within the Serra do Mar forest continuum, the largest remnant of the Atlantic Forest (>1,000,000ha overall, but subdivided by roads and associated habitat clearance). In these cases, the size of study plots does not represent the actual patch size of the forest, so we assigned an arbitrary patch size of 500,000ha, following Ribeiro et al. (2009).
Morphological traitsWe collected data on four biometric traits – body mass, gape width, wing length, and first-secondary length (supplementary data 2, Table S2) – each capturing different dimensions of the ecological niche related to seed dispersal. Body mass is a standard ecological trait and at least partly reflects the amount of fruits that can be consumed, and seeds dispersed, by frugivorous organisms (Jordano and Schupp, 2000). Gape width provides an estimate of the upper limit to seed size that can be swallowed and, consequently, dispersed by a bird (Wheelwright, 1985). Wing length (WL) and first-secondary length (FSL) in combination provide an estimate of wing shape related to dispersal (Dawideit et al., 2009). Specifically, following Claramunt et al. (2012), we calculated hand-wing index (HWI) using the equation HWI=100×(WL−FSL)/WL, where WL is the distance from the carpal joint to the tip of the longest primary feather, and FSL is the distance from the carpal joint to the tip of the first secondary feather. HWI is a standard index of flight and dispersal ability in birds, and thus potentially reflects the potential for long-distance seed dispersal, which in turn is crucial role for metacommunity dynamics and seed dispersal in fragmented forests (Hamilton, 1999).
We compiled body mass data for all study species based on published literature (Dunning, 2007; Wilman et al., 2014). The other three biometric traits were measured from specimens at several museum collections, including the Natural History Museum, Tring, UK, and in the Zoology Museum of University of São Paulo, Brazil. When possible, we measured four adult individuals of each species (two males and two females), and used average values at species level in our analyses. In total, we compiled a complete dataset of four biometric traits for all bird species in our sample. We note that HWI was only very weakly associated with body mass (Pearson's correlation: r=−0.01) and gape width (r=−0.12), while gape width was partially correlated with body mass (r=0.48).
Functional richnessNumerous functional diversity measures have been proposed and used in ecological studies, but because we are interested in variation in extreme values, we used the Functional Richness (FRic) metric developed by Villéger et al. (2008).
Unlike some other functional measures, FRic does not require abundance data, and is thus the most suitable index in the context of our study. FRic represent the amount of functional space filled by the community (Villéger et al., 2008) in a T-dimensional space, where T is the number of functional trait axes incorporated. Thus, we calculated FRic using a convex hull with each functional trait as one dimension. FRic is the volume of this hull, which encompasses all species in an assemblage, bounded by the extreme trait values in each dimension. To calculate FRic, we entered all three functional traits—body mass, gape width and HWI—into the function ‘dbFD’ of the package ‘FD’ (Laliberté et al., 2014), available on R 3.1.1 (R Core Team, 2016).
Analytical approachOne standard technique for assessing relationships between variables involves using mean values in least square regressions. However, this approach is insensitive when the mean undergoes no change, thus masking effects on more extreme values (Cade and Noon, 2003). Given that we are interested in effects of land-use change on different components of trait variation, we explored the relationship between morphological trait composition and patch size using quantile regression, which allows us to model shifts in extreme trait values (Cade et al., 1999; Cade and Noon, 2003). A quantile τ (0<τ<1) of one sample represents a point in which 100τ% of the sample values are smaller than it. For example, the 0.9 quantile for body mass represents the value at which 90% of the bird species weigh less, and 10% weigh more. We used quantile regression to model patterns of association between morphological traits and patch size for a range of different quantiles. The associations—which we refer to as ‘trends’ following Elsner et al. (2008)—are estimated using the slope coefficients for each quantile. We defined significant quantiles as those for which the trend values were higher than the confidence interval of the linear regressions. For further details of the rationale and application of these methods, see Elsner et al. (2008). We performed quantile regressions using the ‘quantreg’ package (Koenker, 2016), available on R 3.1.1. For both FRic and quantile regression analyses, we log-transformed body mass and patch size to ensure that variables were distributed normally.
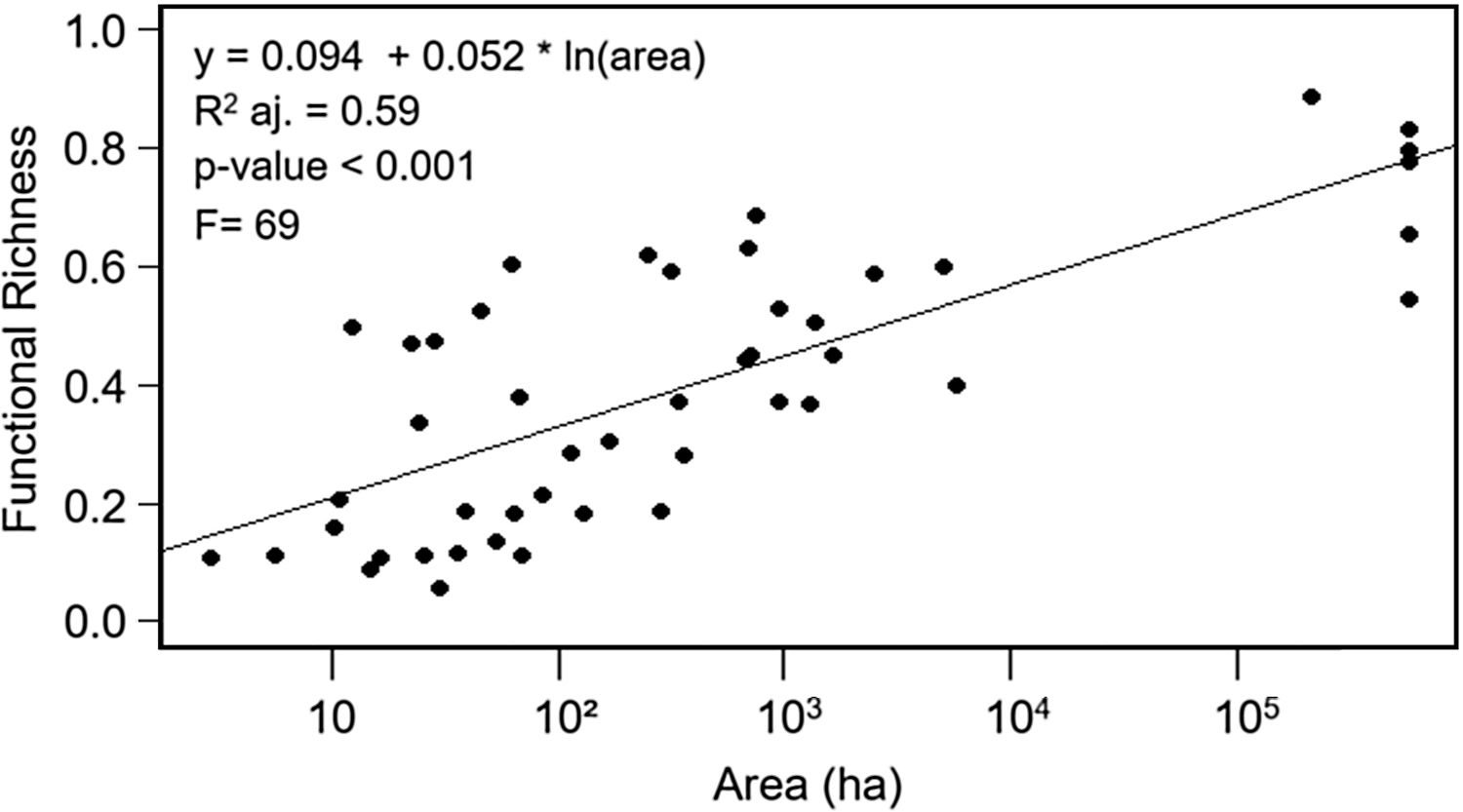
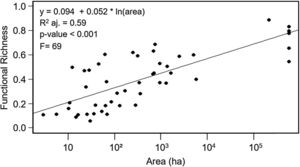
ResultsEffects of fragment sizeFRic values for frugivorous bird assemblages ranged from 0.05 to 0.88 (mean=0.40). Patch size positively influenced the filling of functional space in frugivorous bird assemblages, with values of FRic increasing in larger fragments (Fig. 2; adjusted-R2=0.59, F=69, p<0.001).
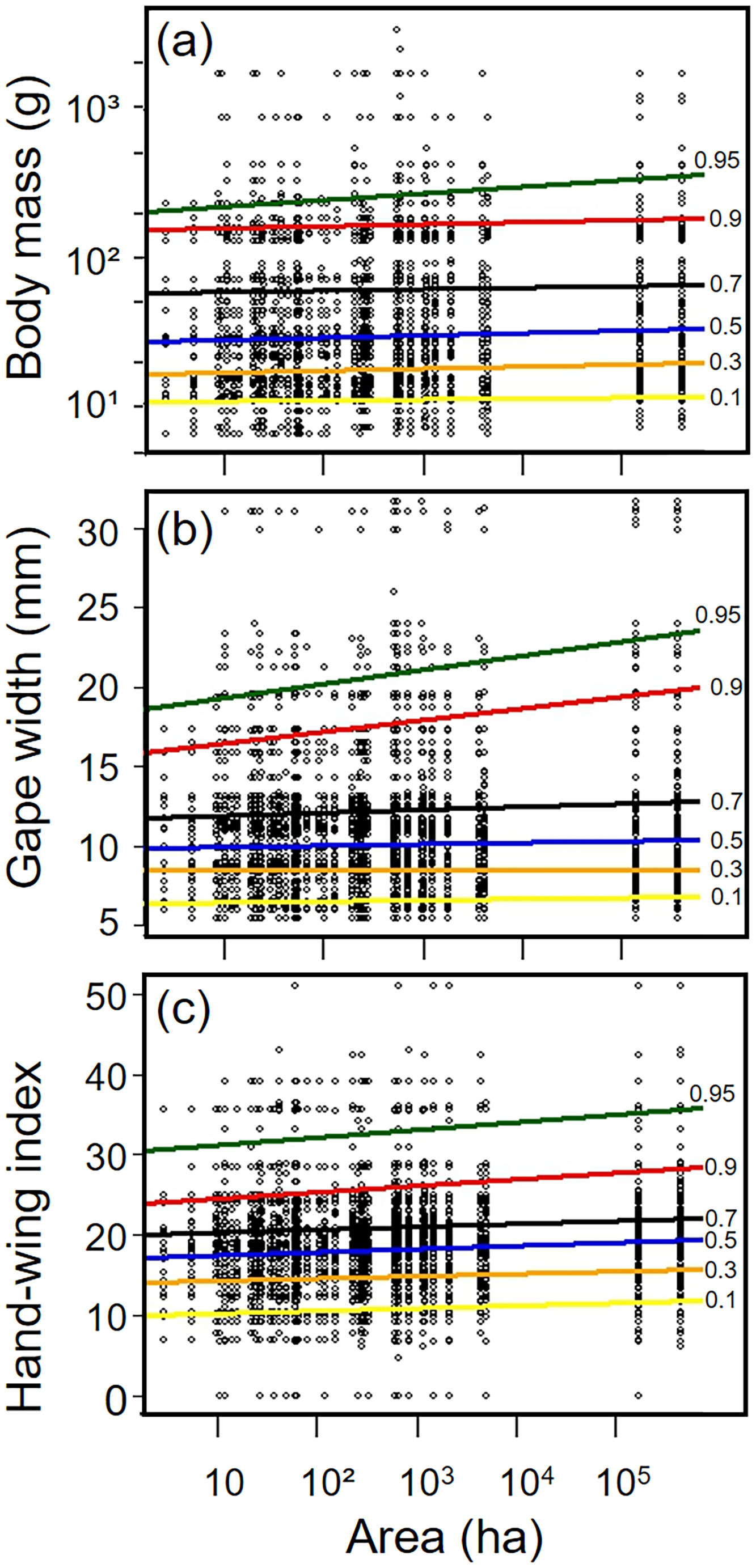
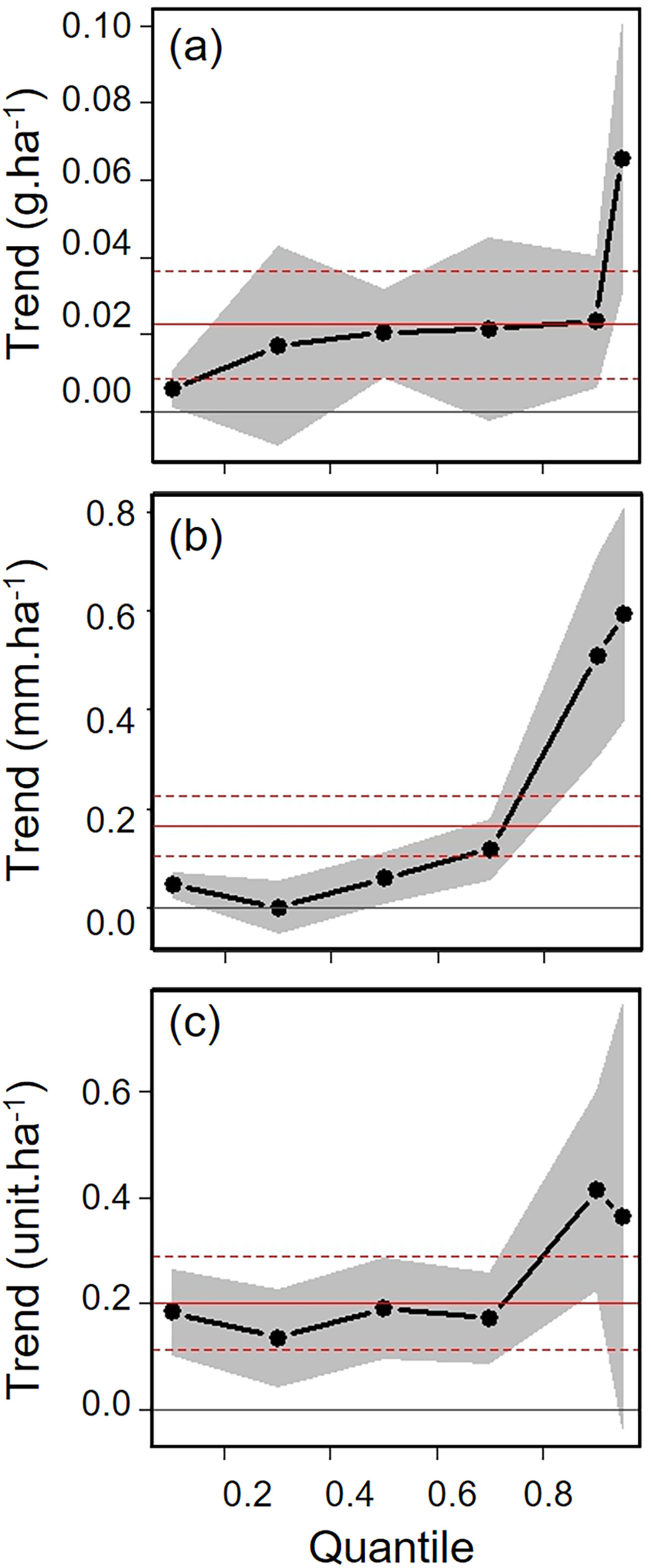
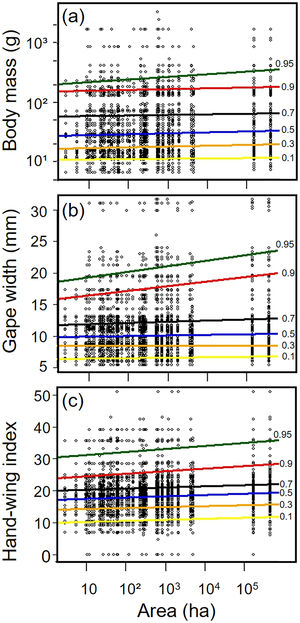
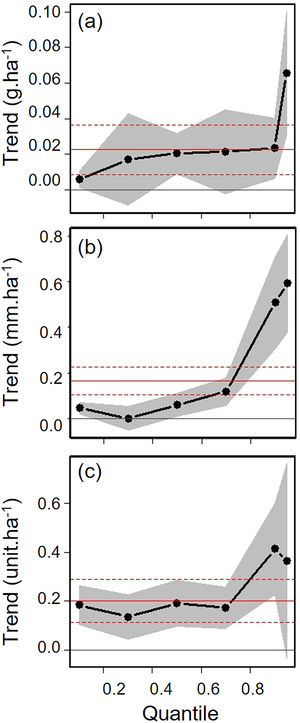
Biometric trait variationHWI and body mass were negatively affected by patch size reduction, with both traits showing an asymmetric decrease in their highest trait values (Fig. 3a and c, supplementary data, Table S3), and consequently in the upper (0.9 in HWI and 0.95 in both) quantiles (Fig. 4a and c, supplementary data, Table S3). In contrast, lower values of HWI and body mass were not affected by patch size reduction, with values below the cited above showing no significant changes. Similarly, gape width was affected by patch size reduction only for species with the widest gapes. The slope of associations was increasingly steep above the 0.9 quantile (Figs. 3b and 4b), where we detected an almost 5mm average gape width difference between frugivore assemblages in the smallest and largest forest fragments.
Relationships between habitat patch size and three biometric traits—(a) body mass; (b) gape width, and (c) hand-wing index (HWI)—in frugivorous bird assemblages. Black dots represent trait values for each bird species of an assemblage (y-axis) in each forest patch. Colored lines show the quantile regression for six different quantiles: τ=0.1 (yellow), τ=0.3 (orange); τ=0.5 (blue); τ=0.7 (black); τ=0.9 (red) and τ=0.95 (green). (For interpretation of the references to color in this legend, the reader is referred to the web version of the article.)
Trends between patch size and three biometric traits—(a) body mass; (b) gape width, and (c) hand-wing index (HWI)—estimated for frugivorous bird assemblages, and plotted for six different quantiles (τ=0.1, 0.3, 0.5, 0.7, 0.9, 0.95). Gray shadows represent 90% confidence intervals. The continuous horizontal red line shows the strength of association using linear regression, and the dashed red lines represents the 90% confidence interval of the linear regression. Association values outside the confidence interval of the linear regression were treated as significant, occurring above the quantiles 0.95 for body mass, 0.9 for gape width, and 0.9 for HWI (more information on supplementary data, Table S3). (For interpretation of the references to color in this legend, the reader is referred to the web version of the article.)
Our results indicate that the structure of frugivorous bird assemblages was affected by patch size reduction: the smaller the forest fragment, the smaller the range of values for body mass, HWI and gape width when compared to those found in larger fragments. The same pattern was observed for FRic, with lower values associated with small areas. Our findings add to a growing body of work suggesting that larger-sized seed-dispersers are predisposed to extinction in degraded or fragmented woodland, with implications for seed dispersal (Terborgh et al., 2008; Galetti et al., 2013; Bregman et al., 2016; Bomfim et al., 2018). In particular, the functional impoverishment of frugivore communities in smaller forest patches may have severe negative consequences for plant species that depend on vertebrate seed dispersers.
Local extinction and species turnover in the face of land-use change may have no impact on the functioning of ecosystems if species are ‘functionally redundant’ and simply replaced by other species with similar ecological function (Schwartz et al., 2000). However, our results suggest that this is not the case for seed dispersing birds because species with large gapes are replaced in smaller forest patches by smaller-gaped species which likely perform different roles. In particular, smaller-gaped frugivores can presumably only swallow smaller fruits and seeds (Wheelwright, 1985), and thus are unlikely to meet the demand for dispersing large-seeded trees, many of which are high-stature, commercially valuable hardwoods.
When we analyzed traits separately, we found consistent increased sensitivity of the highest trait values to extinction in smaller habitat patches. For body mass, the strongest effect of land-use change was detected in large-sized species, many of which decline in or disappear from small forest fragments (Bregman et al., 2015; Burivalova et al., 2015). Indeed, the disappearance of large species from small habitat patches is one of the most pervasive impacts of tropical forest fragmentation, partly because tropical organisms with larger body size have larger area requirements, lower population density and lower reproductive output (Tobias et al., 2013), as well as being the target of increased hunting pressure in fragmented environments (Peres and Palacios, 2007).
Previous studies have found evidence that average HWI tends to increase in small isolated fragments (Bregman et al., 2015), presumably because species with weaker dispersal ability are less capable of crossing gaps between fragments, and thus suffer greater negative impacts of fragmentation. In contrast, our analyses using quantile regression reveal that frugivores at the upper extreme of HWI variation (above the 0.9 quantile) are also negatively affected by decreasing habitat patch size. This has potential implications for seed dispersal because frugivores with highest HWI are likely to travel greatest distances between foraging patches, and thus to transfer seeds between isolated habitat patches, as well as into the deforested matrix.
The shifts in bird community structure detected by our analyses may be caused by a range of processes associated with fragmentation mediated by both patch size, isolation, and edge effects, as well as distance to larger forest blocks and the permeability of the deforested matrix (Fahrig, 2003; Banks-Leite et al., 2010). In part, the shifts can be interpreted as responses to available foods, because fragmentation causes changes in the abundance and guild structure of plants. For example, some families considered important sources of fleshy fruits, e.g., Myrtaceae and Lauraceae, are replaced by ruderal species, such as Compositae, Euphorbiaceae and Solanaceae (Tabarelli et al., 1999). Nonetheless, this does not mean that the bird community is necessarily meeting the ecological demands of the plant system because it is clear that larger size classes of frugivores disappear long before their food plants, causing a seed dispersal deficit (Galetti et al., 2013).
Implications for seed dispersalLarge seeds cannot be consumed, or dispersed, by narrow-gaped frugivores (Wheelwright, 1985), whereas wide-gaped frugivores can consume and disperse seeds of all sizes (Pigot et al., 2016). The wider dietary scope of wide-gaped birds means they disperse seeds of larger numbers of plant species, and are therefore integral to the structure and stability of mutualistic interaction networks, acting as ‘network keystones’ (Pigot et al., 2016). Importantly, we have shown that wide-gaped species tend to undergo non-random local extinction in smaller forest fragments, where assemblages of avian frugivores are primarily composed of smaller passerine species such as tanagers, flycatchers and thrushes. The largest of these are thrushes, with gape-width close to 12mm. Thus, in small fragments, plants with seeds larger than 12mm appear to have lost their natural dispersers (e.g. guans, toucans, cotingas), thereby suffering reduced recruitment or strong selection on seed size (Wotton and Kelly, 2011; Galetti et al., 2013), potentially leading to local extinction over longer timeframes.
Although slightly weaker than the effects on gape width, we also found that large-bodied species disappear from small forest fragments. Body mass predicts the strength of species interaction and feeding specialization in seed dispersal networks (Pigot et al., 2016), and has also been used as a proxy to measure how effectively species perform specified trophic functions (De Coster et al., 2015), which tend to be biomass-dependent. For instance, Ramphastos vitellinus (360g) and Aburria jacutinga (1240g) are far heavier than the largest thrush (Turdus rufiventris, 69g), meaning that their intake of fruits and seeds is also larger, at least per individual. In addition, larger frugivores are likely to roam much further to access their food supply (Wotton and Kelly, 2012).
Taken together, these findings suggest that frugivore assemblages in small habitat patches will disperse relatively few seeds and for shorter distances, particularly in the case of larger seeds (>12mm width) which may fall in a high-density concentration below the parent plant (Wotton and Kelly, 2011). The consequent reductions in seed dispersal distance are likely to be problematical both because seed rain is too limited in scope to allow regeneration of cleared areas (Holl, 1999), and also because density-dependent (e.g. Jansen-Connell) effects reduce seedling survival below or near the parent tree (Bagchi et al., 2014). Further research should explore the link between patch size, isolation, food availability, dispersal limitation and matrix permeability in determining the trait structure and functioning of fragment communities.
Relevance to conservation strategiesPrevious studies have concluded that habitat patch sizes above 2050ha maintain high functional diversity of medium- and large-sized mammals (Magioli et al., 2015), and that 30–50% of forest cover maintain vertebrate richness in the Atlantic Forest (Banks-Leite et al., 2014). Similarly, it has been proposed that tropical forest birds are particularly reliant on the preservation of larger fragments (Tobias et al., 2013) and a landscape matrix conducive to dispersal, such as logged or secondary forest rather than pasture and agriculture (Edwards et al., 2014). Our results add weight to these findings, and provide further evidence that forest fragmentation has severe negative impacts, not only on species richness, but ecological function. In particular, we use a trait-based approach (Gagic et al., 2015) to reveal that seed dispersal by larger, more dispersive, and wide-beaked frugivores is severely impaired in small fragments, with negative implications for metacommunity dynamics and gene flow (Hamilton, 1999). In effect, forest fragmentation results in habitat patches with reduced resilience and lower capacity for forest regeneration in surrounding cleared areas. These results highlight the importance of maintaining large protected areas, coupled with effective strategies for improving connectivity among habitat patches, supporting current efforts to restore forests and corridors in the Atlantic Forest and elsewhere (Melo et al., 2013; Tobias et al., 2013; Banks-Leite et al., 2014; Magioli et al., 2016).
FundingThis study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) by grants to AAAB (#2013/24929-9 and #2014/23809-2), ERA (#2010/05343-5 and #2014/14925-9), KMPMBF (#2011/06782-5 and #2014/09300-0), MCR (#2013/50421-2) and MM (#2014/10192-7). We also thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) by grants to AAAB and MM and CNPq for funding KMPMBF (productivity fellowship grant #308503/2014-7) and MCR (312045/2013-1; 312292/2016-3). Trait data were collected with support from the Natural Environment Research Council, UK (NE/K016431/1, NE/I028068/1) to JAT.
Conflicts of interestNone.
We are grateful to the Forest Science Department of “Luiz de Queiroz” College of Agriculture, University of São Paulo (Brazil), Wildlife Ecology, Management and Conservation Lab (LEMaC), the Postgraduate Program in Forest Resources (PPGRF) and Zoology Department, University of Oxford (United Kingdom). We are grateful to Mark Adams, Hein Van Grouw, Robert Prys-Jones (Natural History Museum, Tring), James Van Remsen (Louisiana State University Museum of Natural History), Luis F. Silveira (Zoology Museum of University of São Paulo – MZUSP), and Maria de Fatima Cunha Lima and Alexandre Aleixo (Museu Paraense Emilio Goeldi) for specimen data, access to specimens and logistical assistance. For help collecting and analysing biometric data, we thank many people, including Nico Alioravainen, Vivien Chua, Bianca Darski, Sam Jones, Hannah MacGregor, Monte Neate-Clegg and Catherine Sheard. We also thank Bruno Santos and Rafaela Naves for useful discussion.