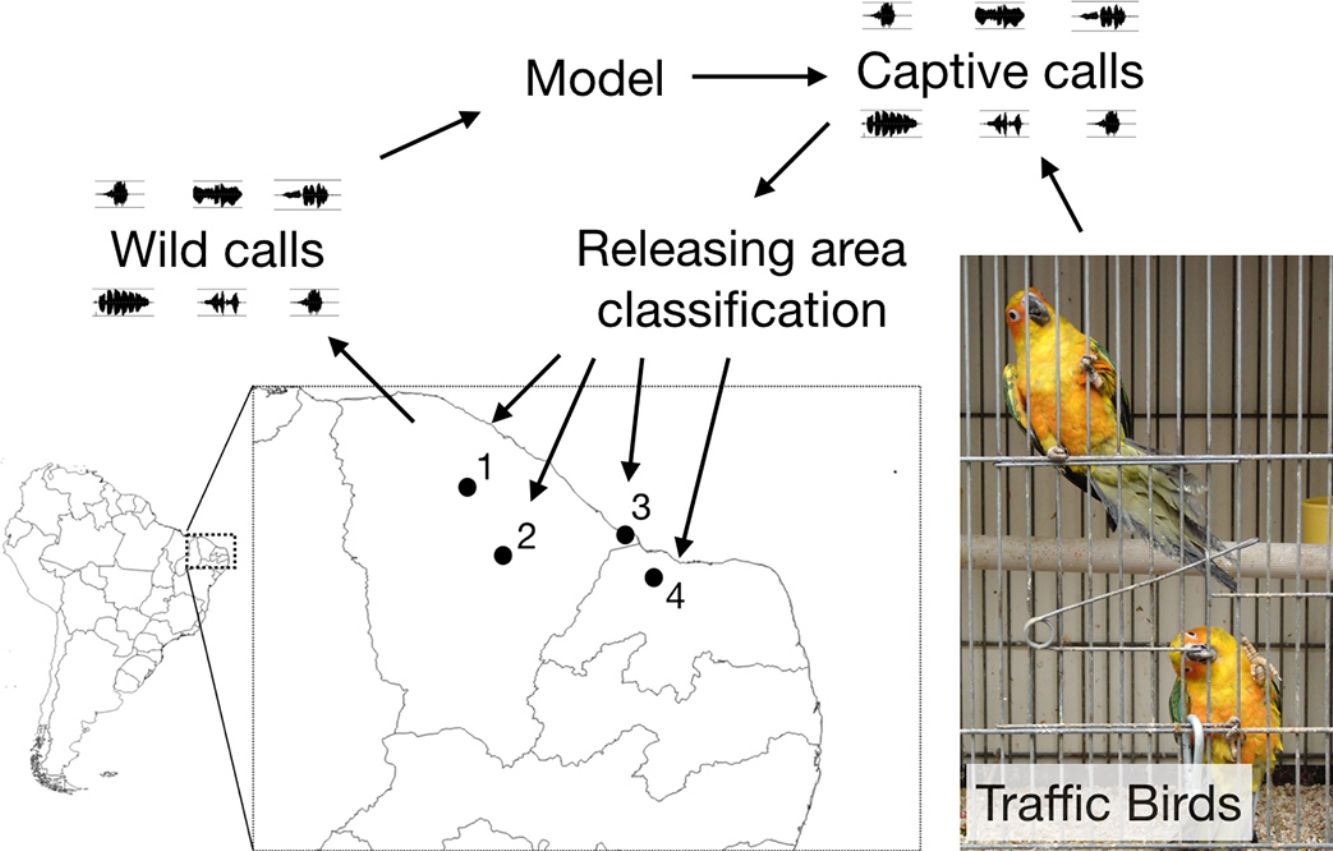
Parrot species are a common target of animal trafficking, and the animals recovered from anti-trafficking operations are generally reintroduced into nature. However, these reintroductions usually fail to consider geographical vocal differences that are known to be present in some Parrot species. We investigated patterns of geographical variations in Eupsittula cactorum vocalizations and used those data to infer the geographical origins of recovered birds and thus predict the most appropriate reintroduction sites. We recorded four wild populations in northeastern Brazil (between the western region of Rio Grande do Norte State and northeastern Ceará State), and three groups of captive individuals seized from traffickers. We considered seven acoustic parameters to classify the flight calls of the different native populations and used a multinomial model to classify the recovered animals according to the native populations sampled. Our results indicated the existence of geographical dialects. Individual birds had been released in Quixadá, where local calls are acoustically distinct. Acoustical parameters can provide important clues about the origins of captured individuals as well as reduce acoustical contrasts between released individuals and native populations. The application of this methodology could potentially improve the efficacy of reintroduction efforts, by reducing vocal distances between released individuals and the native population.
Vocal learning may be defined as the ability to imitate, with or without modification, the vocalizations of conspecifics (or even other species) (Bradbury and Balsby, 2016). Diverse taxa are capable of vocal learning, ranging from birds to mammals (Sewall et al., 2016). In birds, learned calls seem to be restricted to three groups: songbirds, parrots, and hummingbirds (Jarvis et al., 2000). Among the many advantages of learned calls is the possibility of coding individual (Berg et al., 2011) and acquisition of information on populations (Salinas-Melgoza and Wright, 2012; Wright, 1996), as well as vocal labeling (Wanker et al., 2005) – which could allow a bird to address a single individual within a flock (which could have special importance among social birds such as parrots). The learning process is hypothesized to often lead to rapid repertoire changes through cultural evolution (Lachlan and Servedio, 2004) and, although subject to morphological restrictions (Medina-García et al., 2015), learning processes are widely believed to lead to labile acoustical call structures (Vielliard and Silva, 2010).
The vocal abilities of parrots have long captivated the human imagination. In the famous Robinson Crusoe novel (Defoe, 1719), the main character had long “conversations” with a parrot. In fact, the ability to imitate human voices is one of the features that make parrots very desirable as pets (de Araújo, 2011), making parrots’ natural populations susceptible to the pet trade. Habitat destruction and animal trafficking are the main factors threatening parrot conservation throughout the world (Berkunsky et al., 2017; Collar, 2000), and many wild South American parrot species are still being harvested from nature because of the pet trade (Daut et al., 2015; Fernandes-Ferreira et al., 2012; Nobrega Alves et al., 2013).
Many countries, including Brazil, have prohibited the commercialization of wild birds, and have curbed that practice by launching large-scale operations to arrest dealers and recover wild specimens – as exemplified by the efforts of IBAMA (the Brazilian Institute of Environmental and Renewable Natural Resources) in Brazil. Nevertheless, the practice of keeping wild birds represents a deeply-rooted cultural tradition, and the over-harvesting of specimens can cause severe populational reductions (Fernandes-Ferreira et al., 2012). Even thought government operations appear to be helping to change cultural habits and reduce bird harvesting, they produce another serious short-term problem: what should be done with the birds seized from traffickers? Currently, birds are usually sent to Wild Animal Screening Centers (CETAS) and eventually released back into the wild. Because of the difficulty in determining the geographical origins of captured birds (i.e. traffickers who are caught are rarely willing to point out the geographical locations of their harvesting sites or accomplices) individuals are usually released outside their natural ranges. This type of reintroduction can create serious threats to natural populations (Marini and Garcia, 2005), including the risk of establishing a new invasive species, the transmission of diseases and parasites, and the hybridization of otherwise genetically distinct races or subspecies (IUCN, 2000).
Considering this complex background, researchers have been attempting to develop novel methodologies that could determine the origins of recovered birds. Molecular analyses coupled with phylogeographic approaches have shown promising results, but assigning animals to exact geographic locations using molecular data still presents various difficulties. Large portions of the distributions of many species, for example, have not yet been sampled, making it difficult to identify the exact geographic origins of confiscated individuals – and the inferred origins of those confiscated birds could easily change if additional data were incorporated into the data set (Presti et al., 2015). Additionally, as mitochondrial DNA provides no information about the adaptive natures of genetic similarities, it could have little or no direct connection to variations in traits relevant to local adaptations, or to genetic incompatibilities between populations (Fernandes and Caparroz, 2013). Finally, molecular analyses require an invasive procedure that takes a considerable time to be achieved, and although those procedures could provide important information about the genetics of avian taxa, they are not feasible at large scales and with the necessary speed at this time – even though the information gathered would be especially valuable in terms of endangered populations.
Magroski et al. (2017) employed Ecological Niche Models (ENM) to estimate continental distributions of species in isolated patches, using specimen calls to test those ENM, and assign the specimens to appropriate patches. Despite some successes with classifying species with innate calls, the methodology demonstrated serious shortfalls when considering species capable of vocal learning (such as parrots). Wide geographic variations among those species prevented the use of call parameters to infer their broad-scale geographical origins. Those authors observed that populations of species such as the Red-shouldered macaw (Diopsittaca nobilis) that were separated by just a few kilometers could show considerable vocal differences, so that even populations within a given patch (but separated by hundreds of kilometers) would encompass large portions of the achievable vocal variations and mask inter-patch variations (Magroski et al., 2017). The presence of geographic dialects can difficult the reintroduction of released individuals in nature, for the ability of a bird to integrate (i.e. mate) local flocks seems to depend on their ability to acquire the unfamiliar local dialect (Wright and Dahlin, 2017).
Magroski et al. (2017) envisioned a continental analysis, and proposed that intra-patch variations prevented vocal classifications among large-scale patches. In this study, we focus on the existence of small-scale vocal variations and test if these can be used to determine the most suitable place for the release of captured E. cactorum, either by determining the original natural range of the individuals, but also by determining the locality with the smallest vocal divergence between the released individuals and the natural population. As geographical dialects might hinder reintroductions reintroduction (Wright and Dahlin, 2017), the reduction of the acoustical differences among reintroduced and natural populations could help guarantee the efficiency of the process.
Materials and methodsTarget species and samplingThe Cactus Conure (Eupsittula cactorum) is a small (25cm long) psittacid that is very common in the dry northeastern forests of South America. Its distribution encompasses the Caatinga dryland vegetation in the northernmost regions of the states of Piaui, Ceará, and Rio Grande do Norte, throughout Paraíba, Pernambuco, to Bahia, and reaching central Minas Gerais (Forshaw, 2010; Sick, 1997). It forms flocks, as do most psittacid species, which shape their social relationships through complex acoustical communication systems (Guerra et al., 2008). The species is commonly raised as pets in Northeastern Brazil (Alves et al., 2016) and is known to be sold in many localities (Nobrega Alves et al., 2013). In fact, psittacids are among the most confiscated species within Brazil (Destro et al., 2012) and illegal traffic is one of the major problems for its conservation (Berkunsky et al., 2017). Understanding the problems associated with wildlife traffic is crucial if we are to mitigate its negative effects.
Our data consisted of two distinct sets of sound recordings: the first set comprised four wild populations recorded directly in the field; the second set consisted of captive individuals recorded at the Wild Animals Screening Center (CETAS) facility in Fortaleza (CE).
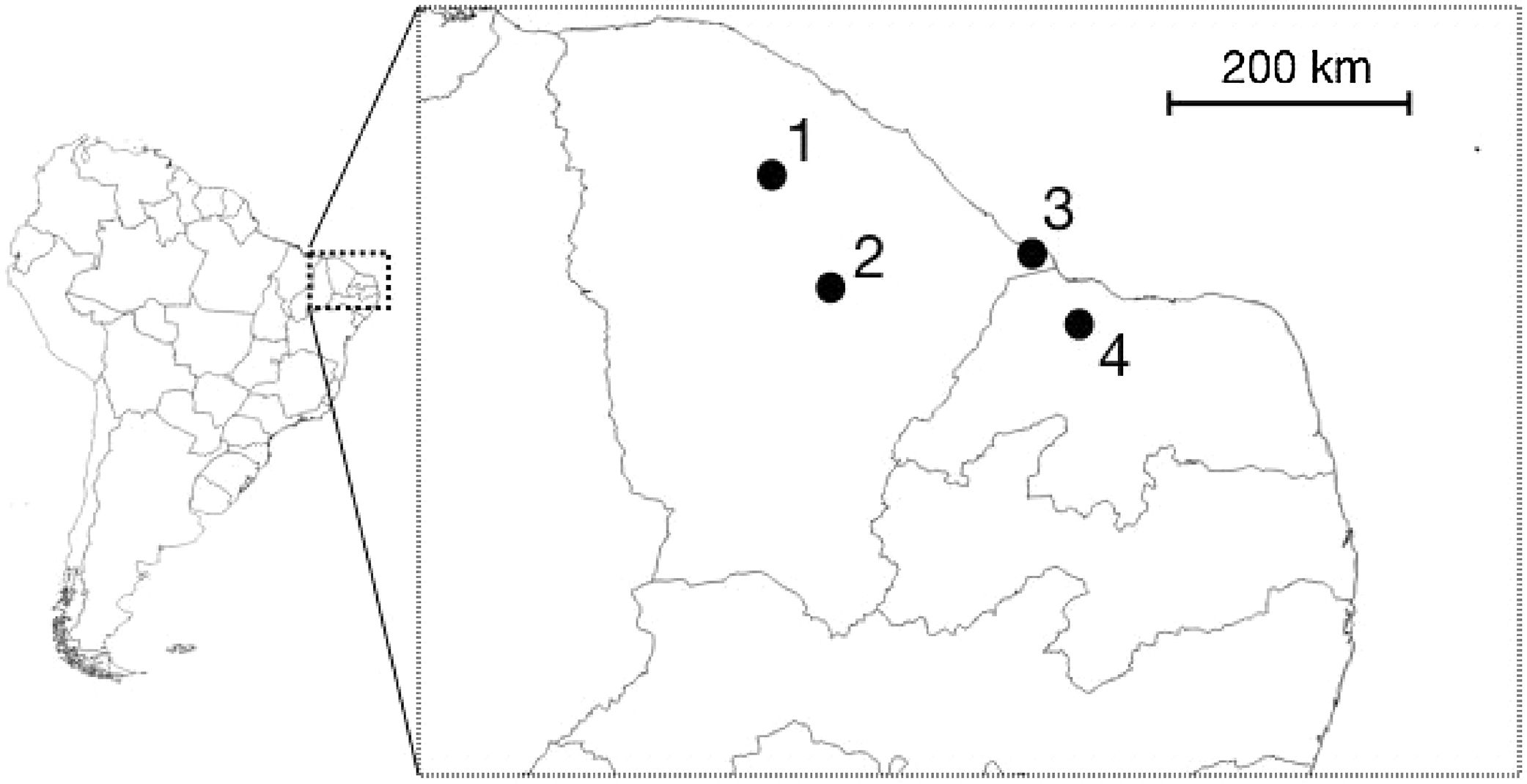
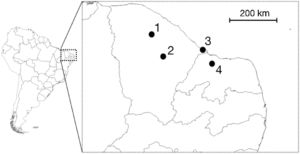
We undertook expeditions to make sound recordings of wild individuals in the field from September/2015 to August/2016. Four distinct populations of Cactus Conure in the northernmost portion of their distribution range were considered, located at: Paramoti (CE); Quixadá (CE); Icapuí (CE); and Serra do Mel (RN) (Fig. 1). The recordings were made between 05:00–08:00 and 15:00–18:00, times when psittacids are most vocally active. All recordings were made using a Tascam-DR05 digital sound recorder coupled to a Shure Beta58a microphone mounted on a 50cm diameter parabola with a 19cm focus (Fig. 2A). All recordings were built with a 48kHz sampling rate and 24-bit resolution.
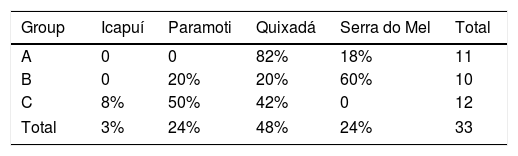
During the sampling period, IBAMA (CETAS) informed us of new arrived groups of recovered Cactus Conures. The specimens were kept together in a large cage, and no information on their geographical origins was available. Captured specimens were placed in small individual cages and recorded using a Tascam-DR05 digital sound recorder coupled to a Shure Beta58a microphone. We kept individuals in visual contact with other captive birds so that they would be stimulated to call; a close-range recording setup was used to reduce background noise (Fig. 2B). We were able to record three different groups of individuals, referred to here as A (11 individuals), B (10 individuals), and C (12 individuals). Following the experiments, the specimens were released at Quixadá, where IBAMA maintains one of its Wild Animal Release Area bases (ASAS).
Sound analysisMost parrot species exhibit vast vocal repertoires (e.g., de Araújo et al., 2011; de Moura et al., 2011; Fernandez-Juricic et al., 1998), with the flight (contact) call being the most studied vocal component in psittacids (Magroski et al., 2017; Medina-García et al., 2015; Salinas-Melgoza and Wright, 2012; Wright, 1996; Wright et al., 2008). In order to avoid pseudoreplication, only a single flight call of each Cactus Conure was selected from each sound recording (McGregor et al., 1992), based on its signal-to-noise ratio and overlap with acoustical artifacts. All selected calls were run through a high pass filter (300Hz) and normalized to 0dB preceding the analyses. This procedure was necessary to reduce problems arising from comparisons of recordings made at different amplitudes that could reduce the precision of acoustic comparisons (Zollinger et al., 2012).
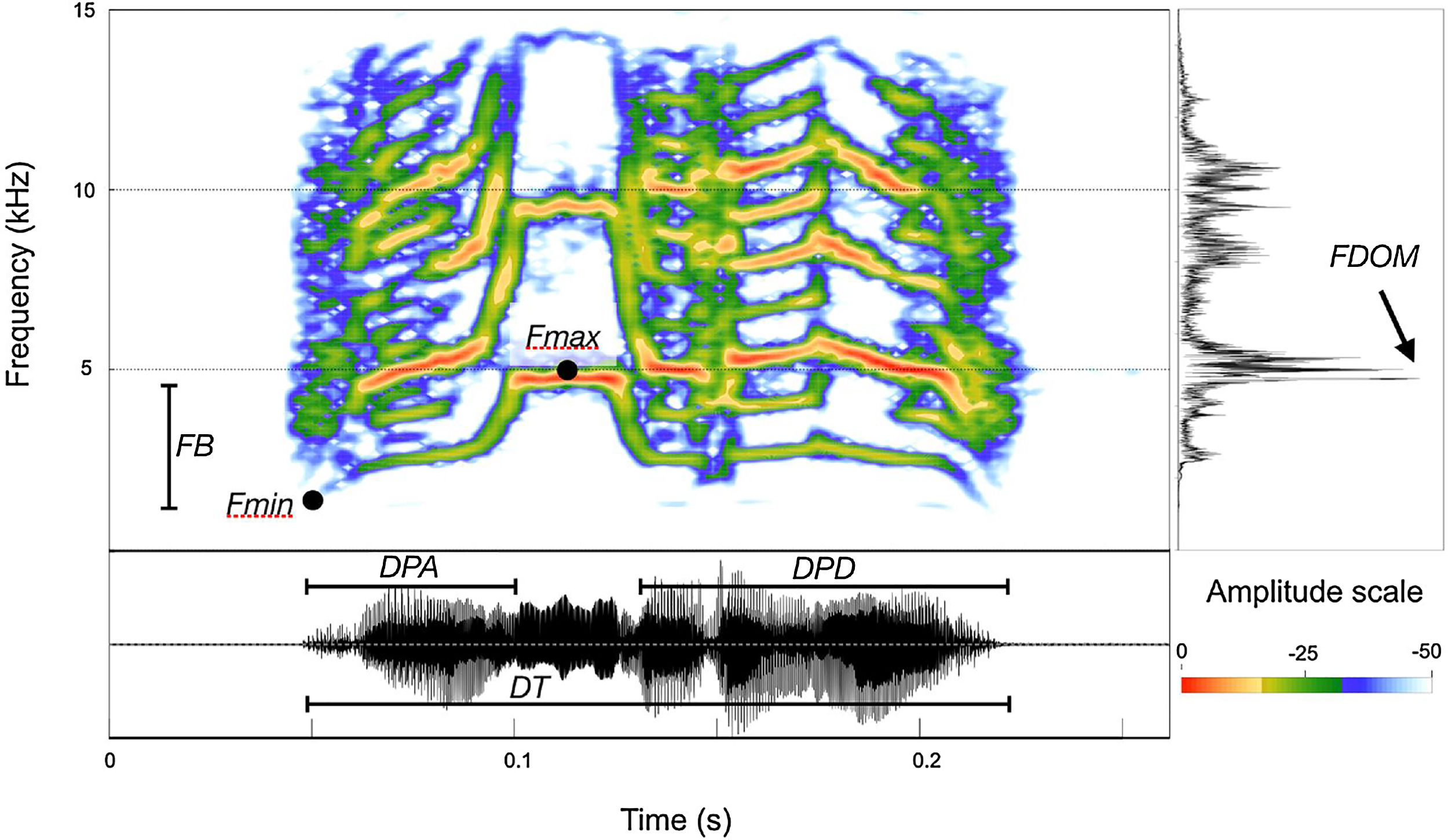
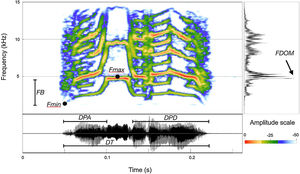
Selecting which acoustical parameters to be measured is not a simple process, and depends upon the physical structures of the signals to be analyzed. Therefore, before we undertook actual measurements, a small dataset sample was examined to ensure the proper selection of homologous parameters that could be measured throughout the entire dataset. The homologous call structures within the dataset allowed us to divide the call into three portions, from which we selected seven acoustic parameters (Fig. 3): Total duration – DT; duration of the ascending portion – DPA; duration of the descending portion – DPD; dominant frequency – FDOM; maximum fundamental frequency – FMax; minimum fundamental frequency – FMin; frequency band (FMax−FMin). All measurements and spectrograms were built using SoundRuler 0.9.6 software (Gridi-Papp, 2007) with a FFT size of 512 points and 0.40 contrast.
Measurement map depicting the acoustical parameters measured for the calls of Cactus Conure (Eupsittula cactorum). Total duration – DT; duration of the ascending portion – DPA; duration of the descending portion – DPD; dominant frequency – FDOM; maximum fundamental frequency – FMax; minimum fundamental frequency; FB – frequency band.
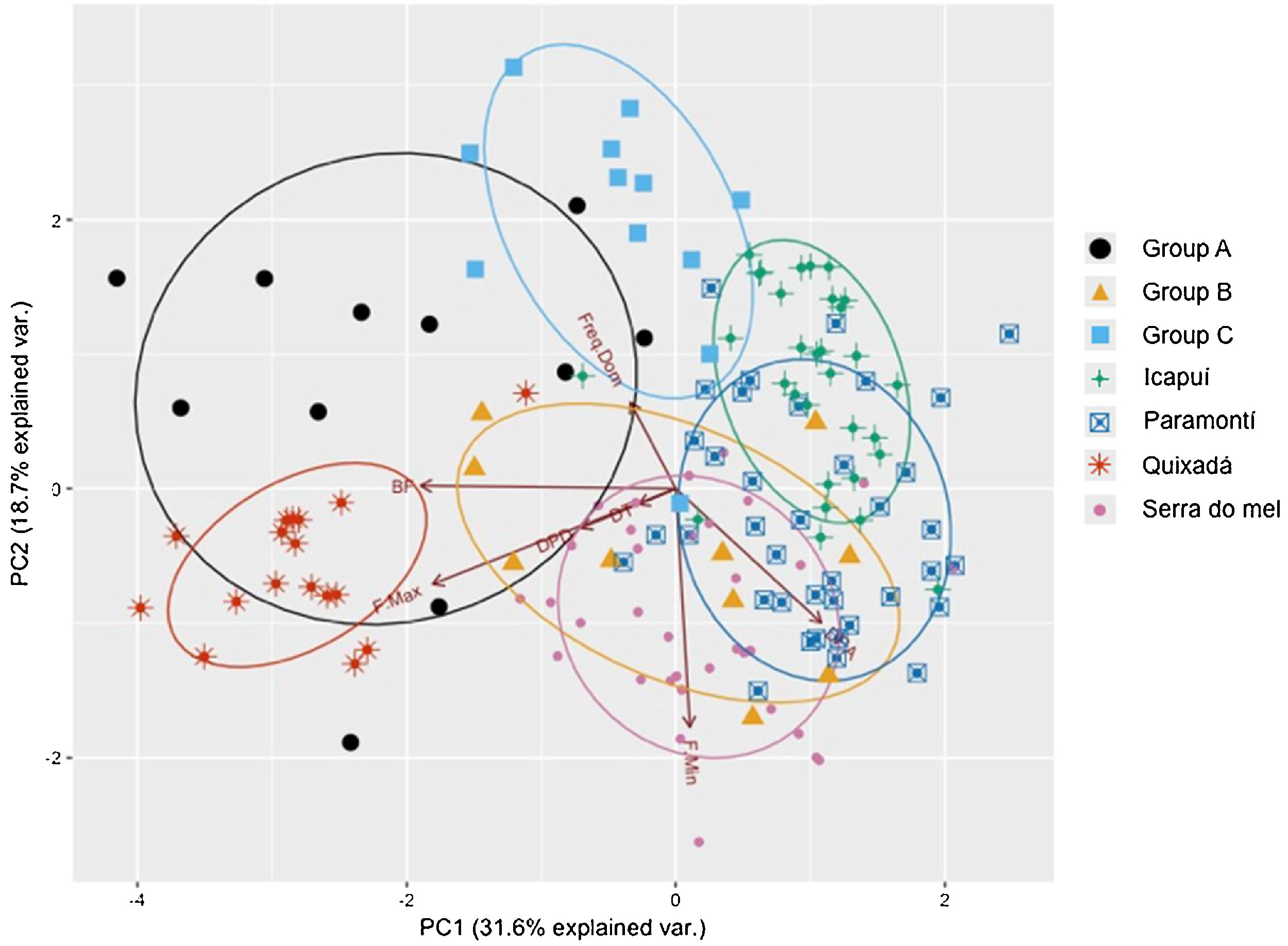
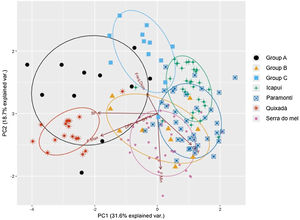
In order to visually identify any geographic variations among the four populations of Cactus Conure, a PCA was built using acoustic parameters, which we considered a proxy of the acoustical space. To avoid problems arising from different acoustic parameter scales, the PCA was built using a correlation matrix. Since the acoustic space is defined as the set of parameters within the call, the existence of geographical dialects would imply the segregation of the acoustic space used by each of the populations analyzed. Additionally, the relative positions of the acoustical spaces of each of the captive group (A, B, C) in relation to native populations could provide clues about the best places for releasing them. Any acoustic spatial segregation, on the other hand, would indicate that captive individuals had calls distinct from those of sampled natural populations, even though the classification would still indicate the best releasing areas due to reduced vocal differences.
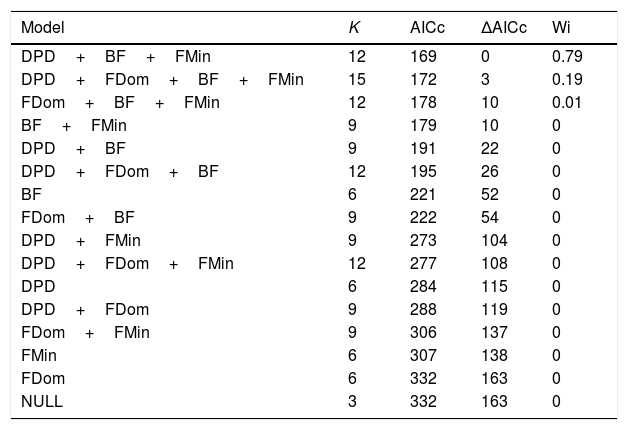
We then built a multinomial log-linear model via neural networks based on the parameters of the four natural populations to classify CETAS captive groups in relation to natural populations. As collinear variables could make it more difficult to detect any effects and reduce the efficiency of multinomial classifications, prior to the analysis we removed variables with high collinearity by calculating the Variance Inflation Factor (VIF) within a full multinomial model, and dropping the covariate with the highest VIF. We re-ran the analysis and recalculated VIF values until all VIFs values were smaller than 2 (Zuur et al., 2010), so that all variables included in the model presented low collinearity. The non-collinear parameters list is represented in the full model of Table 1.
The multinomial regression model selections of flight call localities in relation to call parameters.
| Model | K | AICc | ΔAICc | Wi |
|---|---|---|---|---|
| DPD+BF+FMin | 12 | 169 | 0 | 0.79 |
| DPD+FDom+BF+FMin | 15 | 172 | 3 | 0.19 |
| FDom+BF+FMin | 12 | 178 | 10 | 0.01 |
| BF+FMin | 9 | 179 | 10 | 0 |
| DPD+BF | 9 | 191 | 22 | 0 |
| DPD+FDom+BF | 12 | 195 | 26 | 0 |
| BF | 6 | 221 | 52 | 0 |
| FDom+BF | 9 | 222 | 54 | 0 |
| DPD+FMin | 9 | 273 | 104 | 0 |
| DPD+FDom+FMin | 12 | 277 | 108 | 0 |
| DPD | 6 | 284 | 115 | 0 |
| DPD+FDom | 9 | 288 | 119 | 0 |
| FDom+FMin | 9 | 306 | 137 | 0 |
| FMin | 6 | 307 | 138 | 0 |
| FDom | 6 | 332 | 163 | 0 |
| NULL | 3 | 332 | 163 | 0 |
K, number of estimated parameters; AICc, second-order Akaike's information criteria; ΔAICc, variation of AICc in relation to the best model; Wi, Akaike's weight; NULL, null model; Vocal parameters: duration of the descending portion – DPD; frequency band – BF; dominant frequency – FD; maximum fundamental frequency – FMax; minimum fundamental frequency – Fmin.
We built several multinomial models, accounting for all possible combination of parameters. We selected the models based on the second order Akaike criterion (AICc), by comparing the models to a null model, and selecting those with the lowest ΔAICc. As ΔAICc values lower than 4 indicate support for multiple models (Burnham et al., 2011), we tested the classification efficiency of models with ΔAICc lower than 4. The multinomial models were built using R software (R Core Team, 2015) by implementing the nnet package (Ripley and Venables, 2011); AICc comparison tables were through the implementation of the AICcmodavg package (Mazerolle, 2011).
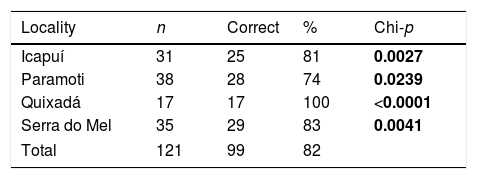
We first used the best model to predict the original population of the call, and if there was a high degree of call variation regionally, as hypothesized by Magroski and collaborators (2017), we expected call differences to be enough for their proper classifications among the studied populations. In order to examine if the model classification efficiency was better than expected by chance, we built a confusion matrix that expressed the percentages of correct classifications of the calls among patches, and compared them to the correct classification expected by chance through a chi-squared test. Hence, even though we used the second order Akaike criterion (AICc) for model selection, the chi-square test was used to evaluate the efficiency of model classification.
Finally, we used the best multinomial model to classify the vocalizations of captive individuals (A, B, C) among the sampled populations. The classification of captive groups among natural populations would indicate the best possible locations for their release regardless of their geographical origins, for the vocalizations of the released individuals would be the closest (among a limited set of possibilities) to the native vocalizations.
ResultsThe first two axis of the principal component analysis explained 50.3% of the variation in the acoustical parameters. While some overlap was observed between the Icapuí, Serra do Mel, and Paramonti populations, the Quixadá population showed a very distinct call structure, with considerable segregation from other populations within the acoustical space. Paramonti calls fell between those of Icapuí and Serra do Mel, while the latter two populations appeared to be segregated within the acoustical space. Overall, the PCA supported the idea of high call parameter variability and the presence of vocal dialects within close populations such as Quixadá and Paramonti (∼100km) or Icapuí and Serra do Mel (∼70km).
Variance Inflation Factor (VIF) analysis (Zuur et al., 2010) led to the removal of three collinear variables from the model: the maximum fundamental frequency (FMax), total duration (DT), and the duration of the ascending portion (DPA). Two models obtained a ΔAICc value higher than 4 (Table 1), indicating that both had empirical support. We therefore tested the classification efficiencies of those models based on the percentage of correct call classifications within their original populations. While the first model had a high Akaike weight (Wi), which suggests greater empirical support, the second model was found to be more efficient at classifying the calls. We therefore opted to use the second best model for the analysis, as follows: Locality∼DPD+FD+BF+FMin (Table 1).
The multinomial analyses showed different efficiencies in classifying calls of the populations examined. The calls of Quixadá had a perfect classification of 100%, indicating its distinctiveness. Other localities, such as Paramonti, showed lower classification values of 74%. Overall, the multinomial model had a correct classification of 82%, and the calls of all of the populations studied were classified better than expected by chance (Table 2). Our results demonstrate clear geographical differences in vocal repertoires supporting the predictions of Magroski et al. (2017).
Confusion matrix showing multinomial analyses of call classification efficiencies.
| Locality | n | Correct | % | Chi-p |
|---|---|---|---|---|
| Icapuí | 31 | 25 | 81 | 0.0027 |
| Paramoti | 38 | 28 | 74 | 0.0239 |
| Quixadá | 17 | 17 | 100 | <0.0001 |
| Serra do Mel | 35 | 29 | 83 | 0.0041 |
| Total | 121 | 99 | 82 | |
n, sample size; Correct, the number of correct classifications; %, the percentage of correct classifications; Chi-p, p value of the Chi-square that determines if the classification efficiency is higher than expected by chance. Bold values represent significant differences.
The classification of the calls of captive individuals (Table 3) reinforced the results graphically presented in the PCA (Fig. 4), and indicated that individuals released in Quixadá were released outside their region of origin. Nevertheless, given different possible locations for releasing those individuals, Quixadá was actually the most appropriate locality – for in 48% of the cases it showed the smallest vocal difference. Group A demonstrated a vocal structure closer to the dialect of the Quixadá population, and 82% of the individuals were classified for that region, while only 18% were classified for Serra do Mel. Group B, on the other hand, used a portion of the acoustic space closer to the Serra do Mel dialect (60%) while the remaining individuals could be divided between the Paramoti (20%) and Quixadá (20%). Nearly half the individuals of Group C were classified for Quixadá (42%) or Paramonti (50%), but only a small portion for Icapuí (8%; Table 3). Overall, 48% of the captive birds were classified for Quixadá, followed by 24% for Paramonti and Serra do Mel, with only 3% for Icapuí.
Our data indicated the presence of geographic call variations at regional scales at distances less than 100km. Similar results can be found in the literature, where geographical call variations where reported at similar ranges (Bond and Diamond, 2005; Bradbury et al., 2001; Kleeman and Gilardi, 2005; Reynolds et al., 2010; Wright, 1996). Efficient communication is essential for translocation, reintroduction, and release success (Wright and Dahlin, 2017), as individuals within larger social organizations have greater chances of surviving stress situations (Guerra et al., 2008). In Parrots, good flock social organization is directly reflected in the efficiency of foraging, resource acquisition, migratory movements, and anti-predatory behavior (Brightsmith et al., 2005). In that sense, geographic dislocations can negatively influence reintroduction efficiencies as vocal differences can hinder social interactions and preclude proper flock organization, thus reducing the success of reintroduction protocols (Bradbury et al., 2001; Wright and Dahlin, 2017).
Ideally, reintroductions should be made within the original locality of the specimen. However, determining the exact origins of individuals from animal trafficking apprehensions is almost impossible because they typically occur along trade routes, far from the region where the individuals were captured and little information is retrieved from arrested traffickers. Even though efforts have been recently made to determine the geographical origins of individuals through the use of bioacoustics (Magroski et al., 2017), it failed to do so within species with learned calls, such as Parrots. We also failed. The success of the method presented here to determine the geographical origins of recovered specimens would depend on a herculean sampling of the species geographical variation – samples from every single dormitory of the species along its distribution – what would be virtually impossible. Only then we would have the necessary information to determine, with reasonable certainty, the geographical origins of the specimens. In fact, this was not our main objective here.
Under such a scenario of methodological shortfall, immediate actions that could reduce the negative effects of translocations would be very welcome, especially until a permanent solution is available. Our results allowed for the definition of areas with similar vocal dialects, making it possible to reduce overall vocal differences between reintroduced birds and native populations. Group A, for example, in which 82% of the calls were classified for Quixadá, had vocalizations structurally similar to the wild birds at that locality (even thought calls seems more similar to the calls of B and C). Releasing them at Quixadá and not Paramonti should therefore increase their chances of successful adaptation, reducing the potential negative effects of vocal differences. On the other hand, only a few individuals of Group B were classified for Quixadá, so that releasing those individuals in that area would not be the best option. In the case of Psittaciformes, which are known to show great dependence on vocal communication in shaping social and cultural ties, considerations of geographical call variations is extremely important (Guerra et al., 2008), and reducing the vocal differences should improve the efficiency of the releasing process (Wright and Dahlin, 2017).
Everyday realities at CETAS installations scattered throughout Brazil oblige them to release many groups of birds with distinct repertoires in a single locality. Even though the present logistical limitations of CETAS makes such an approach unavoidable, it is important to note that 52% of the released individuals were classified for areas other than Quixadá. By using the methodology presented here it would have been possible to classify those individuals prior to their release and to choose more suitable locations based on the geographical variations of their calls.
We have shown that the Cactus Conure possesses geographical variations on their calls (dialects) throughout its distribution range. The high degree of variation observed between calls ∼70km apart precludes them for large-scale determinations of the geographical origins of captured specimens, but comparing the vocalizations of individuals to be released with vocalizations of possible releasing areas could reduce vocal differences and improve the overall success of returning them to nature. We still lack data, however, about how vocal differences can interfere with socialization processes and affect post-release success. It is known that parrots are open-ended learners, capable of learning throughout their lives (Salinas-Melgoza and Wright, 2012), so that the long-term consequences of releasing specimens with distinct vocal repertoires are still unclear.
We thank the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA-CE) for their permission and kind attention while this work was being carried out in the CETAS (Wild Animals Screening Center) at Fortaleza. We would like to thank the two anonymous reviewers for their suggestions and comments. We are also grateful to Lucas Barros for providing sound recordings of wild individuals of Areia Branca, and Flávio Torres for allowing access to his farm at Quixadá. Bruno Martins and Giovanna Rodrigues were supported by CAPES Masters Fellowships, while Carlos B. de Araújo was supported by a CAPES-PNPD Post Doc Fellowship.