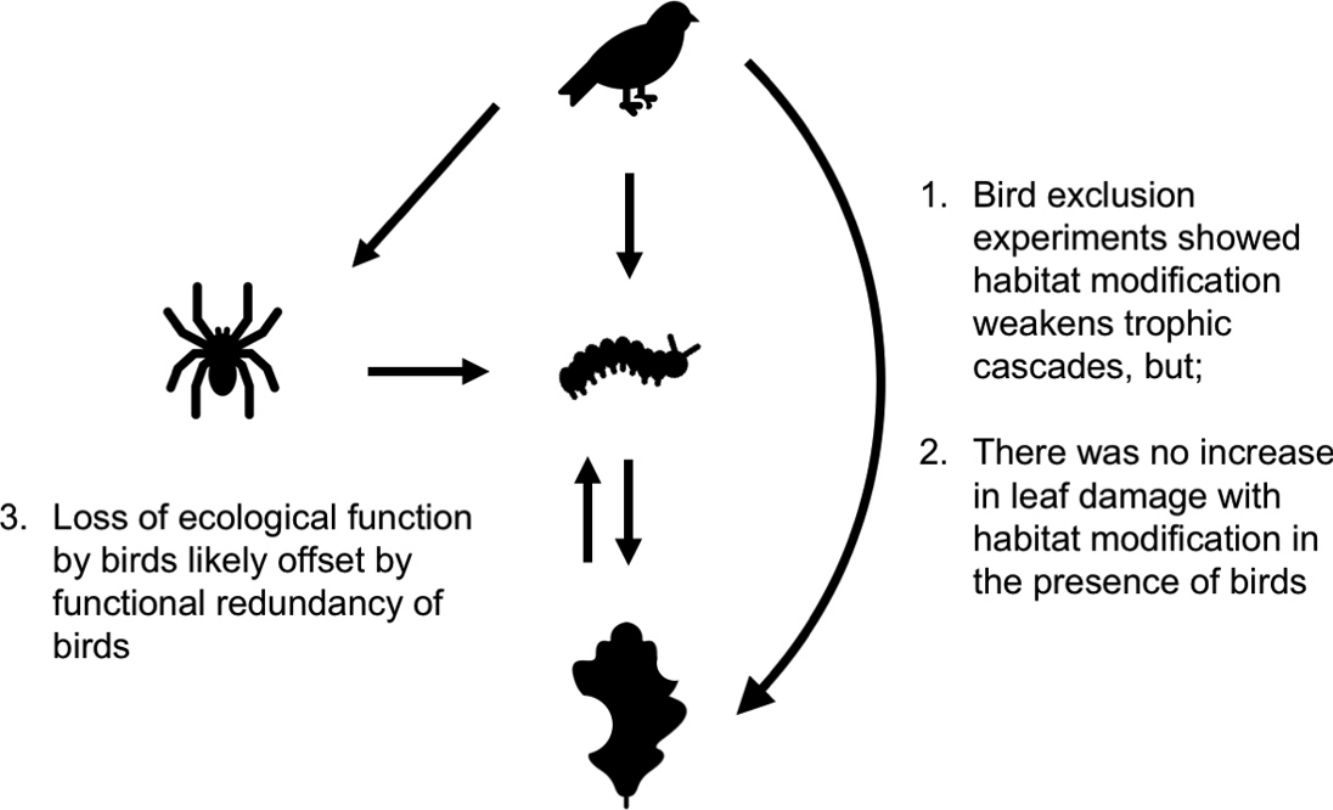
Habitat modification is now a widespread phenomenon, impacting landscape structure, biophysical processes, food webs and biodiversity. These changes have trickle-down effects on trophic cascades: predators often become rarer, increasing prey populations, which then subject plants to higher levels of herbivory. How habitat modification mediates this trophic cascade, however, is poorly understood, and this is particularly true for temperate forests. Here we investigate if the strength of trophic cascades, defined as the magnitude of the effect of bird exclusion on leaf damage, varies along a gradient of increasing habitat modification, from forest interior to forest edge to open habitat, through an experimental manipulation of bird exclusion. We found that habitat modification reduces the number of bird observations, with trophic cascades being three times stronger in the forest interior than edge and open habitats. However, there is no corresponding increase in leaf damage with habitat modification in the presence of birds, suggesting that other taxa or factors may mediate leaf damage in modified habitats. Our findings suggest that even though habitat modification disrupts the functions that birds perform in the ecosystem, overall ecosystem function is not dramatically altered, possibly due to the functional redundancy of birds.
Vast masses of natural landscape have now been converted to human use, transforming the earth's land surface (Foley et al., 2005). Increasingly, forested areas are becoming smaller and more fragmented (Haddad et al., 2015), exposing them to threats such as microclimatic changes and invasion of non-forest species (Broadbent et al., 2008). Consequently, modified landscapes exhibit not only altered landscape structure: biophysical processes, food webs and biodiversity are impacted too (Tylianakis et al., 2007; Broadbent et al., 2008).
Habitat modification has important consequences for the functional integrity of ecosystems (De Coster et al., 2015), and one ecological function often disrupted in modified habitats is arthropod predation by insectivorous birds (Sekercioğlu, 2006). Without top-down control of arthropod populations, plants are subject to defoliation by predator-mediated release of leaf-chewing arthropods (Holmes et al., 1979). This defoliation can cause a reduction in plant biomass and even plant mortality (Mäntylä et al., 2011). Besides the impact on individual host plants, arthropod defoliation can cause larger scale impacts such as a reduction in net ecosystem productivity (Medvigy et al., 2012). The loss of top-down predators can thus negatively affect plants via the increase in herbivore populations (Mäntylä et al., 2011).
Trophic cascades are the effects of predators/prey on the abundance, biomass or productivity of a trophic level more than one trophic link away (Pace et al., 1999). A meta-analysis of studies on trophic cascades by avian predators of herbivorous invertebrates found that in general, plants performed better in the presence of birds, where measured plant responses such as leaf damage were 1.44 times higher when birds were absent (Mäntylä et al., 2011). Trophic cascades are also dependent on strong interactions between trophic levels (Pace et al., 1999). Strong seasonality in temperate regions limits the period of optimal conditions for growth, driving a strong synchrony between host plants, herbivorous invertebrates and birds (van Asch and Visser, 2007). During the onset of spring in temperate zones, eggs of more than 100 species of moths, laid in the winter, hatch as caterpillars to feed on newly emerged oak leaves (van Asch and Visser, 2007; Tyler, 2008). These caterpillars meet the sizeable demand for food by many nesting passerine birds, whose arrival from migration and breeding is timed to coincide with the peak in caterpillar population (Both et al., 2006; Møller et al., 2008; Burger et al., 2012). Consequently, without vertebrate predators, the intensity of arthropod herbivory on oak trees can increase substantially (Marquis and Whelan, 1994; Böhm et al., 2011).
Although it is well-known that the exclusion of birds negatively impacts plants (Mäntylä et al., 2011), how habitat modification mediates this trophic cascade is still poorly understood, especially in temperate regions. Previous studies on the multitrophic relationship between birds, arthropods and plants in the tropics found that loss of forest cover and forest fragmentation were associated with a decrease in insectivorous bird abundance and an increase in herbivory (Karp et al., 2013; Peter et al., 2015; Morante-Filho et al., 2016). The strength of trophic cascades, a measure of the degree to which effects of changes in one trophic level are passed on through the food chain, is highly variable among ecosystems (Heath et al., 2014), revealing insights into the effects of removing top-down predators on an ecosystem and how its stability is affected by human disturbance (Pace et al., 1999).
In this study, we investigate if the strength of bird-arthropod-plant trophic cascades on oak trees varies along a gradient of increasing habitat modification, from forest interior to forest edge to open habitat, through experimental bird exclusion. We hypothesise that numbers of birds will decrease with increasing habitat modification, resulting in an increase in herbivory. Therefore, we predict that pairwise differences in arthropod abundance and leaf damage between bird exclusion and control will decrease from forest interior to forest edge to open habitat. We further hypothesise that the strength of trophic cascades, which we define here as the magnitude of the effect of bird exclusion on leaf damage, will weaken with increasing habitat modification, as the effect of bird exclusion is likely to be attenuated down the food chain with lowered bird numbers.
Materials and methodsStudy siteWe carried out this study at Silwood Park, Berkshire, United Kingdom from April to June 2019, in the spring when many nesting passerine birds depend on arthropod larvae for food (Burger et al., 2012). Silwood Park is made up of 100ha of diverse habitats, predominantly comprising young, naturally regenerated oak woodland, with fragments of ancient woodland (Crawley, 2005). Mean temperature during the study period was 12.2°C, with an average daily rainfall of 2.3mm/day (Silwood Park Weather Station, 2019).
Using a database of more than 3500 uniquely tagged oak trees in Silwood Park, we overlaid locations of Quercus robur trees with a map of canopy cover in QGIS (QGIS Development Team, 2019). We calculated the area of each patch of trees with a continuous canopy cover and classified trees located in patches with areas smaller than 20,000m2 as belonging to open habitat, based on limitations in site size to ensure sufficient representation of trees in each habitat type. For trees located in patches larger than 20,000m2, we calculated the distance of each tree to the nearest edge. Subsequently, we classified all trees less than 10m away from the nearest edge as belonging to the forest edge and those more than 45m away as forest interior. Using a random number generator, we selected 15 trees in each of the three habitat types. We ensured that each tree was located at least 50m away from each other to ensure spatial independence, consistent with previous results on the territoriality of breeding great tits (Krebs, 1971).
Experimental setupWe constructed bird exclusion cages using plastic garden fencing with 0.02m mesh size – small enough to keep out birds and other vertebrates, while allowing free passage for most arthropods. Each cage was cylindrical with a length of 1m and an approximate diameter of 0.3m, with slight size variations to fit different branch morphologies. At each tree, we selected two adjacent branches between 0.5 and 2m above ground with similar numbers of buds. We randomly assigned one branch as a bird exclusion branch and marked the other as a control. We fitted each exclusion branch with a cage, suspended using jute twine such that leaf growth was not restricted as far as possible. We set the cages up right after bud burst, before leaf extension (see Crawley and Akhteruzzaman (1988) for descriptions of phenological stages), so that all effects on leaf damage observed in this study would have occurred during the study period. Because of variation in bud burst date for each tree, we determined the start dates of the experiment according to individual phenology, but left each cage up for a total period of eight weeks per tree.
Data collectionBird surveysWe conducted point counts of birds between 0700 and 1000h, during the peak period of bird activity, on days of fair weather. At each tree, we counted all birds within a 20m radius of the base of the tree for 20min, based either on visual identification where possible or bird calls when views of the birds were obscured by foliage. Where bird identification was uncertain, we recorded bird calls and identified them after via playback. We did not record birds of the same species within five minutes of the first observation unless we had observed or heard them calling concurrently. We repeated point counts at each tree once every two weeks, for a total of four point counts per tree.
Arthropod surveysTo obtain a measure of arthropod abundance, we conducted standardised beatings on each branch at the end of the experiment. Using a metre-long wooden beating stick, we beat each branch 15 times while a 0.9m×0.7m white tray was held directly beneath the branch. We emptied the tray into a resealable bag and brought the arthropods back to the laboratory for identification. We identified most arthropods to order and Coleoptera to family due to the diverse feeding strategies of this order, and separated adults from larval stages as these tend to have different life histories (Tyler, 2008). Following descriptions of invertebrates on oaks in Tyler (2008), we assigned each taxon to one of five feeding guilds – leaf-chewing, sap-sucking, predatory, fungivorous/detritivorous or omnivorous.
Leaf damage assessmentTo assess leaf damage, we randomly collected 20 leaves on each branch by collecting every fifth leaf starting from the branch tip. We also counted the total number of leaves on the branch to estimate total leaf area as this could confound the number of arthropods collected. Using LeafByte, a mobile application for measuring herbivory (Campbell and Getman-Pickering, 2018), we obtained measures of total leaf area, damaged leaf area and percent leaf damage for each leaf. We calculated total leaf area per branch as the average leaf area of 20 sampled leaves multiplied by the total number of leaves on the branch.
Data analysisTo test our prediction that number of bird observations decreases with increasing habitat modification, we fitted a Generalised Linear Model (GLM) with Poisson errors, with total number of bird observations at each site as the response variable and habitat type (forest interior, forest edge or open habitat) as a categorical explanatory variable.
To test whether treatment (bird exclusion or control) and habitat type had an effect on the total number of arthropods and leaf-chewing arthropods, we fitted separate Generalised Linear Mixed-effects Models (GLMMs) with Poisson errors, with the total number of arthropods or leaf-chewing arthropods as the response variable, treatment, habitat type and their interaction as fixed effects, total estimated leaf area on a branch as an offset and unique tree identity as a random effect. We logit transformed the mean percent leaf damage for each branch to better fit a normal distribution, and fitted a Linear Mixed-Effects model (LME) with logit percent leaf damage as the response variable, treatment and habitat type and their interaction as fixed effects and unique tree identity as a random effect. We conducted post hoc Tukey's Honest Significant Difference (HSD) tests for all models to test for significant pairwise differences between each combination of treatment and habitat type.
We then used confirmatory path analysis in a generalised multilevel context, also known as piecewise structural equation modelling (SEM) (Lefcheck, 2016), to test for the direct and indirect effects of bird exclusion on leaf damage. Piecewise SEM allows for data with a multilevel or hierarchical structure and variables with different sampling distributions to be fitted in the same model (Shipley, 2009). To test whether the effect of bird exclusion, and hence the strength of trophic cascades, differs among habitat types, we used treatment as a binary exogenous variable and leaf-chewing arthropod abundance and logit percent leaf damage as endogenous variables. We conducted a multigroup analysis (Lefcheck, 2019) to test if each path varied by habitat type. We report both unstandardised path coefficients and standardised path coefficients, which reflect the relative magnitude of change of different paths (Lefcheck, 2019). To evaluate model fit, we used the coefficient of determination (R2), which shows the variance explained by the effect of the other variables on each endogenous variable.
We tested all hypotheses at the α=0.05 significance level. Where there was overdispersion in any of the above models, we refitted models using an appropriate error structure (e.g., quasipoisson). We also checked that there were no violations of model assumptions (e.g., heteroskedasticity, normality of residuals) in all models and sub-models in piecewise SEM. We performed all statistical analyses and plotting in R version 3.6.1 (R Core Team, 2019).
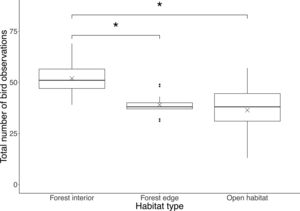
ResultsWe recorded a total of 1908 birds belonging to 33 species in this study, where the most common bird species was the blue tit (Cyanistes caeruleus). Total number of bird observations differed by habitat type (F2,42=10.4, P<0.001), where the forest interior (51.9±8.2) had a significantly higher number of bird observations than the forest edge (39.0±4.9; P=0.0017) and open habitat (36.3±13.4; P<0.001), but there was no difference between the latter two (P=0.71; Fig. 1).
Boxplot showing total number of bird observations at each habitat type. Boxes represent first, median and third quartiles; whiskers indicate maximum and minimum values no more than 1.5 times the interquartile range; black circles represent outliers; crosses indicate means. Significant pairwise comparisons between habitat types from Tukey's HSD tests are indicated with an asterisk.
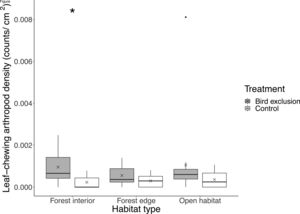
We found 3608 individual arthropods (40.1±24.7 per branch) from the beating samples collected. Of which, 152 were leaf-chewing arthropods (1.69±1.58 per branch), 1856 were sap-sucking, 684 were predatory, 723 were fungivorous/detritivorous and 193 were omnivorous. Of the leaf-chewing arthropods, 70 were Lepidopteran larvae. While the pairwise difference in total arthropod abundance between exclusion and control branches was similar across habitats (χ22,82=0.93, P=0.63), the abundance of leaf-chewing arthropods differed between exclusion (2.27±1.44) and control (0.67±0.81) branches only in the forest interior (P=0.0019; Fig. 2). There was a fourfold increase in the number of leaf-chewing arthropods inside bird exclusion cages in the forest interior.
Boxplot showing leaf-chewing arthropod density at each habitat type and treatment. Boxes represent first, median and third quartiles; whiskers indicate maximum and minimum values no more than 1.5 times the interquartile range; black circles represent outliers; crosses indicate means. Significant pairwise comparisons between treatments from Tukey's HSD tests are indicated with an asterisk.
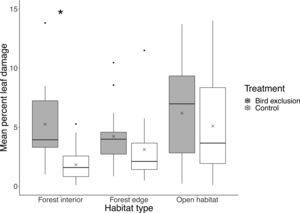
In total, 84% of leaves (N=1800) showed signs of leaf damage, with an average of 4.28%±3.41% (mean±SD) of leaf damage per leaf. There was no significant difference in leaf damage on control branches among habitats (interior-edge: P=0.47; interior-open: P=0.14; edge-open: P=0.98). Mean percent leaf damage differed between exclusion and control branches (F2,42=5.66, P=0.0067) only in the forest interior (P<0.001), but not in the forest edge (P=0.35) or open habitat (P=0.50), with mean percent leaf damage being 3.43% higher inside exclusion cages in the forest interior (Fig. 3).
Boxplot showing mean percent leaf damage at each habitat type and treatment. Boxes represent first, median and third quartiles; whiskers indicate maximum and minimum values no more than 1.5 times the interquartile range; black circles represent outliers; crosses indicate means. Significant pairwise comparisons between treatments from Tukey's HSD tests are indicated with an asterisk.
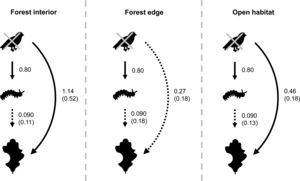
Bird exclusion directly increased the abundance of leaf-chewing arthropods by 2.23 times (t1,44=4.86, P<0.001), and this effect was similar in all habitats (χ22,43=2.60, P=0.27; Fig. 4). The abundance of leaf-chewing arthropods did not affect leaf damage (t2,43=1.33, P=0.19) and this effect was the same in all habitats (χ22,43=1.28, P=0.53; Fig. 4). However, the direct effect of bird exclusion on leaf damage differed among habitats (χ22,43=9.70, P=0.0078), where bird exclusion increased leaf damage only in the forest interior (t13,32=2.96, P=0.011) and open habitat (t13,32=3.04, P=0.0094), but not in the forest edge (t13,32=1.19, P=0.25; Fig. 4). The strength of trophic cascades, judging by the standardised path coefficients of direct effect of bird exclusion on leaf damage, was almost three times stronger in the forest interior than open habitat, and negligible in the forest edge (Fig. 4). Overall, the piecewise SEM explained 35% of leaf-chewing arthropod abundance and 60% of leaf damage.
Results of piecewise SEM showing relationship between bird exclusion, leaf-chewing arthropods and leaf damage among habitat types. Solid arrows depict significant paths; dashed arrows depict non-significant paths. Unstandardised path coefficients are shown beside each arrow; standardised path coefficients are given in brackets where available.
Our findings revealed that birds have an important role in controlling populations of herbivorous arthropods and in reducing herbivory in oak trees in Southern England. Birds’ ecological functions, however, were only important in the forest interior, as (1) edge and open habitats showed much weaker effects of bird exclusion on arthropod abundance and leaf damage compared to the forest interior, and (2) there was no corresponding increase in leaf damage on control branches among habitats. These results suggest that while the ecological functions performed by insectivorous birds, and consequently trophic cascades, are weakened or disrupted in modified habitats, other taxa with redundant functions likely become more abundant, such that overall ecosystem functioning is not impacted.
As expected, we found that forest edge and open habitat had lowered numbers of bird observations relative to the forest interior. Not only does habitat modification cause a direct loss of habitat for birds (Fahrig, 2003), it also alters ecological flows, reducing the quality of remaining habitat (Ries et al., 2004), and affects breeding patterns and social systems (Fischer and Lindenmayer, 2007). These changes, by presenting birds with higher predation risk, competition and disruptions to dispersal, restrict bird populations (Fischer and Lindenmayer, 2007).
Bird populations, indeed, have an important role in controlling insect populations and reducing herbivory. Our results show that while bird exclusion did not affect total arthropod abundance, it was associated with an increase in leaf-chewing arthropod abundance and leaf damage, especially in the forest interior. This suggests a preference for leaf-chewing arthropods, largely comprised of Lepidopteran larvae, by nesting birds in the spring. This suppression of leaf-chewing arthropods is especially prominent in the forest interior, where not only is there higher bird abundance, birds also have higher reproductive success and are better able to maintain their ecological function (Hinsley et al., 1999).
Despite bird exclusion having a direct effect on leaf damage in both the forest interior and open habitat, the much larger effect size of bird exclusion on lower trophic levels in the forest interior compared to the forest edge and open habitat points to weakened or even disrupted trophic cascades in modified habitats. Our results are consistent with findings that vertebrate communities in tropical forest edges also have weakened control over invertebrate density, and consequently leaf damage, compared to those at forest interiors (Harrison and Banks-Leite, 2019). It is possible that bird abundance in the forest edge and open habitat are lowered beyond a threshold at which interactions between birds and arthropods are maintained (Valiente-Banuet et al., 2015). Indeed, the extinction of species interactions, and subsequently ecological functions, can occur even before species are completely lost (Valiente-Banuet et al., 2015).
Despite this weakened trophic cascade, however, there was no significant difference in leaf damage among habitat types in the presence of birds (i.e., control branches). Indeed, there have been contradictory findings regarding the effect of habitat loss and fragmentation on leaf damage (Morante-Filho et al., 2016), suggesting that the effects of habitat modification cannot be simplified into a linear relationship between birds, arthropods and plants. Polis and Strong (1996) suggested that because food webs tend to be reticulate, or have high connectivity, simplifying webs into linear food chains does not appropriately capture responses of one trophic level to changes in another. This is certainly true in this study, where only 35% and 60% of leaf-chewing arthropod abundance and leaf damage respectively were explained by the tritrophic relationship between birds, arthropods and plants.
These findings imply missing relationships at each level of the food chain which have not been accounted for. For instance, predatory arthropods can act as intermediate predators to consume leaf-chewing arthropods, although they themselves may be controlled by birds acting as intraguild predators (Mooney et al., 2010). Indeed, even with similar levels of leaf damage among habitats, the effect of bird exclusion differs, suggesting different factors mediating leaf damage in these habitats. In the relatively undisturbed forest interior, leaf damage is likely to be facilitated predominantly by top-down control by birds, i.e., high abundances of birds suppress leaf-chewing arthropod populations and leaf damage (Morante-Filho et al., 2016). Conversely, in the forest edge and open habitats, top-down control by other trophic groups, i.e., predatory arthropods (Mooney et al., 2010), or bottom-up control, i.e., plant defences preventing herbivory (Coley and Barone, 1996), could be more dominant.
Despite the suppression of leaf-chewing arthropods by birds in the forest interior, there was no direct effect of leaf-chewing arthropod abundance on leaf damage in all habitats. Yet, it is unsurprising as estimates of leaf damage reflect accumulated herbivory in the eight-week experimental period, while arthropod abundances from beating samples reflect only their presence at one time point. Leaf-chewing arthropods, especially caterpillars, may move within, or even between, plants to feed as partially eaten oak leaves produce tannin to deter them from causing further damage, while others may have already gone through all their larval stages prior to the end of the experiment (Tyler, 2008). It is also likely that apart from the numerical effects of birds on arthropods and arthropods on herbivory, predator avoidance behaviour by prey can reduce herbivory rate without a corresponding decrease in arthropod abundance (Abrams, 1995).
The consequences of weakening trophic cascades on ecosystem function are thus not necessarily damaging and require further exploration. Our findings point to the functional redundancy of birds, where the function of controlling arthropod populations and thus reducing leaf damage in plants can be fulfilled by other species or processes undisturbed (or even benefited) by habitat modification (Elmqvist et al., 2003). Indeed, this functional redundancy may confer to ecosystems a resilience to disturbance (Elmqvist et al., 2003), where other taxa can replace dominant species in performing ecosystem processes (Ewers et al., 2015). Other ecosystem processes potentially controlling leaf damage need to be studied in tandem with the trophic relationship between birds, arthropods and plants so as to ascertain the effects of the weakening of one trophic cascade on overall ecosystem function.
Historical habitat modification at the Silwood Park study site has resulted in small remnant continuous forest patches, the largest of which is only 0.45km2. As such, the distinction among habitat types used in this study is relatively small. Nevertheless, our findings demonstrate differential effects of habitat modification on trophic cascades even in small fragmented forest patches, with the forest interior showing stronger trophic cascades than forest edge and open habitat. Indeed, Banks-Leite et al. (2010) showed that fragmented secondary forests with an already impoverished bird community exhibit similarly strong ecological responses to edge and area effects as primary forests. Our study therefore extends this finding beyond individual species responses to how multitrophic interactions respond to habitat modification in small fragmented forest patches. Undeniably, the study of ecological functions and trophic interactions in less pristine forest patches may be especially important in a time when 70% of the world's remaining forests are already subject to the damaging effects of fragmentation (Haddad et al., 2015).
FundingThis work was supported by the Department of Life Sciences at Imperial College London.
Conflict of interestsNone declared.
For help in the field, we thank Sebastian Ow, Kong Ze Hui and Jason Wong. We thank Aiko Leong, Sammi Lai and Janet Chik for logistical support. We would also like to thank Dr Jonathan S. Lefcheck, author of R package piecewiseSEM, for advice on model-fitting.