Habitat loss is the primary driver of the decline of biodiversity in ecological communities. However, which ecological processes are implicated in the removal of species following habitat loss, i.e. the disassembling of the community, remains unclear in many ecosystems. We address this question by investigating how the taxonomic and functional diversity of bird assemblages are related to fragment size in the Atlantic Forest from northeastern Brazil. We used complementary metrics of diversity and a randomization procedure to test whether changes in diversity result from either random or deterministic processes, such as limiting similarity or habitat filtering. The species–area (SAR) and functional richness–area (FAR) relationships were positive, as expected. However, the FAR had a lower slope than the SAR, which indicates a slower loss of functional richness than that predicted by the loss of species richness. Communities in smaller fragments contain more functionally overdispersed species and a more even distribution of abundance. These results indicate that limiting similarity drives the disassembling of bird assemblages in small fragments of the Atlantic Forest, which presumably reflects increased competition. This dynamic tends to reduce functional redundancy in the impoverished assemblages, with potentially deleterious consequences for ecosystem functioning and forest conservation.
Habitat loss is the leading cause of the decline in biodiversity and ecosystem functioning worldwide (Brooks et al., 2002) as species diversity is dependent on the amount of habitat available (Watling et al., 2020). Around one million species may become extinct by the end of the 21st Century (IPBES, 2019), with half of these extinctions occurring in tropical forests (Pimm and Raven, 2000). However, which species will be lost remains unclear, and the understanding of this process, known as community disassembling, is a central theme in community ecology (Murphy and Weiss, 1992; Ostfeld and LoGiudice, 2003). Species may not be eliminated randomly, and some may be more vulnerable to extinction than others (Owens and Bennett, 2000), depending on their specific functional attributes (Almeida et al., 2018; Suding et al., 2005). A possible consequence of this non-random loss of species is the removal of unique ecological functions from the ecosystem, such as pollination or seed dispersal, leading to an impoverishment of the functional characteristics of ecological communities (Cadotte et al., 2011).
Traditional ecological indices used to characterize communities, based solely on the numbers of species and individuals, are not sufficient for a systematic understanding of the impacts on ecosystem functioning (Gagic et al., 2015). A more suitable approach would include functional traits that describe the ecological functions of the species (Violle et al., 2007). The concept of functional diversity, based on the ecological, morphological, and physiological traits of the species, has been a fundamentally important advance in the description of the variation found in ecological communities (Ding et al., 2013; Tilman, 2001; Valdivia et al., 2017). The functional structure of a community reflects the ecological and biogeographic processes that support the formation of assemblages, including biotic interactions and the abiotic factors that influence the characteristics of regional pools of species (Cadotte et al., 2011; Mouillot et al., 2013). These aspects of species diversity and functional structure also reflect the response of ecological communities to anthropogenic pressures on an ecosystem, and are thus essential for the effective conservation of habitat remnants (Mouillot et al., 2013).
The dominant processes that structure ecological assemblages involve a combination of abiotic, biotic and stochastic factors (Webb et al., 2002). Abiotic factors select for species and lineages with specific adaptations (i.e., functional traits) that favor their survival under certain conditions, which act as environmental filters. The result of this process is the formation of local assemblages with species that are more similar to one another (i.e., clustered) regarding their functional traits (Kraft et al., 2015). Biotic factors affect the structure of assemblages through biotic interactions, in particular, competition. Species with similar resource requirements (i.e., taxa that are functionally similar) will compete, leading to the exclusion of those with a lower competitive capacity or reduced population size, a process known as competitive exclusion by limiting similarity (MacArthur and Levins, 1967). Assemblages in which this process predominates tend to comprise species with more distinctive functional traits (i.e., over-dispersed; Kraft et al., 2015). Finally, neutral theory posits that stochastic processes (e.g., dispersion and ecological drift) will prevail over trait-dependent processes in determining community structure (Hubbell, 2001).
Most research that has focused on the relative influence of competition and environmental filters on community assembling has dealt with broad spatial scales. Most of the studies have identified a predominance of environmental filters over limiting similarity (Kraft et al., 2015; Mouchet et al., 2013; Weiher and Keddy, 1999). However, the findings of some studies indicates that the process of communities assembling is scale-dependent, with environmental filters being more influential on community structure at a regional scale, with species interaction becoming more important at the local scale (Mouchet et al., 2010; Smith et al., 2013). Local-scale studies that encompass the functional aspects of the species are necessary to shed light on the interplay of ecological processes that structure ecological communities. This perspective can provide profound insights into the processes that underly the disassembling of local ecological communities, which may be potentially valuable for the effective management of biodiversity (Almeida et al., 2016; Cadotte and Tucker, 2017; Ding et al., 2013).
Bird communities are an essential component of terrestrial ecosystems, where they provide critical ecological functions, such as pollination, seed dispersion, nutrient cycling, and the control of insect populations (Mahendiran and Azeez, 2018; Morante-Filho and Faria, 2017). Local bird diversity is related primarily to the distribution of feeding and nesting resources (Whelan et al., 2008). As habitat is lost, these local resources decrease (Watling et al., 2020) and new conditions are created (e.g., edge habitats or open areas), which may attract opportunistic species, increasing competition for the limited resources (Gascon et al., 2000; Pimm and Raven, 2000). The size of a forest patch has a positive effect on bird species richness (Watling et al., 2020), which is one of the parameters most affected by the reduction of the area of forest in tropical regions (Betts et al., 2019).
The understanding the effects of habitat loss on forest bird assemblages at a local scale requires data on multiple aspects of the diversity of functional traits, especially the traits linked to the essential factors that determine community assembly, such as the exploitation of different feeding resources and microhabitats by the bird species (Cadotte et al., 2011; Mazel et al., 2014). In the present study, we investigated different aspects of the taxonomic (i.e., species richness and uniformity) and functional (i.e., richness, uniformity, divergence, and dispersion) diversity of bird assemblages along a gradient of fragment size in the Atlantic Forest of northeastern Brazil. We tested whether the functional and species richness varied at the same rate along the fragment size gradient. We expected that smaller fragments have lower taxonomic and functional richness, given that these two parameters are naturally correlated (Mouchet et al., 2010). However, we hypothesize that, if the species that are absent from the smaller fragments have similar functional traits to the species that persist in these fragments (i.e., they are functionally redundant), the slope of the functional richness–area relationship (FAR) will be lower than that of the species–area relationship (SAR). This would indicate the limiting similarity is the leading process of community disassembling along the gradient of fragment size (Mouchet et al., 2010). We further predict that one consequence of this effect would be an increase in functional dispersion, given that, as the size of the fragments decreases, taxonomic and functional evenness will both increase, together with the functional divergence in the smaller fragments.
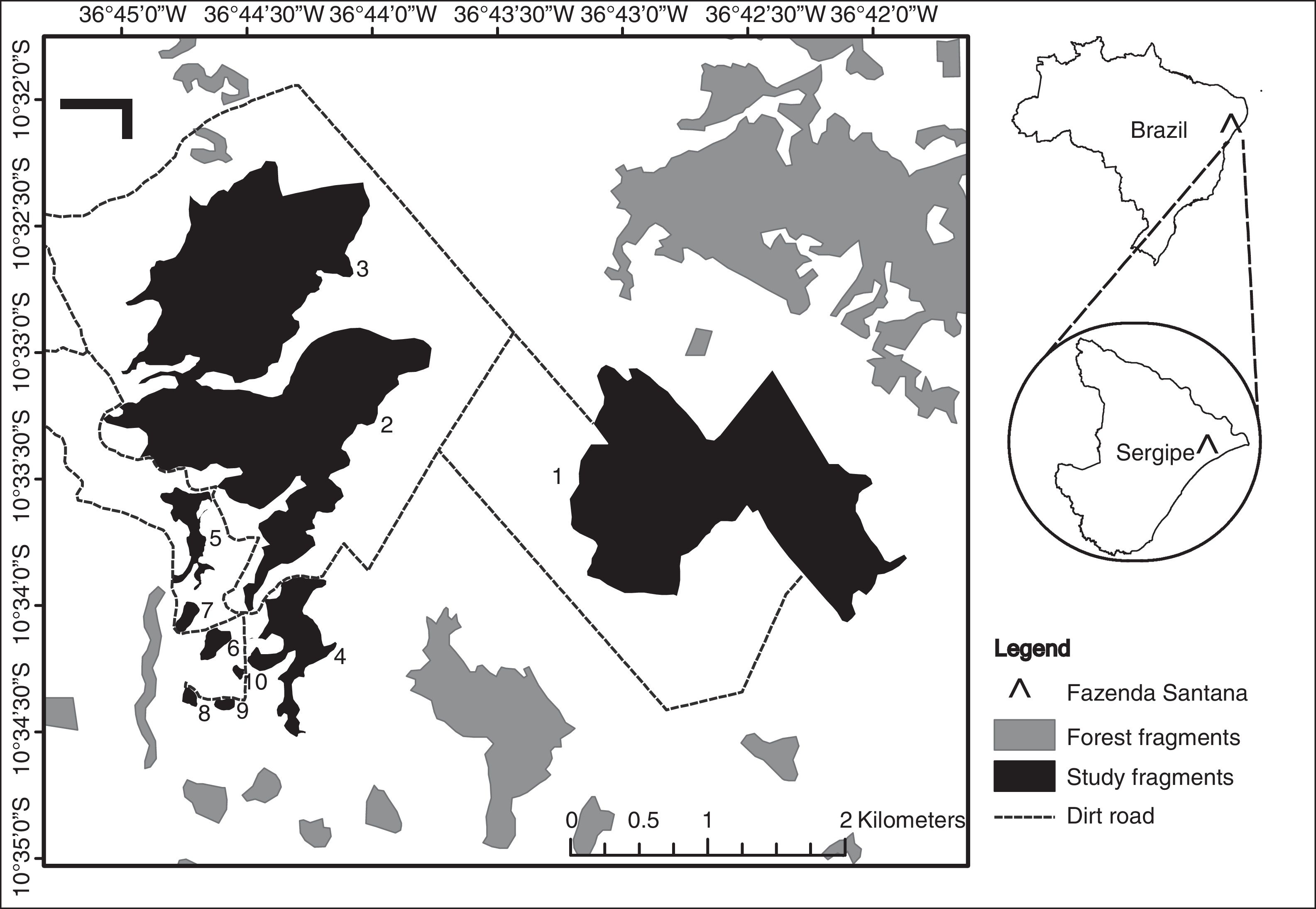
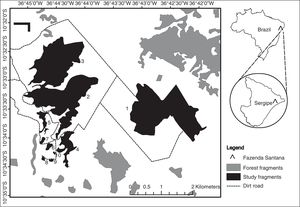
Materials and methodsStudy areaWe surveyed fragments of Atlantic Forest located on a farm, Fazenda Santana (10° 32′ S, 36°45′ W) located on the northern coast of Sergipe state, Brazil (Fig. 1). The local climate, according to Köppen-Geiger classification, is As (i.e. tropical with dry summers) with annual precipitation between 1000 mm and 1400 mm, and a mean temperature of 22.7–26.5 °C (Aragão et al., 2013; Kottek et al., 2006). The local soils are quartzite sandy with terrain varying from flat to undulating at altitudes between 40 m and 150 m above sea level (Jacomine et al., 1975).
We selected 10 forest fragments ranging in area from 0.73 ha to 241 ha (Supplementary Material, Table S1). These fragments are distributed within a homogeneous matrix of sugarcane plantations. The largest fragments are also more complex in shape (r = 0.8, p = 0.01) and are less isolated than the smallest fragments (r = -0.82, p = 0.01; Fig. S1). Because of this, the collinearity of shape and isolation with size, and the prevailing effect of habitat size on species diversity, we focused our study on the effect of fragment area alone. As the two largest fragments (2 and 3) are connected by an ecological corridor approximately 150 m wide, we considered them to be separate, but with 0.0 m of isolation from each other.
Bird surveyWe conducted six sampling events in each fragment between March-2017 and February-2018. Each sampling event lasted 10 days, with one fragment sampled per day (morning and afternoon). The surveys were based on MacKinnon lists of 10 species (Bibby et al., 1998), which were conducted between sunrise (ca. 5:00 h) and 10:00 h and from 15:00 h to sunset (ca. 18:00 h). In this method, the observer compiles lists of species by recording each new species until the predetermined standard number of 10 species is reached. A specie cannot be repeated in the same list, nor can the same individual be included in successive lists (Bibby et al., 1998). The total sampling effort was 480 h, with a total of 48 h of observation for each fragment. During the study period, we compiled as many lists as possible in each fragment according to the number of individuals of the different species present in the fragment (Bibby et al., 1998). The survey was undertaken by only one observer (H.S.O.), avoiding bias provoked by different bird identification capacity.
The transects were surveyed by walking along the trails located at the edge and the interior (core) of each fragment, to ensure the sampling of species in different microhabitats. The order in which the edge and core trails of a given fragment were sampled was alternated among sample events. That is, if the core was sampled in the morning and the edge in the afternoon on one day, the next survey was conducted in the reverse order. Birds observed flying over the fragments at a cruising height, typically vultures and egrets, were not included in the lists, given that their presence was not associated with the forest fragment per se.
This method also allows us to obtain the Index of Frequency in the Lists (IFL), an indicator of relative abundance, which is obtained by the equation: IFL = ni/LM, in which ni is the number of times the species i was recorded in the lists, and LM is the total number of Mackinnon Lists made for the site (Ribon, 2010). Therefore, the more common a species, the more frequent it will be in the lists and the larger will be its IFL. We used the IFL of each species per fragment as a measure of its relative abundance for the calculation of other diversity metrics.
Diversity indexFunctional diversity was determined by functional traits related to resource partitioning, given that the diversity of ecological functions and the coexistence of bird species are related to the exploitation of resources, in particular food and nesting sites (Whelan et al., 2008). We used the seven functional traits from Wilman et al. (2014), which reflect the various requirements of the species and how they obtain resources (Table 1; Supplementary Material, Table S2). Biomass is related systematically to other traits, such as metabolic rates and behavior, and dictates the amount and size of the feeding items exploited (Luck et al., 2012). The trophic guild reflects the dominant type of feeding items exploited by the species and, together with the foraging stratum, are related to regulatory ecosystem functions, such as the control of insect pests, pollination, and the transport and cycling of nutrients (Whelan et al., 2008).
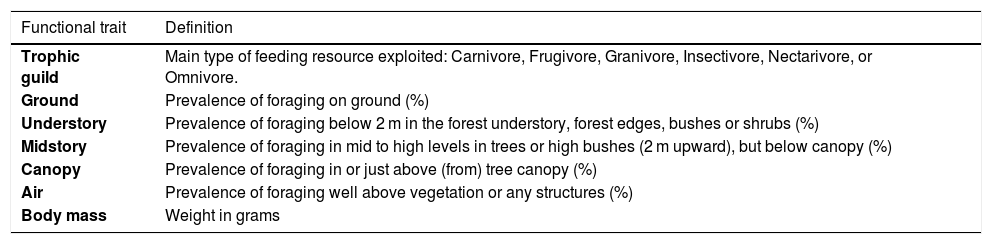
Functional traits used to describe the functional diversity of the bird assemblages in fragments of Atlantic Forest found at northern coast of Sergipe state, Brazil.
| Functional trait | Definition |
|---|---|
| Trophic guild | Main type of feeding resource exploited: Carnivore, Frugivore, Granivore, Insectivore, Nectarivore, or Omnivore. |
| Ground | Prevalence of foraging on ground (%) |
| Understory | Prevalence of foraging below 2 m in the forest understory, forest edges, bushes or shrubs (%) |
| Midstory | Prevalence of foraging in mid to high levels in trees or high bushes (2 m upward), but below canopy (%) |
| Canopy | Prevalence of foraging in or just above (from) tree canopy (%) |
| Air | Prevalence of foraging well above vegetation or any structures (%) |
| Body mass | Weight in grams |
We used six metrics to analyze bird diversity. We compiled two metrics of taxonomic diversity: species richness (S), given as the number of species observed per fragment, and Pielou’s evenness (J’), which evaluates the uniformity of the abundance among the species recorded in a given fragment (Pielou, 1966). We calculated these indices with the vegan package of the R software 3.6.0 (R Core Team, 2018). We measured four indices of functional diversity, following Laliberté and Legendre (2010) and Villéger et al. (2008): FRic (functional richness), FEve (functional evenness), FDiv (functional divergence), and FDis (functional dispersion). The FRic is the only index that correlates with S (Laliberté and Legendre, 2010; Mouchet et al., 2010), although these metrics may have different relationships (slopes) with fragment size. The indices of functional diversity are acknowledged to be the best descriptors of the ecological roles played by the species present in a given ecosystem (Gagic et al., 2015).
We used Gower’s distance (Gower, 1971) to create a matrix of trait distances between pairs of species, which we used to calculate the different metrics. Gower’s distance is appropriate here because it considers different types of variables simultaneously (Legendre and Legendre, 2012). We applied a Principal Coordinates Analysis (PCoA) to this functional distance matrix, whose first three axes explained 78% of the variation in the distances. We then used the PCoA axes as new functional traits, which, together with the matrix of relative abundance (IFL) of each fragment (Supplementary Material, Table S3), allowed us to calculate the indices of functional diversity. For this procedure, we used the dbFD function of the FD package (Laliberté and Legendre, 2010) of the R software 3.6.0 (R Core Team, 2018).
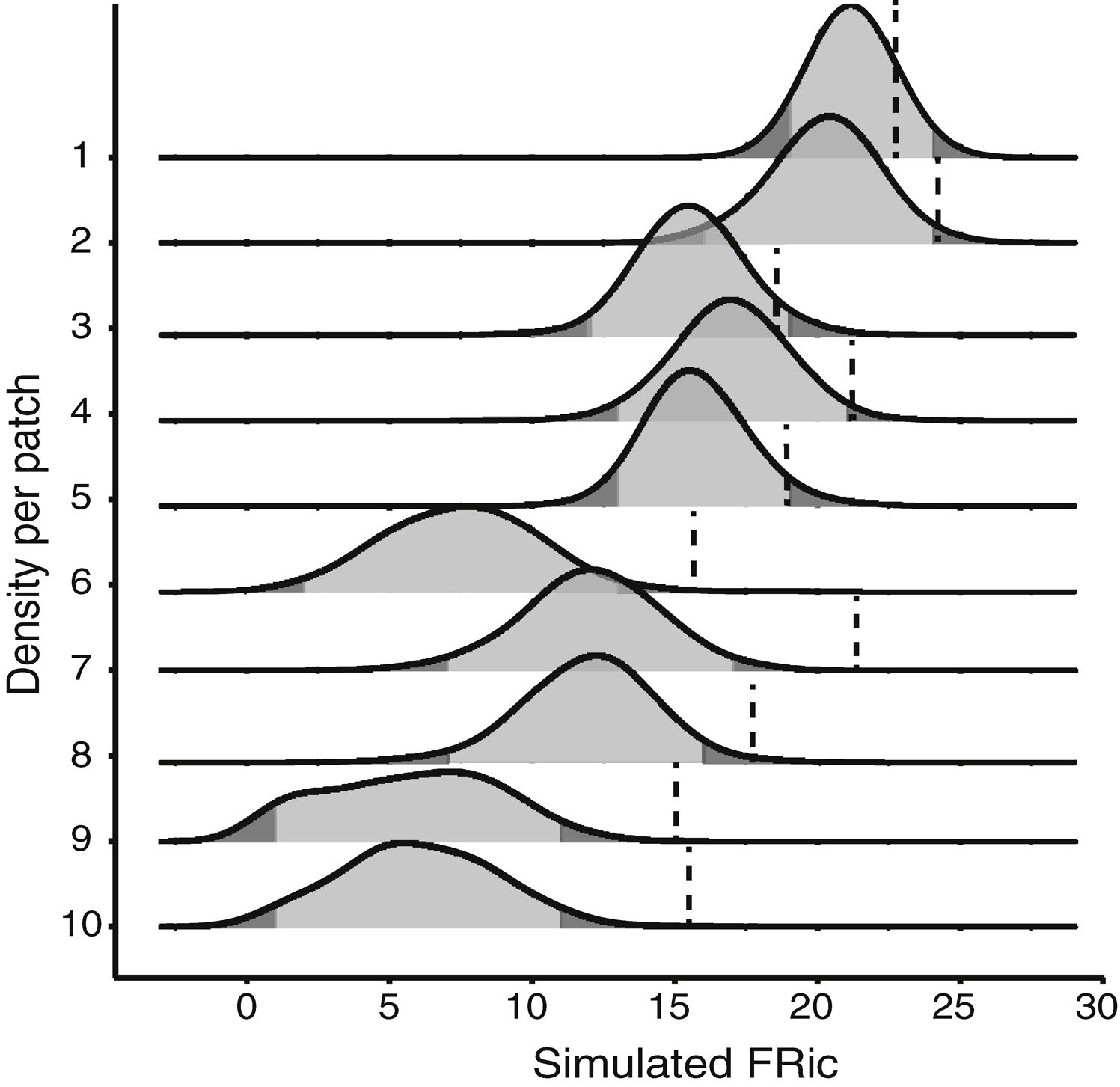
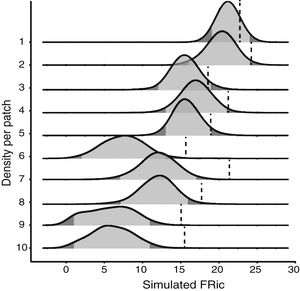
Null modelTo test whether the estimates of FRic differed from the values expected by chance, we compared the observed values with the values determined by a matrix-swap null model (Manly and Sanderson, 2002; Mason et al., 2013), using the standardized effect size (SES) of Gotelli and McCabe (2002). The simulated assemblages were created by randomizing (999 permutations) all the species recorded in all the fragments, while maintaining the abundance and species richness observed in each fragment. We calculated the SES of functional richness (SESFRic) for each fragment, which measures the number of standard deviations that the observed metric is above or below the mean value obtained for each simulated community, based on the formula: SESFRic = Obs-Exp/SDexp, where Obs = the observed FRic, Exp = the mean of the 999 simulated values, and SDexp = the standard deviation of this mean (Gotelli and McCabe, 2002).
Under a normal distribution, 95% of the SESFRic values will be between -1.96 and 1.96 standard deviations of the mean. Values outside this confidence interval are considered to be significantly different from the null model, at α = 0.05 (Wittman et al., 2010). When SESFRic is significantly lower than the null model (SESFRic < -1.96), the assemblage is characterized by functional clustering, which indicates that environmental filters are the dominant assembling rule. When functional diversity is higher than expected (SESFRic >1.96), by contrast, the assemblage has species that are functionally more distinct from one another, which indicates that limiting similarity is the predominant force in the community (Mouchet et al., 2010).
Data analysisWe evaluated the sampling efficiency by estimating species richness using the Chao 1 estimator (Chao, 1984), run in EstimateS 9.1 (Colwell, 2013). We used linear regression models to relate the diversity indices (S, J’, FRic, FEve, FDiv, FDis, and SESFRic) to the area of the fragments. All the variables were log10-transformed prior to the regression analyses to ensure the normality and linearity of the data (Scheiner, 2003; Zuur et al., 2009). To test for the difference in the rate of change between functional richness and species richness relative to fragment area, we employed an analysis of covariance (ANCOVA), which interaction term tests for the difference between the slopes of the two relationships (McDonald, 2014). A slope of the FAR significantly lower than that of SAR indicates retention of functional traits despite the loss of species at smaller fragments. We ran all the analyses in the R software, version 3.6.0 (R Core Team, 2018).
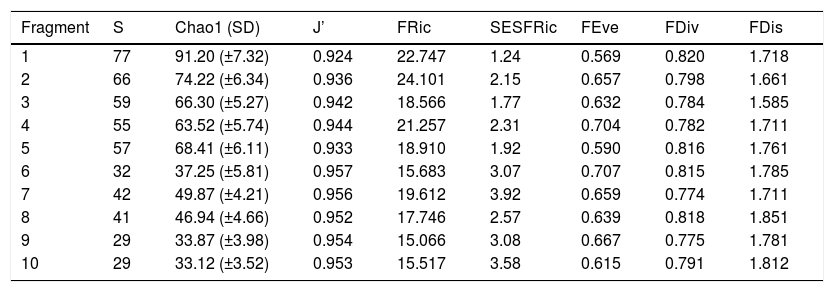
ResultsWe obtained 1896 records of 92 bird species in a total of 192 McKinnon lists. The observed species richness corresponded to 91% (∼99sp. ± 6.43SD), of that estimated by the Chao 1 procedure, with a total of between 29 and 77 species being recorded per fragment. Sample completeness was similar in all fragments, at ∼83–89% (Table 2).
Metrics of the bird taxonomic and functional diversity and the spatial characteristics of the fragments of Atlantic Forest found at northern coast of Sergipe state, Brazil.
| Fragment | S | Chao1 (SD) | J’ | FRic | SESFRic | FEve | FDiv | FDis |
|---|---|---|---|---|---|---|---|---|
| 1 | 77 | 91.20 (±7.32) | 0.924 | 22.747 | 1.24 | 0.569 | 0.820 | 1.718 |
| 2 | 66 | 74.22 (±6.34) | 0.936 | 24.101 | 2.15 | 0.657 | 0.798 | 1.661 |
| 3 | 59 | 66.30 (±5.27) | 0.942 | 18.566 | 1.77 | 0.632 | 0.784 | 1.585 |
| 4 | 55 | 63.52 (±5.74) | 0.944 | 21.257 | 2.31 | 0.704 | 0.782 | 1.711 |
| 5 | 57 | 68.41 (±6.11) | 0.933 | 18.910 | 1.92 | 0.590 | 0.816 | 1.761 |
| 6 | 32 | 37.25 (±5.81) | 0.957 | 15.683 | 3.07 | 0.707 | 0.815 | 1.785 |
| 7 | 42 | 49.87 (±4.21) | 0.956 | 19.612 | 3.92 | 0.659 | 0.774 | 1.711 |
| 8 | 41 | 46.94 (±4.66) | 0.952 | 17.746 | 2.57 | 0.639 | 0.818 | 1.851 |
| 9 | 29 | 33.87 (±3.98) | 0.954 | 15.066 | 3.08 | 0.667 | 0.775 | 1.781 |
| 10 | 29 | 33.12 (±3.52) | 0.953 | 15.517 | 3.58 | 0.615 | 0.791 | 1.812 |
S = species richness; J’ = Pielou’s evenness; FRic = functional species richness; SESFRic = standardized effect size of functional richness; FEve = functional evenness; FDiv = functional divergence; FDis = functional dispersion.
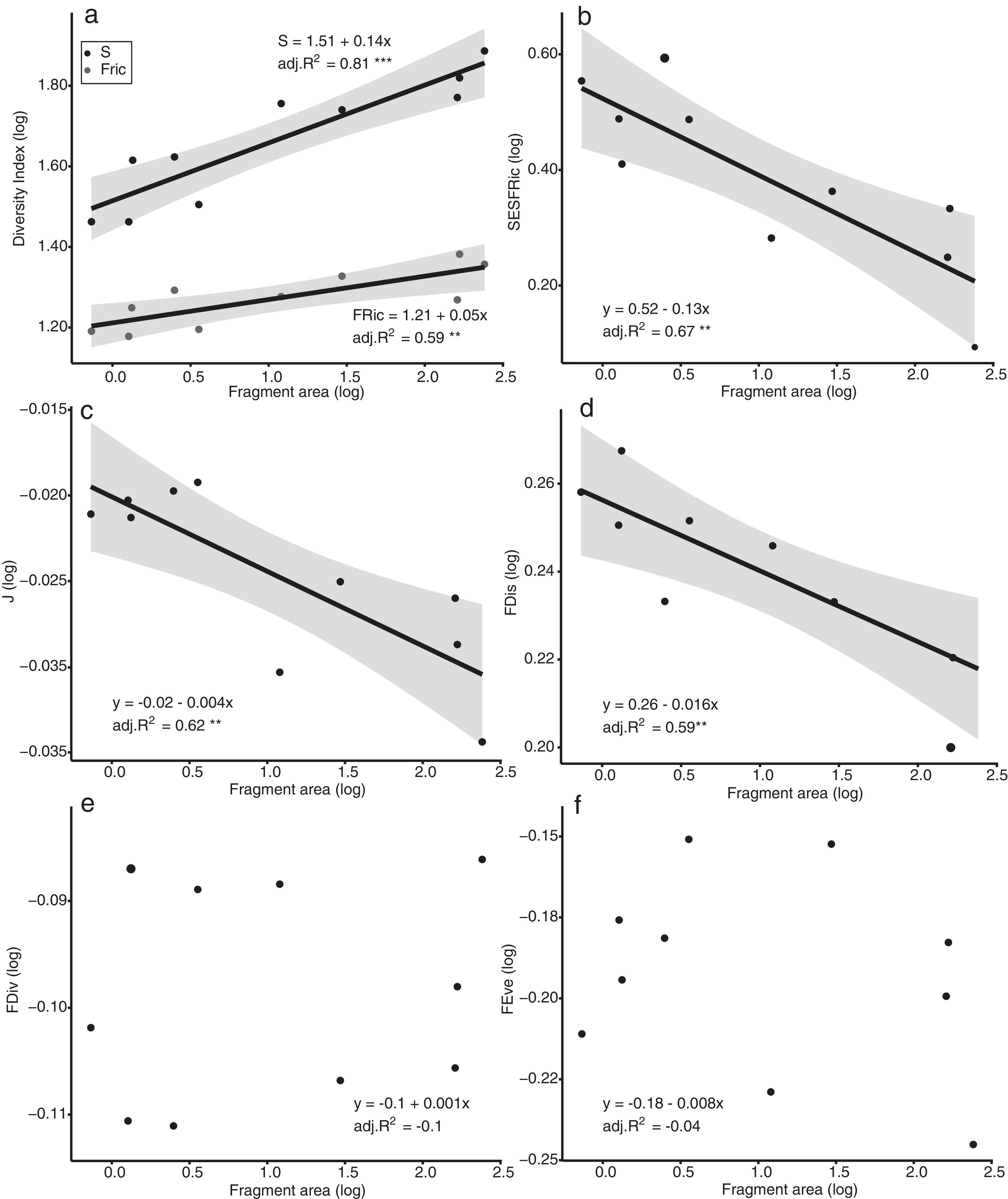
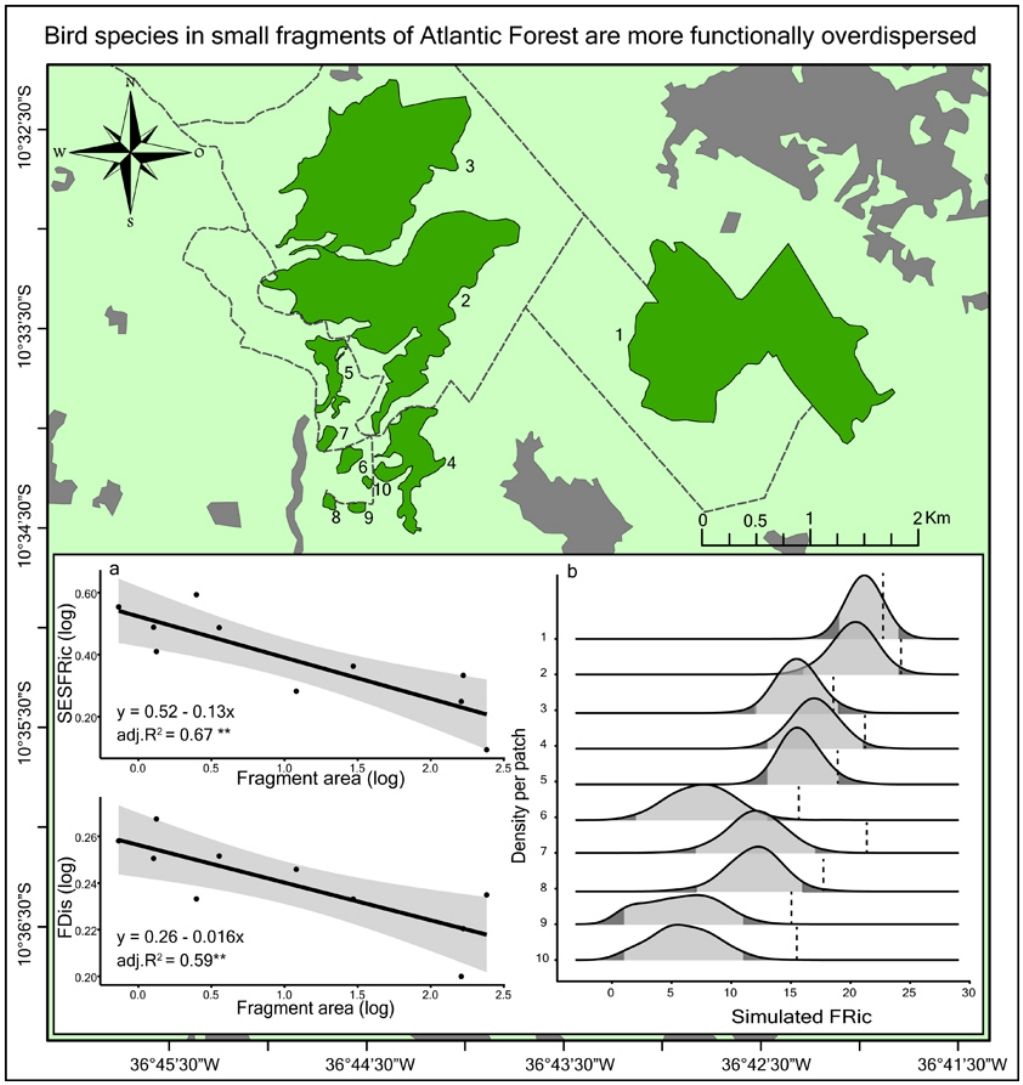
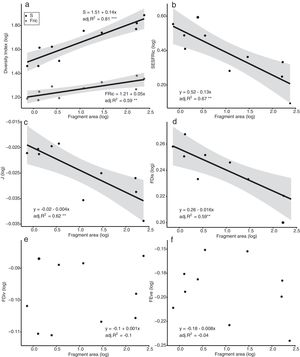
The models of linear regression between the diversity indices and the area of the fragments revealed that both the SAR and the FAR were positive and significant (Fig. 2a). By contrast, uniformity (J’) and functional dispersion (FDis) were related negatively with the area of the fragment (Fig. 2c, d). Neither functional divergence (FDiv) nor functional uniformity (FEve) were related significantly to fragment area (Fig. 2e, f). The ANCOVA used to compare the slopes of the SAR and FAR models revealed that functional richness decreases more slowly with fragment size than species richness, i.e., with a significantly lower slope (F = 9.7302, p = 0.0066; Fig. 2a).
Linear regression plots with 95% confidence intervals (shaded areas) showing the predicted relationship between the diversity indices and the forest spot area gradient. S = Species Richness, J = Pielou’s evenness, FRic = Functional Richness, FEve = Functional evenness, FDiv = Functional divergence, FDis = Functional dispersion, SESFRic = Standardized Effective Size of Functional Richness. * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
Seven assemblages had a SESFRic higher than the upper limit of the confidence interval, which indicates that their FRic values were significantly higher than those predicted by the null model (Fig. 3, Table 2). The linear regression between the SESFRic values and the area indicate a significantly negative relationship (R² = 0.67, p = 0.0022, Fig. 2b). That is, although larger fragments had more species than smaller ones, they are functionally less diverse than expected according to their species richness. This reflects the accumulation of redundant species in larger areas, as also indicated by the result of the ANCOVA, above (Fig. 2a).
DiscussionWe found that fragment size affects both the taxonomic and functional diversity of the bird assemblages surviving in remnants of the Atlantic Forest in northeastern Brazil. More notably, we found that, as the area of the fragment decreases, functional richness decreases at a rate slower than that of species richness, indicating that the species that are absent from the smaller fragments share functional traits with those that persist in these fragments. A high proportion (70%) of the assemblages had significantly higher functional richness than expected according to their species richness, i.e., their SESFRic. This metric was also related negatively with fragment area, indicating that the dissimilarity among species tends to increase in smaller fragments. Although other features of a forest fragment may affect the local diversity, including its shape, isolation, and the characteristics of the matrix (Barros et al., 2019; Watling and Donnelly, 2006), the effects of these factors on bird diversity was determined by their collinearity with the area of the fragment (Supplementary Material). Future studies should investigate these effects on bird diversity more systematically while controlling for the dominant effect of fragment area.
These findings suggest a high level of interspecific competition in the community (Cadotte and Tucker, 2017; Sobral and Cianciaruso, 2015), which is consistent with the hypothesis that limiting similarity is the dominant assembling rule in these bird assemblages (MacArthur and Levins, 1967; see also Almeida et al., 2016; Mouchet et al., 2010). This conclusion is also supported by other results of the present study. For example, the intensification of competition among species will be expected to exclude the less competitive taxa, while leaving the more abundant ones, which will tend to reduce the disparities in the relative abundance of the more persistent species (Cadotte and Tucker, 2017; Hillebrand et al., 2008; MacArthur and Levins, 1967). We also recorded a significantly higher species evenness (J’) in the smaller fragments, which indicates that the less abundant species are more likely to be excluded, which is consistent with the role of competition as the principal driver of local diversity (Cotgreave, 1994; Hillebrand et al., 2008). One other important finding is the significant negative relationship found between functional dispersion (FDis) and fragment area, which indicates that the few species that persist in smaller fragments are ecologically more different from one another than expected by chance (Karadimou et al., 2016; Mouchet et al., 2010).
Functional evenness (FEve) and divergence (FDiv) were not associated with fragment area, although these metrics do provide some useful insights. Functional evenness expresses how evenly the abundance of individuals is distributed in functional space, and this metric decreases as the number of functionally unique and rare species increases (Sitters et al., 2016). The generally low values of FEve recorded in the present study indicate a lack of functionally rare species in the study area, even in the larger fragments (Mason et al., 2005; Villéger et al., 2008). At less than 250 ha, in fact, even the largest fragments surveyed in the present study may be too small to support a full local bird assemblage, which implies that local extinctions may already have occurred at all sites. The FDiv, in turn, describes how the traits of the most abundant species differ from those of the other taxa (Villéger et al., 2008). Low FDiv values reflect the recurrence of common species, which are usually the more abundant in the fragments. In the present study area, these species include Coereba flaveola (Linnaeus, 1758), Columbina talpacoti (Temminck, 1810), and Tyrannus melancholicus (Vieillot, 1819), which were the most abundant species in five, six, and seven fragments, respectively.
The predominance of limiting similarity as a result of habitat size is consistent with that observed in other groups of organisms (Mason et al., 2013, 2007; Slingsby and Verboom, 2006; Valdivia et al., 2017) including birds from islands (Ding et al., 2013), dense rainforest (Almeida et al., 2016), different vegetation types (Lee and Martin, 2017), and at varying spatial scales (Sobral and Cianciaruso, 2015). Even so, little is known about how habitat loss affects the functional structuring of tropical forest bird communities (Bovo et al., 2018; Si et al., 2017; Ulrich et al., 2016). Previous research has emphasized the assembling, i.e., the formation, of ecological communities, with substantial theoretical and empirical developments (e.g., Diamond, 1975; Morin, 2011). However, as habitat modification and other threats intensify, it remains to be seen whether analogous processes – i.e., limiting similarity and habitat filtering – drive the disassembling of ecological communities in an equivalent way. In other words, does the subtraction of species from an assemblage following impact follow a similar sequence to that observed when the community is formed?
Perhaps even more importantly, the results of the present study have a number of implications for the conservation and management of the local bird communities and the Atlantic Forest remnants they inhabit. Firstly, our results underscore the well-known negative effect of forest loss on bird species diversity (Brooks et al., 2002; Watling et al., 2020). The preservation of both species diversity and the redundancy of the ecological functions of ecologically-similar species tends to require the maintenance of much larger tracts of forest (Cadotte et al., 2011). However, our findings do indicate that the presence of a few larger fragments in the landscape may not reduce the chances of local extinction in the smaller remnants. Local extinctions in the smaller fragments might nevertheless be avoided by adopting measures that ensure a reduction in competition in these remnants. These measures may include not only an increase in the connectivity between the fragments within the landscape, but also between landscapes, that is, by ensuring greater species dispersal, which would lower local extinction risk (Newmark et al., 2017). Birds also have essential ecological roles in forest ecosystems, including seed dispersal, pollination, and the control of insect populations, which means that the preservation of bird diversity is fundamental to the maintenance of forest dynamics, including plant reproduction and recruitment, which are essential for the regeneration of the habitat (Bovo et al., 2018; Mahendiran and Azeez, 2018; Morante-Filho and Faria, 2017).
Overall, then, we found that the reduction in species diversity with decreasing fragment size in a Atlantic Forest landscape involves primarily the loss of species with similar ecological functions, which indicates the influence of interspecific competition as the process driving the local community disassembling. In this context, we would point out that the analysis of species richness alone may not be sufficient to guarantee the understanding of shifts in bird species diversity in response to habitat impacts, in particular the loss of taxa (Gagic et al., 2015). Given this, a functional perspective would appear to provide a more conclusive perspective on the interactions among species and their environment (Mason et al., 2013; Tilman, 2001; Violle et al., 2007). Finally, our study underscores the need for the maintenance of large and interconnected fragments of Atlantic Forest, at both local and regional scales, in order to guarantee the preservation of bird species diversity, the functional diversity of the community, and the stability of ecosystem functions.
Conflict of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
FundingThis study is part of HSO's master degree, which was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. SFG and SFF by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 302854/2019-3 and 310852/2017-0, respectively). This project was also supported by CAPES/FAPITEC grants (#88881.157451/2017-01 and #88881.157961/2017-01) provided to SFF and SFG. SFG is a member of the National Institute of Science and Technology - Ecology, Evolution, and Conservation of Biodiversity (INCT-EECBio, CNPq/FAPEG, grant 380733/2017-0).
Data availability statementThe authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Ethical approvalThis article does not contain any studies with human participants or animals performed by any of the authors.
Credit authorship contribution statementHelon Simões Oliveira: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing. Sidney F. Gouveia: Data curation, Validation, Formal analysis, Project administration, Writing - review & editing. Juan Ruiz-Esparza: Methodology, Writing - review & editing. Stephen F. Ferrari: Validation, Writing - review & editing, Supervision.
We thank the managers at Companhia Brasileira de Açúcar e Álcool, for authorizing fieldwork at Fazenda Santana and all field logistics, our collaborators at the UFS Laboratory of Conservation Biology, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), for the masters scholarship to HSO. Also at Conselho Nacional de Desenvolvimento Científico e Tecnológio (CNPq), CAPES and Fundação de Apoio à Pesquisa e Inovação Tecnológica do Estado de Sergipe (FAPITEC) for SFG support. Thanks to the anonymous reviewers for providing constructive comments.