The Andean Condor (Vultur gryphus) is a globally threatened species. Its highly mobile capability presents important challenges for conservation planning, especially in extremely geographically complex regions such as Colombia, where little is known about its ecology. Over the past three decades, financial and technical conservation efforts have primarily focussed on reintroduction and local management strategies. However, these initiatives did not properly prioritize the various conservation measures undertaken. We utilized roosting locations across Colombia to identify suitable roosting distribution with high risk because of the anthropogenic impact on a Systematic Planning Tool for decision-making based on robust spatial habitat modelling to define where and how should focus the Andean condor conservation actions in the country. Specifically, we aimed to develop a conservation planning tool to facilitate spatially explicit decision-making. Our results showed that Colombia has at least 19,571.33 km2 of suitable roosting habitat for this species, but over 30% of this area is currently considered to be under conservation risk due to severe anthropogenic impacts. Considering this, we suggested different actions for each proposed area according to potential threats generated by human communities.
One of the greatest challenges facing conservation planning (Knight et al., 2006) is deciding how and where to invest limited financial and technical resources to conserve biodiversity (Wilson et al., 2007). This situation becomes even more complex for mobile threatened species that are highly mobile with large home ranges, which are usually susceptible to large-scale threats throughout their distribution range (Nandintsetseg et al., 2019). For this reason, single actions at specific local scales are often insufficient to secure species persistence, and large-scale approaches, including transboundary management and conservation strategies, are required (Lambertucci et al., 2014; Runge et al., 2014).
The Andean Condor (Vultur gryphus) is one of the largest and most mobile species in the Neotropical region, with its distribution spanning most of the Andes. Classified as Vulnerable (VU), its populations are in decline (BirdLife International, 2020) and the situation is particularly critical in the northern part of its range (Naveda-Rodríguez et al., 2016; Padró et al., 2023). In Colombia, the Andean Condor is considered Critically Endangered (CR) (Renjifo et al., 2016): This critical situation was identified in Colombia in the 1980s, a decade during which it was believed to be extinct in several localities (Rodríguez et al., 2006). Consequently, a reintroduction program was implemented between 1989 and 2013 and 71 individuals were released at eight repopulation sites (Sáenz-Jiménez, 2020). However, to date only one successful reproduction of the released condors was reported in Los Nevados Natural Park (Restrepo-Cardona et al., 2018). At present, little is known about the ecological requirements and survival of reintroduced individuals and the threats they may face, hindering the development of effective technical and financial strategies for their long-term conservation in the country.
Andean Condors can travel more than 300 km/day (Lambertucci et al., 2014; Padró et al., 2023) and prefer to roost on cliffs and steep mountain slopes, which offer refuge from threats and adverse weather conditions (Lambertucci and Ruggiero, 2013). Condors regularly frequent the same roosting sites (Padró et al., 2018), with conditions that offer safety and facilitate easy take-off and landing (H.J. Williams et al., 2020). The roosting sites are preserved within Priority Conservation Areas (PCAs) to guarantee safe habitats that facilitate efficient take-off and landing, nesting, and the overall survival of Andean Condors (Plaza and Lambertucci, 2020). Additionally, these sites serve as vital conservation and gathering spots for other bird species, supporting their populations and the ecosystem services provided (Lambertucci and Ruggiero, 2016). These areas also create stepping-stone corridors between regions, promoting gene flow among populations (Padró et al., 2023), Thus, the protection of roosting sites should help to reduce discrete loss of genetic variability (Padró et al., 2018), and reduce the effects of inbreeding (Padró et al., 2020).
Here, we highlight the significance of modelling suitable roosting sites as an effective systematic planning tool to inform condor conservation strategies in Colombia, a region characterized by substantial research gaps on habitat use and movement ecology of the Andean Condor. This study aims to identify PCA’s for the Andean Condor in Colombia based on the available information on confirmed roosting areas used by the species and the potential risks defined by the Human Footprint Index (HFI, Correa Ayram et al., 2020). The delineation of these areas will serve as a valuable decision-making tool, providing guidelines for better prioritization of Andean Condor conservation efforts and effective mitigation of population threats at the landscape scale.
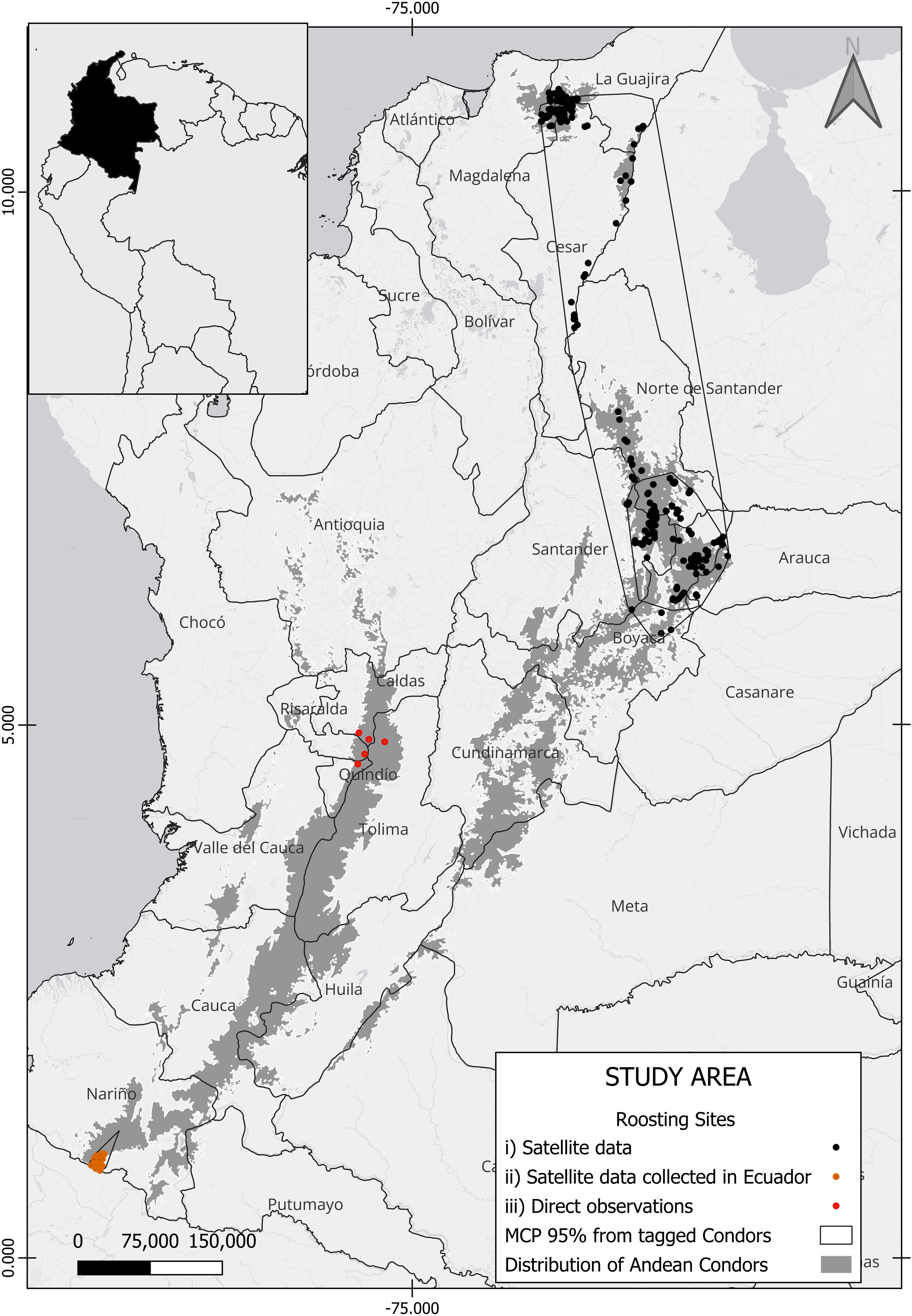
Material and methodsStudy AreaThe study was carried out within the historical distribution of the Andean Condor in the Colombian Andes (Rodríguez et al., 2006) in an area located between 1800 and 5500 m asl. The study area was defined according to the most up-to-date data on the presence and distribution of Andean Condors in Colombia (Sáenz-Jiménez, 2020), and comprises an area of 83,808 km2.
We gathered roost site data from three sources: (i) satellite data from two tagged wild condors, a non-breeding adult female and an immature male, tracked in north-eastern Colombia between 2019 and 2021 using Geotrack 65 G Solar PTT trackers (eight GPS fixes/day); (ii) data collected from three condors tagged between 2014–2019 in Ecuador, comprising one juvenile male and two subadult females, using microwave telemetry satellite trackers (PTT-100 50 gram solar patagial tags) programmed to provide one fix per hour from 05:00 to 19:00 local Ecuador time (GMT-5); and iii) direct observations at communal and occasional roosts, and one nesting site, between 2014 and 2021 across different parts of the Colombian Andes (Fig. 1).
Study Area. Roosting sites identified, dots in colours represent the origin of the data; polygons correspond to Minimal convex polygons for tagged wild condors. The Gray area represents potential Andean condor distribution (Sáenz-Jiménez, 2020).
Roosting condor locations were identified using the satellite tracking data collected between sunset and sunrise (18:00 to 05:00 h), when condors are less active and birds with movement speeds of zero knots could be assumed to be resting (Perrig et al., 2020). Roost sites in areas without satellite tracking data were confirmed by observation between 18:00 and 05:00 h (Lambertucci and Ruggiero, 2013). GPS errors potentially resulting from multiple closely spaced roosting locations were eliminated by aggregating all observations within a 100 m radius and assuming that they corresponded to the same roost sites. We identified the roosting sites using Package tidyr in R (Wickham and Girlich, 2022) and assumed that all the identified roosting sites had the same importance.
Selection of predictive variablesWe explored climatic and geomorphological variables to identify those that could explain roosting site selection (data for all records are shown at doi: 10.17632/trgd5tnwxp.1). The climatic variables selected were expressed at a spatial resolution of 50 m and included wind speed, air density (air mass per unit volume) (Badger et al., 2015), and solar radiation on inclined surfaces (Solargis, World Bank Group, 2019) all of which are known to influence the flight and soaring capabilities of condors (H.J. Williams et al., 2020), or to provide protection against extreme weather conditions (Lambertucci and Ruggiero, 2013).
In addition, six geomorphological variables were included at a spatial resolution of 90 m, including roughness (topographic complexity), convergence (dissected terrain with valleys and ridges), elevation, degree of northerly orientation, degree of easterly orientation (measures of orientation combined with slope to the north or to the east), and slope (rate of change of elevation) (Amatulli et al., 2020). These variables are associated with cliff structures (Amatulli et al., 2020), which provide condors with refuge from predators and adverse weather conditions (Lambertucci and Ruggiero, 2013) (SI 1). All variables with a spatial resolution less than 90 m were resampled to 90 m using the resample function with bilinear interpolation method in the Package raster of R (Hijmans et al., 2023). Data were collected in areas within the extent of the potential distribution of condors in Colombia.
Distribution patterns of potential roosting sitesWe analysed roosting sites used by condors (denoted 1) and 500 random points (denoted 0) located between 2000 and 5500 m asl within the potential condor distribution in Colombia and randomized the data using the resample function with Package caret in R (Wickham, 2017). We tested for correlations among all variables and excluded those that were highly correlated (>0.7) (SI 2) (Hosmer and Lemeshow, 2000). All variables were standardized to mean zero and unit variance. We analysed the potential influence of variables using the Generalized Linear Model (GLM) approach based on the Binomial Logistic family and logit link to relate the species presence-absence, using the ‘glm’ function using Package stats in R (Venables and Ripley, 2002) and generated 93 models, including all the potential variable combinations without interactions.
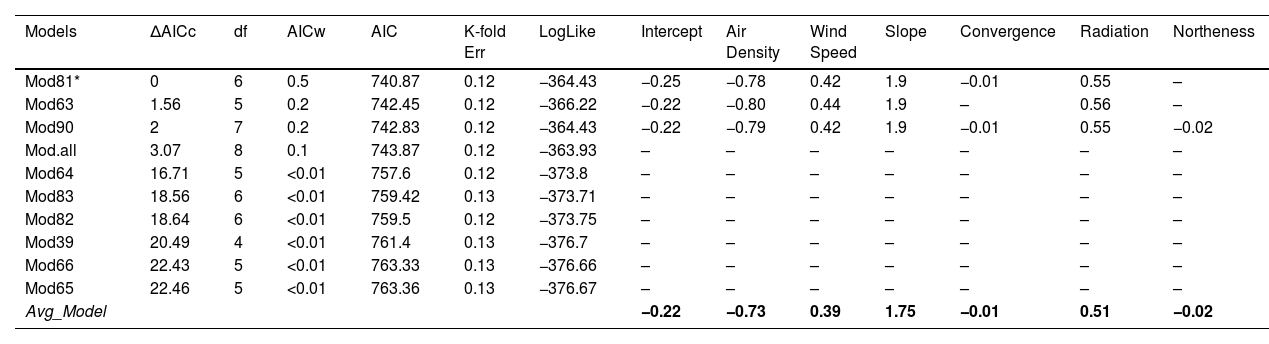
We used the Akaike information criterion (AIC) corrected for small samples, Delta AIC, and AIC weight (AICw) values using the bbml R package (Bolker et al., 2009) to choose the most parsimonious models based on delta AIC values (ΔAICc < 2) as the best-performing models for downstream analyses (Table 1). Where a predictive model was identified as competitive, we averaged the selected models using AICw and estimated the weighted regression coefficient values of each variable (Table 1) (Imam and Kushwaha, 2013). To validate the most competitive models, we used K-fold cross-validation with a training set and data test set (80%–20%), repeated in 10 cross-validation runs (Yates et al., 2023), and calculated the prediction error for GLMs using R Package boot (Canty and Ripley, 2022). After identifying and averaging a group of models, we extrapolated the model values, considering the coefficient of predictor variables, the AICw, and the Intercept (Table 1), using the raster calculator in QGIS 3.16.16 to generate a predictive map of suitable roosting areas for Andean Condors within their potential distribution in Colombia.
Summary of the Generalized Linear Model (GLM). The table shows the first ten models obtained, along with their Akaike delta value (ΔAICc), Akaike value (AIC), degrees of freedom (df), weight, log-likelihood (LogLike), and prediction error for K-fold Cross-validation (K-fold err). The combined value of the variables corresponding to each model are shown in the variables row. *Indicates the best competitive models.
| Models | ΔAICc | df | AICw | AIC | K-fold Err | LogLike | Intercept | Air Density | Wind Speed | Slope | Convergence | Radiation | Northeness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mod81* | 0 | 6 | 0.5 | 740.87 | 0.12 | −364.43 | −0.25 | −0.78 | 0.42 | 1.9 | −0.01 | 0.55 | – |
| Mod63 | 1.56 | 5 | 0.2 | 742.45 | 0.12 | −366.22 | −0.22 | −0.80 | 0.44 | 1.9 | – | 0.56 | – |
| Mod90 | 2 | 7 | 0.2 | 742.83 | 0.12 | −364.43 | −0.22 | −0.79 | 0.42 | 1.9 | −0.01 | 0.55 | −0.02 |
| Mod.all | 3.07 | 8 | 0.1 | 743.87 | 0.12 | −363.93 | – | – | – | – | – | – | – |
| Mod64 | 16.71 | 5 | <0.01 | 757.6 | 0.12 | −373.8 | – | – | – | – | – | – | – |
| Mod83 | 18.56 | 6 | <0.01 | 759.42 | 0.13 | −373.71 | – | – | – | – | – | – | – |
| Mod82 | 18.64 | 6 | <0.01 | 759.5 | 0.12 | −373.75 | – | – | – | – | – | – | – |
| Mod39 | 20.49 | 4 | <0.01 | 761.4 | 0.13 | −376.7 | – | – | – | – | – | – | – |
| Mod66 | 22.43 | 5 | <0.01 | 763.33 | 0.13 | −376.66 | – | – | – | – | – | – | – |
| Mod65 | 22.46 | 5 | <0.01 | 763.36 | 0.13 | −376.67 | – | – | – | – | – | – | – |
| Avg_Model | −0.22 | −0.73 | 0.39 | 1.75 | −0.01 | 0.51 | −0.02 |
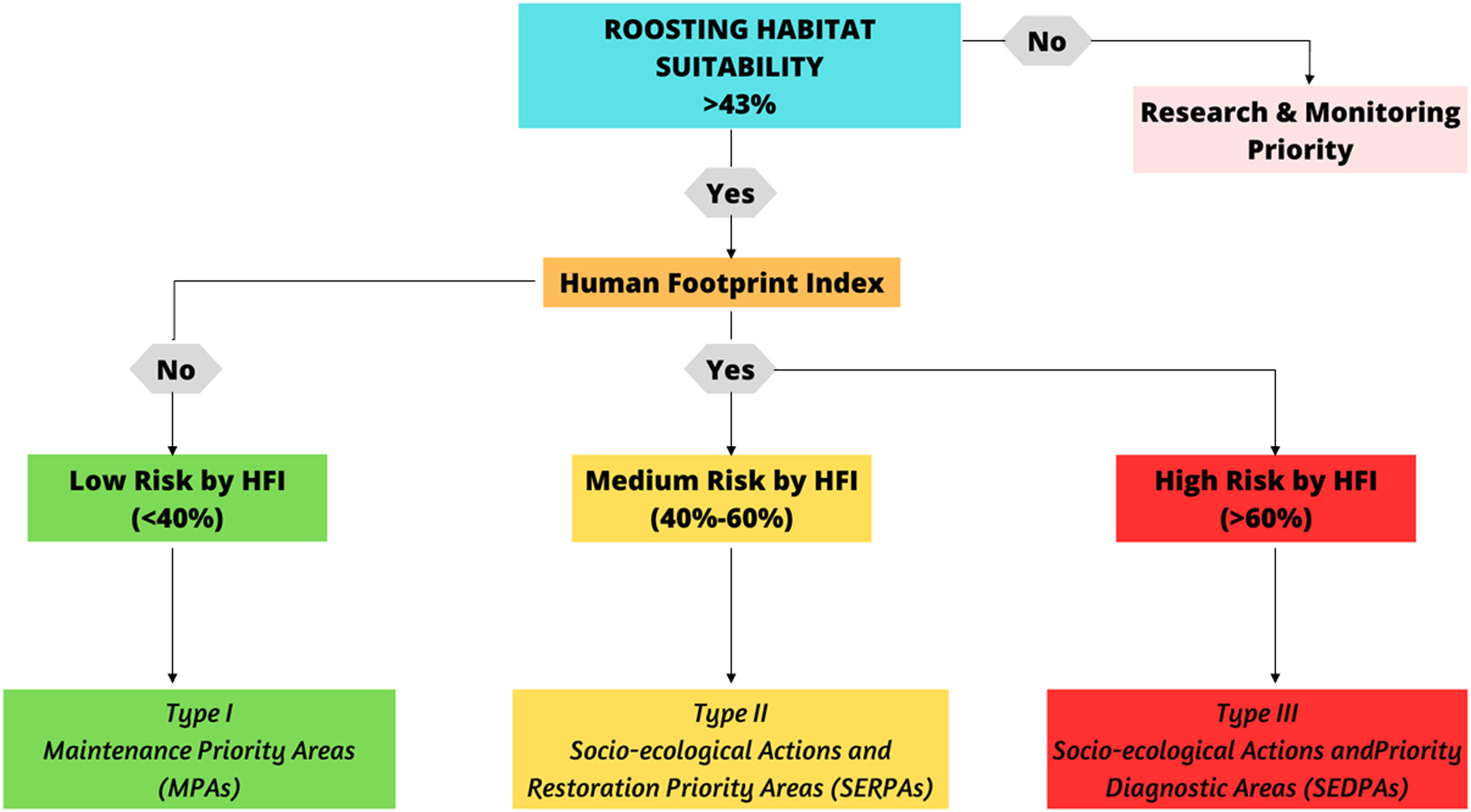
To define priority conservation roosting sites, we first selected areas with values equal or high to 43% of suitability as areas of medium and high suitability for roosting (third quartile of the data) (Allen et al., 2023; De Kerckhove, 2008). Then, we overlapped these areas with the HFI identified by Correa Ayram et al. (2020) using QGis 3.16.16, with a resolution of 300 m, as a proxy of landscape anthropization, such as human population density, land use intensity, and other anthropogenic ecosystem impacts (Correa Ayram et al., 2020).
Given the lack of spatially explicit information regarding direct threats to condors in Colombia, the HFI can be estimated either directly or indirectly from known anthropogenic impacts (Plaza and Lambertucci, 2020). While the HFI is a continuous index that responds to underlying spatial processes, Correa Ayram et al. (2020) defined areas with high anthropogenic impacts as those with an index >40%. We adopted a discrete division of the HFI into three categories: low (<40%), medium (40%–60%) and high (>60%).
Considering the decision tree proposed (Fig. 2), We suggested three types of PCA: (i) Type I Maintenance Priority Areas (MPAs), defined as suitable areas with only natural threats or minimal threats according to their HFI (Correa Ayram et al., 2020); (ii) Type II Socio-ecological Actions and Restoration Priority Areas (SERPAs), defined as areas with medium anthropogenic pressure and suitable roosting sites; and (iii) Type III Socio-ecological Actions and Priority Diagnostic Areas (SEDPAs) (Fig.2), defined as areas with high anthropogenic pressure and suitable roosting sites, probably very close to urban centres or major cities. This final mapping was done using QGis 3.16.16 (Fig. 3). Using the defined PCAs, we proposed a roadmap for the best conservation actions in each PCA type (SI 4), considering the particular threats affecting condors in Colombia (Restrepo-Cardona et al., 2022) and South America (Plaza and Lambertucci, 2020), and more generally for other vulture species elsewhere in the world (Botha et al., 2017).
Proposed Decision Tree for Identifying Actions in Priority Conservation Areas (PCAs) for Andean Condor Conservation. The figure shows a decision tree outlining strategies for Andean Condor conservation within various PCA types. The primary focus is on addressing the intersection of suitable roosting habitats and their Human Footprint Indexes (HFIs), which pose conservation risks to the species. Green areas signify proposed actions designed for Type I PCAs, Yellow areas indicate strategies tailored for Type II PCAs, and Red areas represent approaches for Type III PCAs. The priority decisions highlighted in this figure serve as guidelines for potential actions to be undertaken for the conservation of Andean Condors across various PCAs in Colombia.
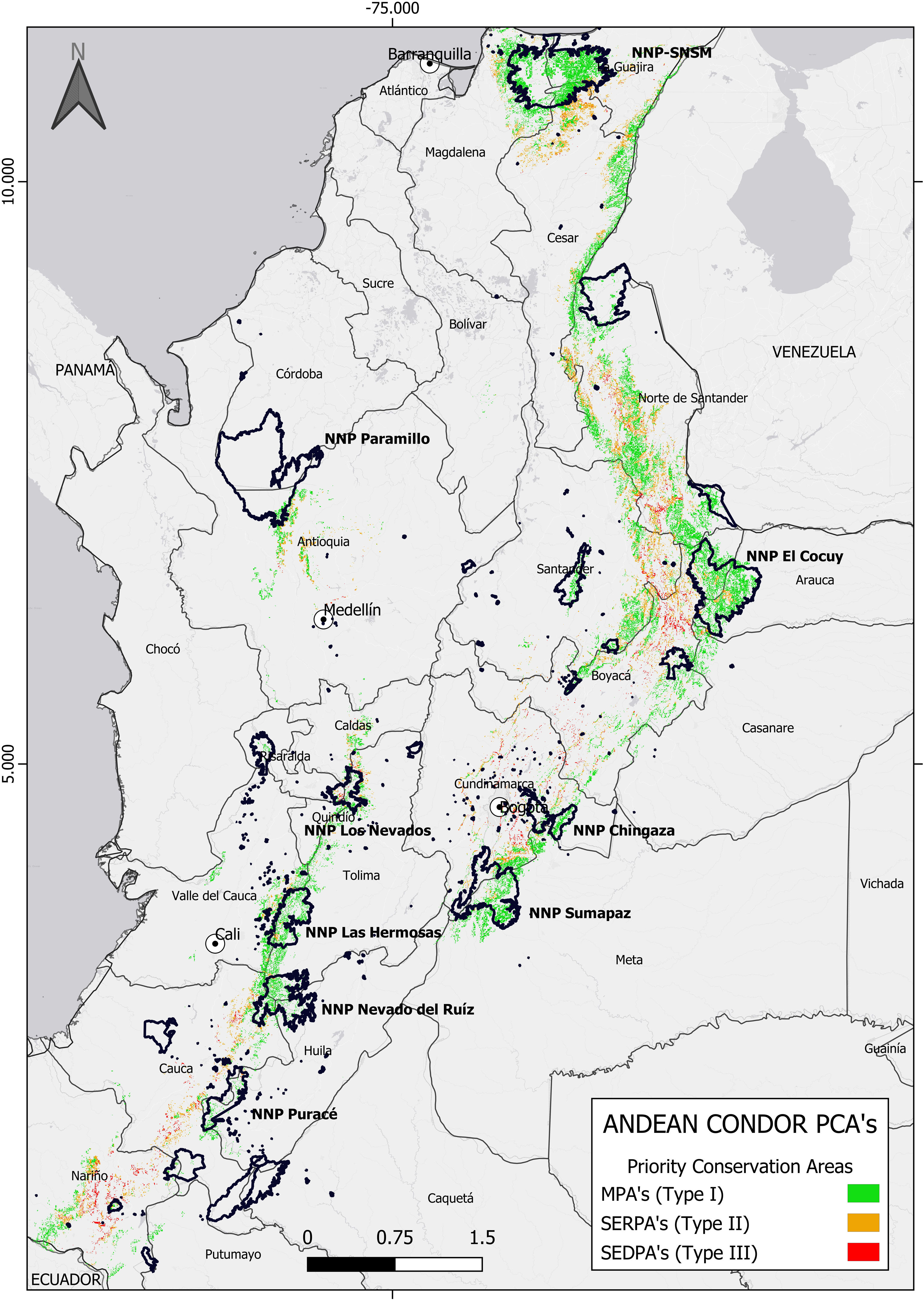
Priority Conservation Areas (PCAs) for Andean Condor Conservation in Colombia. Colours represent priority areas at the landscape scale. Green: low-risk areas with a high probability of roost selection >43% (Type I PCAs); Yellow: areas with medium-risks for conservation and a high-probability of roost selection (>43%) and areas between 40–60% of anthropogenic pressure overlap (Type II PCAs); Red: high-risk areas for Andean Condor conservation where there is a high probability of roost selection (>43%) and high anthropogenic pressure (>60%) (Type III PCAs).
The satellite data recorded 4640 GPS locations, leading to the identification of 461 roost sites in Colombia. Ten of these sites were verified through direct observations and the detailed data for all records are available at doi: 10.17632/trgd5tnwxp.1. Our best model indicated that roost sites were predominantly selected on cliffs with low air density, high wind speed, high radiation, and high slope, in areas such as ridges or cliff ledges, and south-facing (Table 1 and SI 3). The probability distribution of roosting site selection in the three categories was: Low, <40%; Medium, 40%–60%; High, 61%–80%; and Very High, >80%. The resulting map of potential roosts within the potential distribution of the Andean Condor in Colombia is shown in SI 5. The third quartile data (>43%) represents suitable roosting areas for the Andean Condor and, therefore, we only considered areas above this threshold as PCAs.
Priority conservation areas for the Andean Condor in ColombiaThe PCAs for Andean Condor conservation in Colombia covered an area of 19,571.34 km2, which represents 23.35% of the study area. Of this area, 5628.25 km2 (29%) are currently included in National Natural Parks, and of this: 13,715.58 km2 (70%) correspond to Type I MPAs; 4757.59 km2 (24.3%) are Type II SERPAs; and 1098.16 km2 (5.6%) are Type III SEDPAs (Figs. 2 and 3).
Most of the Type I PCAs were in northern Andean region and the National Natural Park Sierra Nevada de Santa Marta (NNP-SNSM). In contrast, Type II and III areas were primarily located outside the NNP-SNSM and the north-eastern Andean region of Colombia (Fig. 3). However, the paramo corridor in the eastern Andes Mountain range, spanning the departments of Cundinamarca, Boyacá, Santander, and Norte de Santander, serves as a critical region with the highest concentration of all PCA types. Our spatial model highlighted the fact that the central and southern regions of the Colombian Andes offer fewer suitable resting and refuge habitats and are characterized by a high HFI. Consequently, these areas have fewer Type I PCAs and a higher prevalence of Type II and III PCAs due to elevated levels of human impact (Fig. 3). Our prioritization exercise enabled us to propose a roadmap with recommendations for conservation actions within each PCA type, addressing potential threats across the territory (SI 4).
DiscussionOur study provides baseline ecological and spatial information and a systematic planning tool for designing and implementing conservation strategies for the Andean Condor in Colombia. These are key for decision-making and action to reduce the extinction risk for this species and represent one of the first systematic approaches to the selection of critical areas for this Critically Endangered species in Colombia.
We found that south-facing cliffs on high slopes located on ridges and cliff faces with high solar radiation, low air densities, and high wind speeds are more likely to be selected for condor roosting sites (Table 1). Condors seem to choose these conditions to seek refuge from threats and adverse weather conditions (Lambertucci and Ruggiero, 2013), in accordance with previous findings in Colombia (Sáenz-Jiménez, 2020). Other birds of prey, including vultures such as the Griffon Vulture Gyps fulvus (Aresu et al., 2022) and the Bearded Vulture Gypaetus barbatus (Margalida et al., 2008), and other raptors such as Bonelli's Eagle (Hieraaetus fasciatus) (López-López et al., 2006), also select resting sites based on these criteria. Similarly, Peregrine Falcons Falco peregrinus (Wightman and Fuller, 2005) exhibit a preference for high cliffs or steep slopes, which provide favourable conditions for thermoregulation, energy conservation during flight, and refuge from terrestrial predators (Aresu et al., 2022).
Systematic conservation planning tools require ecological and biological knowledge at various scales to design informed, species-specific strategies, resulting in more cost-efficient species conservation over time (Nandintsetseg et al., 2019; Nori et al., 2020). In Colombia, technical and financial efforts have been invested in Andean Condor conservation for over 30 years, including public policies, localized conservation programs, (Rodríguez et al., 2006), and reintroductions of captive-bred individuals (Sáenz-Jiménez, 2020). However, the repopulation nuclei defined for the species between 1989 and 2013 (Sáenz-Jiménez, 2020) do not coincide with the most suitable areas and many threats, such as poisoning, shooting, and power lines, that have caused population declines and impacted wild and reintroduced individuals (Restrepo-Cardona et al., 2022). These factors and the limited information regarding threats and their distribution, have resulted in ineffective conservation strategies (Carwardine et al., 2008; Buechley et al., 2019; Santangeli et al., 2022).
Understanding the conservation and magnitude of the threats faced by condors at different scales in the PCAs, and the magnitude of the pressures they face (Wallace et al., 2021, 2022), will enable to focus conservation action at the landscape scale in both the medium and long term (Guerrero et al., 2013), and will assist in generating Systematic Conservation Planning Tool (Gordon et al., 2011).
Identifying Priority Conservation Areas for the Andean Condor in ColombiaOur results indicate that the 19,570 km2 which could be considered for PCA status (PCAs), represents only 23% of the potential distribution of this species in Colombia (Sáenz-Jiménez, 2020). This limitation may stem from our focusing solely on roosting areas, neglecting suitable feeding and flight areas (Perrig et al., 2020). Of the potential PCAs, only 29% are situated within the national protected areas system (Fig. 3), constituting only a small fraction of the overall PCAs for the species in Colombia. This is consistent with previous studies and emphasises the necessity for concerted conservation strategies on private lands owned by ranchers, farmers, and local communities (Sáenz-Jiménez, 2020). A similarly low representation of PCAs in protected areas has been reported in Ecuador (Naveda-Rodríguez et al., 2016) and the southern distribution of condors in Argentina (Perrig et al., 2020; Plaza and Lambertucci, 2020). This situation represents a significant challenge for condor conservation, especially considering the importance of well-chosen protected areas critical for biodiversity conservation (Tittensor et al., 2014).
Previous studies have found that anthropogenic pressures clearly influence the presence of condors (Lambertucci et al., 2009; Lambertucci and Ruggiero, 2013; Perrig et al., 2020). The HFI in Colombia has increased from 1979 to 2015 and caused considerable loss of natural areas, resulting in many ecosystems becoming vulnerable (Correa Ayram et al., 2020). These conditions are more pronounced in the geographically and climatically diverse Colombian Caribbean and Andean regions because of human population growth and urban expansion and the resulting increase in land devoted to providing food security for human communities (Etter and Wyngaarden, 2016). This increasing human footprint may have caused the Andean Condor decline in the Colombian Andes (Rodríguez et al., 2006). Because of the local population extinctions of condors in Colombia, a reintroduction program was developed, with 71 individuals bring released between 1989 and 2013 (Sáenz-Jiménez, 2020). However, only 55% of the condors released survived to 2010 (Sáenz-Jiménez, 2020) and many died from anthropogenic causes (Restrepo-Cardona et al., 2022). This high mortality rate could be due to poor planning and a lack of proper identification of suitable areas for condor reintroduction. Better spatially explicit models guiding reintroductions could reduce the risks due to human persecution and ensure that birds are released into more suitable habitats and have better survival chances (Coz and Young, 2020).
Our strategy for identifying PCAs for Andean Condors in Colombia centres on pinpointing suitable roosting sites in areas where the species is more vulnerable. Roosting sites in PCAs in Colombia serve as vital refuges (Lambertucci and Ruggiero, 2013), safeguarding against low genetic variability (Padró et al., 2020) and facilitating connectivity and gene flow between robust populations within Colombia and across the continent (Padró et al., 2023).
Our findings show that Type II and III PCAs in the central and southern Colombian Andes lie outside the national protected areas scheme and have medium and high HFIs (Fig. 3), which could explain the species’ decline in these localities (Renjifo et al., 2016). Conserving roosts in southern Colombia could facilitate gene flow between populations north and south of the equator, potentially reducing the genetic impoverishment observed in the country’s condors (Padró et al., 2023, 2020). The Type I PCAs in the north-eastern Andes are located in the NNP-SNSM, which is home to the largest wild Andean Condor population in Colombia (Rodríguez et al., 2006) and is under the protection of the Kogui, Wiwa, Arhuaco, and Kankuamo indigenous people. Multicultural and community actions to reduce the threats inside these territories should be considered (Prieto, 2014). Furthermore, satellite tracking data has shown that condors move between the NNP-SNSM and the NNP El Cocuy, using a dispersal corridor of paramo complexes throughout Boyacá, Santander, Norte de Santander, and Cesar, where over 70% of the human persecution events for Andean Condor have been reported (Restrepo-Cardona et al., 2022).
To avoid misguided conservation actions, the definition of PCAs should vary based on the measures to be implemented in them (Carwardine et al., 2008). For this reason, we developed a roadmap with strategies for maintaining or improving the conditions in the various PCA types (SI 4). Suitable roosting areas with low HFIs were termed Type I MPAs, where action should prioritize to maintain viable natural low-risk conditions. Type II SERPAs, where socio-ecological and community programs are essential, especially on private land, and Type III SEDPAs (Fig. 2), should focus efforts on assessing population and habitat status to determine the viability of direct technical and financial condor conservation (refer to Fig. 3, SI 4).
While our study has provided valuable insights into the conservation of the Andean Condor in Colombia, it is important to recognize certain limitations inherent in our approach. One notable limitation is our exclusive focus on roosting sites, which may not encompass the entirety of suitable condor habitats (Frans et al., 2018). Our analysis did not include supplementary feeding sites, or take account of the fact that, in common with most obligate avian scavengers (Moreno-Opo et al., 2015; Delgado-González et al., 2022), Andean Condors can cover large distances in their foraging trips, selecting high quality food patches and predictable food resources such as feeding sites (Perrig et al., 2020). In this context, it is essential to reiterate that while very little is known regarding the movement ecology of the Andean Condor in Colombia, this study was based on the most complete dataset available. The data in this set clustered within isolated patches in the northern part of Colombia, potentially introducing bias into our model. This clustering phenomenon has also been observed in studies of species with low population densities confined to specific habitat patches, and any bias may intensify in habitats which decline in quality due to human intervention (Greene and Stamps, 2001).
Future research endeavours should investigate additional facets of the species' habitat and behaviour, including foraging and nesting sites, to obtain a more comprehensive understanding of its conservation requirements (Frans et al., 2018; Perrig et al., 2020). Consequently, we urge international collaboration to consolidate data gathered throughout South America pertaining to all areas suitable for Andean Condors. Such a collaborative effort is essential to predict Andean Condor PCAs across the whole of South America and to develop a continent-wide spatial decision-making roadmap for the implementation of conservation actions throughout the geographical distribution of the species (Jahn et al., 2017; H.J. Williams et al., 2020).
While our model effectively identified a combination of geomorphological and climatic variables related to roost selection, the exploration of ecological and biological factors remains largely uncharted. For instance, information on food availability, microclimatic variables, and other biological factors could significantly enrich the model's predictive power, because these variables influence population distribution and dynamics (Perrig et al., 2020). Despite the large information gaps, we identified condor PCAs in Colombia for the first time, and proposed conservation actions appropriate to the conditions in each PCA type, which will serve as both regional and national decision-making tools.
Conflict of interestThe authors declare no conflict of interests.
Funding sourcesThis work corresponds to the results of the first author's master's research, and was supported by The Peregrine Fund, [Grant number TPF-COL-1- FY21-FY22); Alejandro Ángel Escobar Fund [Grant Colombia Biodiversa number 2020-I) and Neotropical Ornithological Society [Grant Francoise Vieullimer number, FFV 2020).
We acknowledge The Peregrine Fund and GeoTrak Inc (Keith LeSage) for the donation of satellite trackers and technical advice in obtaining the data on the movement ecology for the south and north of the Colombian Andes. We also thank Juan Sebastián Restrepo for providing the Condor roosting sites monitored in the Central Andes region and Francisco Ciri for taking this research into account in the initial design of the update of the Andean Condor Conservation Program in Colombia 2021-2035.