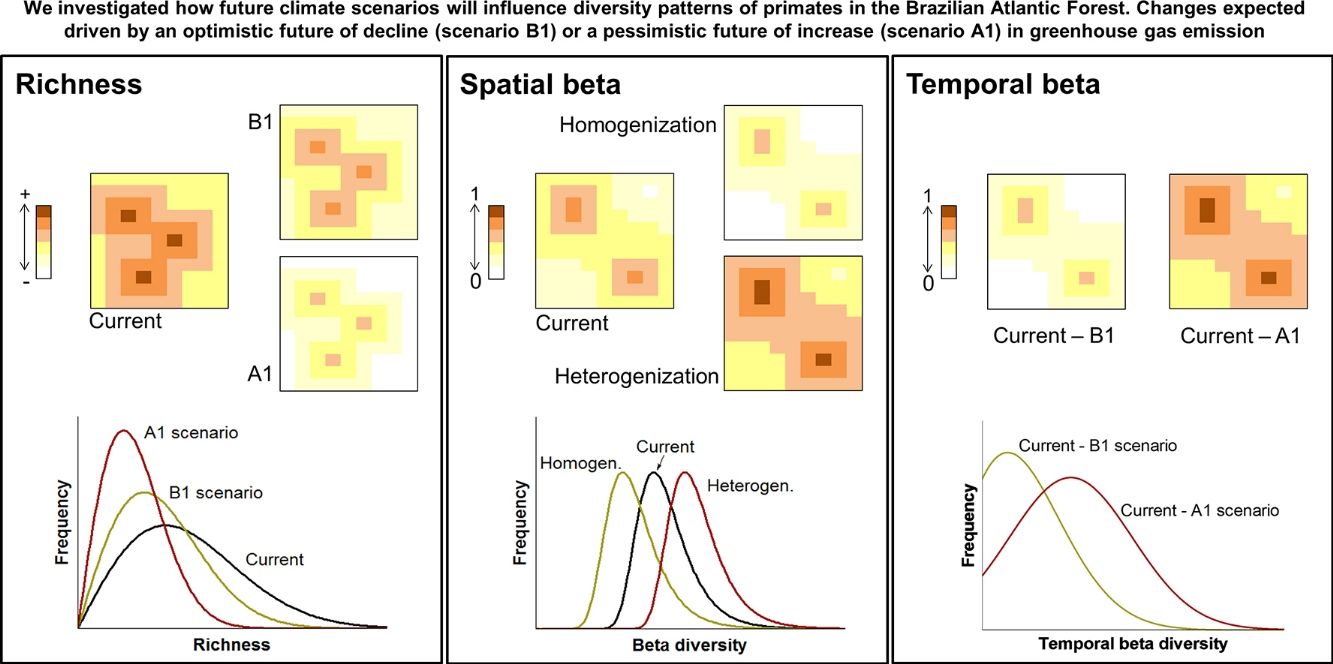
Changes in Earth's climatic conditions may affect the geographic distributions of species causing variations in diversity patterns through space and time. Projections of species distributions over time can measure how future climate scenarios will affect communities. We investigated how climate changes scenarios will influence primate biodiversity patterns in the Atlantic Forest. Specifically: (i) where are the largest changes in species richness expected? (ii) the spatial pattern of beta diversity will undergo homogenisation or heterogenisation? and (iii) where will occur the major changes in temporal beta diversity? We generated current and future species distribution models for Brazilian Atlantic Forest primates. We analysed the changes in the spatio-temporal patterns of alpha and beta diversity. Current high richness patterns will be maintained in future scenarios, with richer areas concentrated in the coastal zones. Regions closer to Cerrado will face a reduction in primate richness. Changes in richness will occur predominantly due to species loss. Communities will be more spatially heterogeneous in the future, with increased beta diversity. The heterogenisation may be driven by the reduction of species geographic distributions. The highest temporal changes will occur mainly in the midwest and the central region of the biome. Climate changes will cause primates diversity changes in both space and time. These changes will not be homogeneous through the Atlantic Forest. Our study is informative at a large spatial scale, providing an outlook on the impact of climate change on primate diversity, and indicating places of primates’ biodiversity maintenance, loss or gain due to climate change.
The increasing emission of greenhouse gases into the atmosphere resulting from human activities such as deforestation, pollution, and burning has accelerated and intensified changes in the climate on Earth (IPCC, 2014). Several climate change scenarios are projected for the future (IPCC, 2014). The most pessimistic scenario forecasts that changes in global precipitation and temperature will be mediated by the continuous increase in greenhouse gas emissions throughout the twenty-first century. In more optimistic scenarios, changes in anthropogenic activities would lead to the reduction in greenhouse gas emissions by 2100, minimising the harmful effects of climate change on life on Earth (IPCC, 2014).
Biodiversity responses to recent climate changes include alterations in phenological events, species abundance, biotic interaction, migration patterns, and species geographical distribution (Parmesan and Yohe, 2003; Pecl et al., 2017; IPCC, 2014). The speed at which climate changes will occur may threaten species survival. Historically, natural climate changes always shaped evolutionary processes, but over a more extended period. Climate changes may lead to species extinction (Waller et al., 2017), and species distribution displacement (Hickling et al., 2006), reduction (Virkkala et al., 2008) or expansion (Sales et al., 2017).
Alfa diversity measures the local diversity at a site, while beta diversity measures the variation of diversity among sites. We can refer to taxonomic diversity measured using presence/absence data, but diversity can be measured on functional or phylogenetic diversity and using proportional abundance or biomass data. Alfa diversity can be measured by species richness, and beta diversity measures the change in species composition among communities, which may occur by species loss or gain among sites (nestedness) and/or by species replacement among sites (turnover) (Baselga and Orme, 2012). Changes in species distributions due to climate changes may have consequences on diversity patterns (Araújo et al., 2004; Ochoa-Ochoa et al., 2012; Parmesan and Yohe, 2003; Tisseuil et al., 2012). Projections over time can measure how communities will be affected by future climate scenarios, allowing us to predict species composition modifications (Hillebrand et al., 2010) or whether future communities are more similar or diversified in a spatial perspective compared to current communities (Tisseuil et al., 2012). Species will be lost from or gained at some places if distributions are resized, or the composition may change by replacement if distributions are relocated, and these processes will be reflected in patterns of spatial and temporal beta diversity (Tisseuil et al., 2012). Changes in spatial diversity due to climate change can result in more homogeneous communities if average size of distributions increase as a result of extinctions or expansions of species distributions, or more heterogeneous communities by decreasing the average size of distributions of species, either by reducing the area or incursion of some species (Ochoa-Ochoa et al., 2012).
Primates inhabiting East Asia, Central America, Amazon and Atlantic Forest are more vulnerable to climate change (Graham et al., 2016). These two last tropical forests put Brazil in the spotlight among the world's priority countries for the conservation of primates in the world (Estrada et al., 2017). Among Brazilian primate species, 48% are in population decline and 39% are threatened (Estrada et al., 2018). The main threats to primates in Brazil are the loss and fragmentation of habitat, hunting for human consumption, collection for pets, predation by domestic animals, interference of exotic species, and climate change (Estrada et al., 2017; Gouveia et al., 2016).
Species distribution models can be used to evaluate changes in species distributions in response to climate change (Williams and Blois, 2018). These models use the relationship among predictor variables (generally climatic predictors at broader scales) and species occurrence to predict species potential distribution through space (Franklin, 2010), generating a habitat suitability surface. Models built with current environmental predictors can also be projected into future using climate scenarios (Williams and Blois, 2018), generally assuming the relationships of species with environmental variables will not change.
Here we used species distribution models to predict how future climate scenarios will influence spatial and temporal diversity patterns of primates in the Brazilian Atlantic Forest. The purposes of our study were: (1) To analyse changes in species richness, expecting differences among current and future scenarios (Fig. 1A); (2) To analyse changes in spatial beta diversity, investigating whether it will undergo homogenisation or heterogenisation (Fig. 1B) and; (3) to analyse temporal beta diversity, estimating changes in species composition from current time to the future, expecting higher changes for more pessimistic scenarios (Fig. 1C). We also take a brief look at changes in the distributions sizes once they partially explain the alterations in beta diversity.
Climate changes acting on diversity patterns. The current and future species richness (A), current and future spatial beta diversity (homogenisation and heterogenisation processes) (B) and temporal beta diversity (C). Changes in the patterns of diversity driven by two climate change scenarios: an optimistic future of decline (scenario B1) or a pessimistic future of increase (scenario A1) in greenhouse gas emission standards by the year 2100.
We compiled records of species occurrence and climatic data to generate current and future climate species distribution models for 25 species of Brazilian Atlantic Forest primates. From these models, we checked the patterns of spatial and temporal changes of diversity. See Supplementary Material for more information on study area (Fig. S1), geographic distribution and occurrence records (Table S1), environmental data (Table S2) and suitability models (Table S3 and Fig. S2).
Spatial and temporal primate diversityA grid with 0.5° latitude/longitude cells was superimposed on the Atlantic Forest distribution (see Fig. S1). Only grid cells with 25% or more Atlantic Forest area were included in the grid, summing 446 grid cells. The α-diversity (richness) for current and future scenarios was calculated as the number of species present in each grid cell. We also calculated the difference between future and current species richness (delta richness). We estimated β-diversity in space and time using the Sorensen similarity index. This index can be partitioned into the turnover (βsim) and nestedness (βsne) components (Baselga and Orme, 2012). Spatial β-diversity for each grid cell was quantified as the mean of β-diversity values between the focal cell and each of the 80 cells located up to the 4th order, that is, up to 200km away from the focal cell. Other studies have already shown that the window size to calculate beta diversity does not change the result qualitatively (Melo et al., 2009; Pinto-Ledezma et al., 2018). We calculated the distribution area of each species, in the current and each of the expected scenarios for the future, once enlargement or reduction in species distributions may explain possible changes in beta diversity. The temporal β-diversity quantifies the difference regarding species composition of a grid cell considering two different times. Then, temporal β-diversity was estimated by comparing, for the same cell, the current species composition with species composition in each scenario of the future.
We compared richness, spatial beta diversity and temporal beta diversity with Friedman's non-parametric test using scenarios (current, RCP 2.6, 4.5, 6.0 and 8.5) as the independent variable, and considering the grid cells as blocks. We also used Friedman's non-parametric test to test whether the range of species (response) is different among scenarios (current, RCP 2.6, 4.5, 6.0 and 8.5) (independent), inserting species as blocks. Friedman's non-parametric test was used in place of one-way ANOVA with blocks, once our data were not normally distributed. We used R software (R Core Team, 2017), the ‘betapart’ package and the ‘betagrid’ script (available at http://rfunctions.blogspot.com.br/2015/08/calculating-beta-diversity-on-grid.html; Melo et al., 2009) to calculate the beta diversity in a grid system. Friedman analysis generates maxT test statistics and p-values (script available at https://www.r-statistics.com/2010/02/post-hoc-analysis-for-friedmans-test-r-code/).
We performed the analysis of spatial and temporal patterns of diversity for all expected climate change scenarios (RCPs 2.6, 4.5, 6.0 and 8.5). However, we will present only the more optimistic and pessimistic scenarios (RCP 2.6; RCP 8.5) in “Results” section. The results for intermediate scenarios of climate changes are shown in Figs. S3–S7.
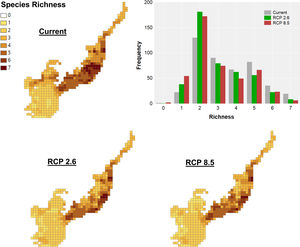
ResultsClimate changes will cause primate richness declineThe current primate richness in the Atlantic Forest is mainly concentrated in the central portion of the biome, in areas located in the south of Bahia, southeast of Minas Gerais, Espírito Santo, Rio de Janeiro and the coast of São Paulo. In general, this pattern will be maintained for future scenarios, with a higher concentration next to the coast. Currently, some areas closer to Cerrado also have high species richness, but projections for the future reveal these regions will have a reduction in the number of species (Fig. 2). Richness differed among scenarios (maxT=14.55, p<0.001, n=446, see Table S4) with higher values in the current scenario compared to the others. Also, the richness in the future RCP 2.6 scenario will be greater than in RCP 8.5.
Climate change acting on spatial patterns of species richness. Species richness of primates in the current, in the optimistic (RCP 2.6) and the pessimistic (RCP 8.5) scenarios of climate changes in the Brazilian Atlantic Forest. Histogram showing the frequency of cells with different richness values in the current and future scenarios.
Observing the delta richness, both optimistic and pessimistic scenarios will lose up to five species, while few grid cells will gain no more than one species (Fig. S4 RCP 2.6 and RCP 8.5, respectively). This gain occurred mainly in grid cells located in the centre and south of the biome. The greatest losses of species are expected in the enclosures near the Cerrado, southwest of São Paulo, at the mid-west and in the central region of the Atlantic Forest, southeast of Minas Gerais.
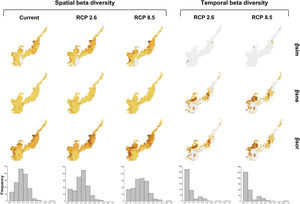
Spatial beta diversity changesNowadays, regions located in the interface with the Cerrado – specifically northeastern Minas Gerais, northwest of São Paulo, west of Paraná and west of Santa Catarina – are more heterogeneous than the grid cells closest to the coast. Besides that, grid cells close to the coast in the southern and northeastern regions of the biome presented lower spatial beta diversity (Fig. 3). This spatial beta diversity pattern will remain in the future, but values in the grid cells located in the central region of the biome will increase, suggesting these places will be more different in relation to surroundings, concerning species composition (Fig. 3). Also, western portions of the biome will have higher values of beta diversity in the more pessimistic scenario (Fig. 3). Many sites will be more heterogeneous in the future in the optimistic (RCP 2.6) and pessimistic scenarios (RCP 8.5) since the frequency of higher beta diversity values will slightly increase and of intermediate values will decrease (Fig. 3). Spatial beta diversity differed among scenarios (maxT=9.13; p<0.001; n=446, see Table S5), with current values smaller than any scenario in the future. However, the optimistic and pessimistic scenarios did not differ (see Table S5). The turnover of species was the component that contributed the most to promote the spatial beta diversity (Fig. 3, see also Fig. S5).
Climate change acting on spatial and temporal patterns of beta diversity. Spatial beta diversity of primates in the current, the optimistic (RCP 2.6) and the pessimistic (RCP 8.5) scenarios of climate change, and temporal beta diversity reflecting the change in primates species composition between current-optimistic (RCP 2.6) and current-pessimistic (RCP 8.5) scenarios of climate changes in the Brazilian Atlantic Forest. Beta diversity (βsor) is fractioned in turnover (βsim) and nestedness (βsne). Histograms represent the frequency values of βsor for each scenario.
We estimate species will exhibit a greater change in distribution area in the pessimistic scenario of climate change (see Fig. S6). Distribution areas will differ between the current and the most pessimistic scenario (maxT=3.85; p<0.01; n=25, see Table S6), being mostly smaller in the future.
Temporal beta diversity changesMany grid cells in the Atlantic Forest will have the composition of species changed in the future (Fig. 3). The highest changes will occur mainly in the midwest and the central region of the biome. Major changes of temporal beta diversity (current-future) will occur in the more pessimistic scenario which differed from all the other ones (maxT=3.49; p<0.001; n=446, see Table S7). Nestedness contributed most to promote the temporal beta diversity (Fig. 3, see also Fig. S7).
DiscussionSpecies richness reductionsClimate change will reduce Atlantic Forest primate richness over space and time. Currently, the richest areas in species are concentrated in the centre of the biome, and a displacement and richness concentration in the coastal areas is projected to the future. These areas at the Atlantic Forest coast are more vulnerable to climate changes, especially due to precipitation decrease and temperature rise (Graham et al., 2016). The west of the biome in the transition areas between the Atlantic Forest and south Cerrado will also suffer the greatest richness reductions. It is expected that these regions will experience changes in the precipitation regime, with less precipitation in the future (Graham et al., 2016). Ecotones are highly dynamic and are susceptible to a strong influence of climate change (Noble, 1993). In Atlantic Forest, amphibians will also experience richness reductions due to climate changes (Lemes et al., 2014; Loyola et al., 2013). Primate species considered in this study have at least 3 million hectares or more than 50% of its distribution in the Atlantic Forest, which reduces chances that species mainly from other biomes bias the results. Climate changes can influence the composition of primate species directly through physiological tolerances of organisms related to climate (Mandl et al., 2018; Muñoz-Delgado et al., 2004), and indirectly through changes in forest cover or resource dynamics (Kamilar and Beaudrot, 2018).
Spatial beta heterogenisationSpecies richness reduction will modify community composition, and consequently spatial beta diversity. The reduction in species distribution area may be related to the increase of spatial beta diversity. This supports the heterogeneity hypotheses, which states that the reductions in the area of distributions and/or local extinction promote heterogenisation (Ochoa-Ochoa et al., 2012). The higher spatial change in species may be explained by high local extinction rates directed by the climate (Lewthwaite et al., 2017). Some Atlantic Forest birds will also suffer range contraction (de Souza et al., 2011). Few primate species will show an increase in the distribution area. The redistributions influence the diversity changes, but the increase in distributions of a few species does not really influence spatial patterns. Our results seem to be dominated by reductions in distributions. Spatial shifts in distributions may also be important (Williams and Blois, 2018), but were not evaluated in this study. The species redistributions also exert influence on the global change drivers (Pecl et al., 2017).
Temporal beta diversityThe highest changes in primate species composition over time are predicted to occur mainly in the central Atlantic Forest and the western of the biome, near the Cerrado, once they have higher temporal beta diversity. The areas with high temporal beta diversity were congruent with those with high delta richness, which reinforces the greater importance of nestedness in temporal beta diversity composition.
Changes in community composition may have important ecological consequences for ecosystems functioning (Barbet-Massin and Jetz, 2015; Pecl et al., 2017). Primates play different ecological interactions, being preys, predators or mutualists in trophic networks (Estrada et al., 2017). Moreover, primates also play important ecosystem functions, such as seed dispersal, plant distribution, nutrient flow, gene flow of plant species (Bueno et al., 2013). Beyond that, reducing interactions among species may compromise ecosystem services, as the system becomes more streamlined and less resilient (Barnes et al., 2018; Brancalion et al., 2018). Changes in primate beta diversity will modify the functional structure of the community. Atlantic Forest sites with higher richness, such as those concentrated in the central portion of the biome, may lose species and still retain the ecosystem functions played by primates, due to functional redundancy. However, in places with low richness, such as the extreme north or the southern region of Atlantic Forest, the loss of a single species can result in loss of important functions, compromising ecosystem functioning. An evaluation of functional diversity patterns may provide more useful insights on the loss of ecosystem services due to climate change.
We used climatic variables as predictors of the occurrence of species, without considering other factors that may also influence their distributions, such as biotic interactions (Leach et al., 2016) and limitations of dispersion (Pearson and Dawson, 2003; Schloss et al., 2012). However, our diversity analyses were performed to the known extent of occurrence of each species, considering only the suitability surfaces within these extensions. This indirectly includes specialists’ knowledge on distribution limitation of these species such as, for example, elevation limits (IUCN, 2018). Beyond that climatic factors should act synergistically with other sources of threats to primates.
Spatio-temporal perspective on species conservationUnderstanding how patterns of diversity will be influenced by changes in climate in a spatio-temporal approach is fundamental to guiding conservation strategies. Such patterns allow us to identify, for example, areas with high species richness both now and in the future, and places where major losses will take place. Additionally, for diversity representation, sites with high beta diversity are important because they are very different in species composition from their neighbours. Although they have low richness, they may contain endemic species or of restricted distribution. When spatial beta diversity is dominated by turnover, this implies that several sites contribute equally to regional diversity, and most of them are important conservation targets. Otherwise, when it is dominated by nestedness, it means few sites that increasingly hold a greater proportion of regional species should be prioritized (Angeler, 2013).
The results of this study help to understand how climate changes influence the diversity patterns in time and space through changes in species geographic distributions, and provide an outlook at a large spatial scale on the impact of climate change on the diversity of primates Brazilian Atlantic Forest. Our approach can be easily used in other biomes around the world to better understand the consequences of climate changes on diversity in time and space.
Conflicts of interestNone declared.
This research was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) scholarship (AAL). MCR is funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (process #2013/50421-2), is supported by fellowship Programa Nacional de Cooperação Acadêmica (Procad/CAPES) (project #88881.068425/2014-01) and receives research grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (processes #312045/2013-1; #312292/2016-3). CEVG is supported by grant from CNPq and by National Institute of Science and Technology (INCT) in Ecology, Evolution and Biodiversity Conservation (MCTIC/CNPq/FAPEG/465610/2014-5). The funding sources had no role in the design, collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the article for publication. We also thanks Leandro Jerusalinsky and Sidney Feitosa Gouveia for previous comments on dissertation version, and two anonymous reviewers for comments and suggestions that really improved the manuscript.