Anthropogenic climate change has been shown to be one of the most pervasive threats to biodiversity. However, few studies have considered its effects on whole communities. Here, using ecological niche models (ENM) and projected future climate scenarios, we analyzed how these environmental changes could promote reductions in the alpha and beta taxonomic, phylogenetic, and functional diversities of mammals in the Cerrado Biodiversity Hotspot. We found that, on average, species richness tends to decrease in most Cerrado areas under future climate scenarios. However, this pattern is not uniform throughout the biome. Overall, southern Brazilian Cerrado may become biotically homogenized – through the extinction of native specialists and expansion of exotic generalists – in the near future, while the rest of biome may become very heterogeneous in taxonomic, phylogenetic and functional aspects. This scenario is very problematic considering that this region has been highly transformed and fragmented by human activities in the past. Based on our ENM approach of species inhabiting present Cerrado, we provided a more accurate analysis about the effects of anthropogenic and/or natural processes at large scales on the communities for this endangered Biodiversity Hotspot. This information could represent invaluable tool to guide future establishment of new and efficient conservation efforts.
Climate change has been shown to affect the distribution of species by determining where they will, or not, occur in response to their climatic tolerances (Lenoir and Svenning, 2014). There is a growing consensus that human activity largely contributed to recent climate changes occurring worldwide. Thus, the Intergovernmental Panel on Climate Change (IPCC) presents different future climate change scenarios depending on projected greenhouse gases concentrations in the atmosphere due to human activity (IPCC, 2014). In this sense, special attention should be given to the consequences of future climate changes to areas that have a high number of endemic species (i.e. Biodiversity Hotspots) and, especially, on those that are heavily threated (e.g. Collevatti et al., 2013; Prieto-Torres et al., 2016). Additionally, there is evidence that some regions will be more quickly affected by climate change than others. This is especially true to flat biomes, such as flooded grasslands and savannas, which will have very fast rates of temperature change due to their lack of topographic complexity (Loarie et al., 2009). Thus, understanding how species will respond to future climate changes in particular flat biomes such as the Brazilian Cerrado should be a priority for conservation biology.
Most studies on climate change usually focus on how populations or species were or will be affected through time (Walther, 2010). In this sense, it is already known that effects of climatic changes on organisms depend on to which biological group they belong, their habitats, and which geographical region they occur (Bellard et al., 2014). In fact, there are evidences that some species can even respond positively (for example, expanding their ranges) to the elevation in temperature or high CO2 concentrations that are expected in the coming years (e.g. Bellard et al., 2012; Prieto-Torres et al., 2016). However, considering that species respond individually to new conditions, it is mostly expected that future climate change will affect species richness worldwide by changing the spatial distribution of environmental conditions faster than species are able to adapt (Bellard et al., 2012; Thuiller et al., 2005; Xenopoulos et al., 2005). For example, plant species richness is likely to decline in most tropical regions in the coming years (Sommer et al., 2010; Zwiener et al., 2018). Considering this scenario, one could then expect communities from warm flat biomes to experience reductions in species richness due to the inability of species to track rapid changes in the spatial distribution of their preferable environmental conditions (e.g. Collevatti et al., 2013; Prieto-Torres et al., 2016).
Several studies have explicitly investigated how future climate changes will affect whole ecological communities (Albouy et al., 2012; Ihlow et al., 2012; Sommer et al., 2010; Thuiller et al., 2005; Walther, 2010; Xenopoulos et al., 2005), including compositional and structural changes in biomes throughout the world (Nolan et al., 2018). These studies showed that, besides reducing local species richness, one of the most pervasive effects of climate change is the decreasing on beta diversity of communities. This is important because they could result in the biotic homogenizing of fauna across entire regions (Clavel et al., 2010; Magurran et al., 2015) due to the extinction of specialist species (usually with small geographic distributions) and the expansion of those generalists (usually with large geographic distributions) (McKinney, 1997; McKinney and Lockwood, 1999). In fact, changes in temperature and moisture conditions can explain the decline in both plant richness and turnover (Thuiller et al., 2005). Also, there is evidence that mammal communities from continental and oceanic islands have been and will become homogenized through time (Longman et al., 2018).
This homogenizing process has negative consequences for the functioning of ecosystems and in the goods and services provided by them (Clavel et al., 2010; Olden et al., 2004). For instance, biotic homogenization can reduce the resilience of communities to environmental disturbances by preventing the colonization of species with locally extirpated traits (Olden et al., 2004). Nevertheless, and despite the relevance about this topic, there are still few studies analysing how climate change could promote the homogenization of communities at large scales, especially in dry and warm flat biomes. For this type of biomes, one could expect communities to experience increases in their compositional similarity due to extinctions of various specialists and narrowly-distributed species but at the same time the expansion of a few generalists, broadly-distributed species (e.g. Collevatti et al., 2013; Prieto-Torres et al., 2016; Siqueira and Peterson, 2003).
Here we project the current ecological conditions for the distributions of 309 mammals from the Brazilian Cerrado under distinct scenarios of climate change in the late 21st century, assessing how the future environmental changes could promote reductions in the alpha and beta diversities across the faunistic community of this endangered biome. It is important to consider that Brazilian Cerrado is a warm flat biome classified as a Biodiversity Hotspot (Mittermeier et al., 2005) and will probably face fast rates of temperature change in the coming years (Loarie et al., 2009). Therefore, specifically, we answer the following questions: (1) Are we going to lose Cerrado mammalian species, phylogenetic clades and functional diversity due to the expected changes in climate? (2) Are mammalian communities going to become more taxonomically, phylogenetically and functionally homogenized? (3) How and where are these changes going to happen throughout the Cerrado biome? and (4) How are the frequency/incidence of future regional extinctions and immigrations among threatened and non-threatened mammal species.
Material and methodsStudy areaThe Cerrado biome covers an area of approximately 2,000,000km2, about 25% of the Brazilian territory. The region is mostly covered by very heterogeneous vegetation (from grassland savannas to savanna-woodlands, as well as few patches of semideciduous forests, evergreen forests, wet grasslands and rupestrian fields). Overall, the Cerrado is under a humid tropical climate, with wet summer and dry winter, and annual rainfall and mean temperature lie around 1745mm and 24.6°C, respectively (Marini and Garcia, 2005). The topography of the Cerrado region varies from flat to smoothly undulating, favoring the practice of mechanized agriculture and irrigation. As a consequence it is considered to be one of the most threatened regions of the world mainly due to habitat destruction and fragmentation (Marini and Garcia, 2005).
Species occurrence data and ecological niche modelingThe first step was to create a complete checklist of mammal species ecologically associated to and inhabiting Brazilian Cerrado, which was compiled from overlapping for all non-volant mammas recorded in Brazil (from the IUCN database available on: http://www.iucnredlist.org/technical-documents/spatial-data) with the grid cells (size 0.25°×0.25°) in which was divided the study area. For our study, we only considered those species that had occurrence covering ≥50% of at least one grid cell (see Keil et al., 2012; Römermann et al., 2007). The final list (see Appendix S1) included 309 Brazilian mammals – including those species occurring in the present time (n=154 spp.) and those predicted to shift into the Cerrado in the future scenario herein analyzed (see below). For all these species we indicated their conservation status according to IUCN categories (IUCN, 2016) as: DD (“Data Deficient”), LC (“Least Concern”), NT (“Near Threatened”), VU (“Vulnerable”), EN (“Endangered”), and CR (“Critically Endangered”).
Because there are distinct algorithms to produce ecological niche models, which may result in different projections of species range shifts, the use of ensemble forecasting is often recommended. This approach combines the projections of distinct models using consensus techniques (Diniz-Filho et al., 2009). Therefore, for each species, we modeled habitat suitability in current and future climate scenarios based on an ensemble of four algorithms that are commonly used in ecological species modeling (Diniz-Filho et al., 2009; Marmion et al., 2008): Generalized Linear Model, Generalized Additive Model, Multivariate Adaptive Regression Splines, and Random Forest. The models were built using the BioEnsembles software (Diniz-Filho et al., 2009) using the default settings and parameters. To characterize the ecological niche of species we used four bioclimatic variables from WorldClim project 1.4 (available in http://worldclim.org/bioclim; Hijmans et al., 2005): annual mean temperature, annual precipitation, temperature seasonality, and precipitation seasonality; which are good determinants of mammal distribution (e.g. Herrando-Pérez et al., 2014). In addition, the potential distributions of mammal species for year 2070 were assessed under three different Representative Concentration Pathway scenarios, which represent diverse (low, medium, and high) future concentrations of greenhouse gases: RCP 2.6, RCP 6.0 and RCP 8.5. We performed the projection models considering four Global Circulation Models: (a) Community Climate System Model (CCSM), (b) Institute Pierre Simon Laplace (IPSL), (c) Model for Interdisciplinary Research on Climate (MIROC), and (d) Meteorological Research Institute (MRI).
To perform the models, the full dataset for each mammal species was randomly partitioned into two random subsets (calibration and evaluation) including 75% and 25% of the data, respectively. This last step was repeated 10 times to make sure that the evaluation procedure was independent of the random splitting procedure. Then, we used a TSS protocol to convert probabilities of occurrence into presences and absences (Allouche et al., 2006). In order to generate a consensus map for each species, we added all model outputs and calculated the relative number of times that species occurrence was predicted by each model in each cell. Considering that averaging across model outputs improve overall projections (Araújo et al., 2005), we used a congruence threshold higher than 0.5 to obtain a final presence/absence map for each species. It is important to note that for each species, we obtained 120 maps of potential distribution for the year 2070 (i.e., four algorithms×10 times×3 RPC scenarios). However, these maps were used, in the same way as in the maps of the present, to produce a consensus map (i.e. frequency of projection >0.5) by each RCP 291 scenario.
On the other hand, future projections were made assuming a limited dispersal scenario, which is a more likely scenario among mammals at the geographical extent of the study area (see Faleiro et al., 2013). This is important because using frequency of projections to generate occurrence maps can make species to occur, in the future, in grid cells more distant from where they could occur considering their biological dispersal capacities. Thus, we estimated the home range of each species based both on its weight and diet according to Kelt and Van Vuren (2001), and calculated its maximum dispersal distance according to Bowman et al. (2002). For this last step, we attributed maximum dispersal distances to the generation length (in days) of each species (Pacifici et al., 2013) and determined the maximum dispersal distance in 71 years (1999 [present scenario] to 2070 [future scenario]). In this sense, if a grid cell where a species occurs in the future was more distant from its nearest grid cell in the present than its maximum dispersal distance value estimated for 2070, the “potential presence” of species was replaced by an absence.
Phylogeny, biological traits, and threatened categoriesWe compiled data for ecological traits (Hidasi-Neto et al., 2015; Safi et al., 2011) and phylogenetic information (Bininda-Emonds et al., 2007; Fritz et al., 2009) for all mammals occurring or projected to occur in the Cerrado (total of 309 species; see Supplementary Material, Appendix S1 for species). The following traits were considered in the study: body mass (in grams), diet (vertebrates, invertebrates, foliage, stems and bark, grass, fruits, seed, flowers, nectar and pollen, roots and tubers; presence/absence), habit (aquatic, fossorial, ground dwelling, above-ground dwelling, aerial; presence/absence), and activity period (cathemeral, crepuscular, diurnal, nocturnal; presence/absence). These traits indicate how much, how, when, and what type of resources mammals use from the environment (Hidasi-Neto et al., 2015; Safi et al., 2011), and are closely related to energy flow through communities (Cardinale et al., 2012). In addition, we added 42 species that were previously absent in the phylogeny as polytomies in their respective genera (see Supplementary Data, Appendix S2). This last step was performed using “add.species.to.genus” function from “phytools” package (Revell, 2012; Supplementary Data, Appendix S1).
Alpha and beta diversity analysesUsing the species occurrence matrices of presence/absence of species for each grid conforming the Cerrado biome in the four climate scenarios evaluated (present and three RCP) we calculated alpha and beta taxonomic, phylogenetic, and functional diversities for mammal communities. First, we estimated the mean phylogenetic (MPD) and mean functional distance (MFD) among all pairs of species (Sobral et al., 2016). For each grid cell we calculated MPD using the cophenetic distances from the phylogeny of all Cerrado species, while the MFD was determinate using a distance matrix generated using Gower's distance representing the functional distances of mammals occurring in the Cerrado. Then, for each cell, we randomly generated 999 assemblages using the independent swap algorithm, maintaining the observed species richness and occurrence frequency in the null communities (Gotelli and Entsminger, 2001). With this step, we calculated the standardized effects sizes (Gotelli and Entsminger, 2001) of MPD and MFD to represent, respectively, the phylogenetic and functional structure (alpha) of Cerrado mammal assemblages. In this sense, positive values indicate that the phylogenetic (or functional) diversity are higher than expected by chance (overdispersion), while negative values indicate they are lower than expected by chance (clustering).
We calculated spatial taxonomic beta diversity as the mean turnover partition of Sorensen's index between a focal cell and its neighbors (up to eight cells) (Baselga et al., 2013; Baselga and Orme, 2012). We calculated spatial phylogenetic beta diversity using the same process to taxonomic beta, but using the turnover partition of PhyloSor index (Baselga and Orme, 2012). The same process was also applied to calculate functional beta diversity but using the Gower's distance and Unweighted Pair Group Method with Arithmatic Mean (UPGMA) method to generate a functional dendrogram. The functional dendrogram was used to calculate the mean PhyloSor between a focal cell and each of its neighbors. To calculate temporal beta diversities, we used presence/absence data to calculate the Sorensen's index (for taxonomic) and PhyloSor (for phylogenetic and functional beta diversity) indices between each grid cell from the present and its respective cell in the future. For all beta diversity calculations, we also calculated the proportion of “turnover/total beta diversity”, where “total beta diversity” is the sum of turnover and nestedness partitions. Turnover indicates how much of the change in beta diversity is related to the spatial or temporal differences in species compositions, while nestedness represents how much is related only to the loss or gain of species.
Between each future RCP and present scenarios, we calculated the values of differences between future and present values of taxonomic (Δrichness), spatial beta (Δspatial taxonomic beta, Δspatial phylogenetic beta, and Δspatial functional beta), and temporal beta (Δtemporal taxonomic beta, Δtemporal phylogenetic beta, and Δtemporal functional beta) diversities. For both spatial and temporal beta diversities we also calculated the differences between the future and present values of the proportions of turnover/total beta diversity, indicating how much of total beta diversity is related to turnover or nestedness in both the present and the future. We then generated maps for alpha, spatial beta and temporal beta diversities for the present and all the future RCP scenarios as well as for the differences observed among the future and present values for each diversity value. Additionally, we also compared the occurrences of species in the future and present scenarios to observe how regional extinctions and immigrations of species were related with species threat status (IUCN categories). As all future scenarios presented qualitatively similar results, here we only show and discuss results for the RCP 6.0 scenario (intermediate concentration of greenhouse gases); however interested readers can directly find the maps for other scenarios in Supplementary Data (Appendix S2). All calculations of diversity values were done in R software (R Development Core Team, 2018).
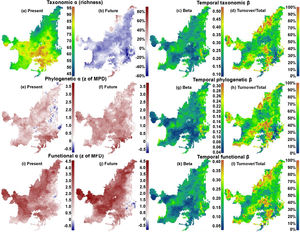
ResultsIn general, we found a reduction in mammal species richness in the future at the grid cell scale (mean of −37.231±15.732 species). This indicates that climate changes are likely to cause local extinctions of several mammalian species throughout the Cerrado biome (Fig. 1; Supplementary Material, Appendix S3). However, despite this average reduction on species richness, we observed heterogeneous patterns of phylogenetic overdispersion and clustering (mean of −0.374±4.59) across the Cerrado (Fig. 1e and f), and a general pattern of functional overdispersion (mean of 0.011±0.011; Fig. 1i and j). Such patterns indicate that even if the total phylogenetic or functional alpha diversity decreases in some areas, some of the regionally extinct species could have been phylogenetically or functionally similar to several species. After the removal of these phylogenetically or functionally redundant species, there could be an increase in the mean phylogenetic or functional distance among species.
On the left side, maps indicating the present values and the percentages of increase or decrease (future-present) in values of taxonomic alpha diversity (“a” and “b” for richness), and present and future values phylogenetic (“e” and “f” for z of MPD), and functional (“i” and “j” for z of MFD) structures. On the right side, maps indicating the temporal beta diversity and percentage of turnover (out of total beta diversity) of taxonomic (“c” and “d”), phylogenetic (“g” and “h”), and functional (“k” and “l”) beta diversities between present time and the near future. Future values were calculated for the year of 2070 under a scenario of intermediate concentration of greenhouse gases (RCP 6.0).
We observed direct relationship among the changes of alpha diversities and the compositions of communities through time (i.e. species temporal turnover). We found that there will be a higher temporal taxonomic turnover in the Northern Cerrado (Fig. 1c and d), despite the general decrease in richness. Although patterns of temporal phylogenetic and functional turnovers were similar to temporal taxonomic turnover, changes in phylogenetic and functional alpha diversities did not follow the same spatial trends. For example, there was an increase in phylogenetic alpha diversity in Eastern Cerrado, a region where there was moderate to high temporal phylogenetic turnover. Moreover, there was a decrease in the functional alpha diversity only in a small region in Southeastern Cerrado, where there was a high temporal functional turnover.
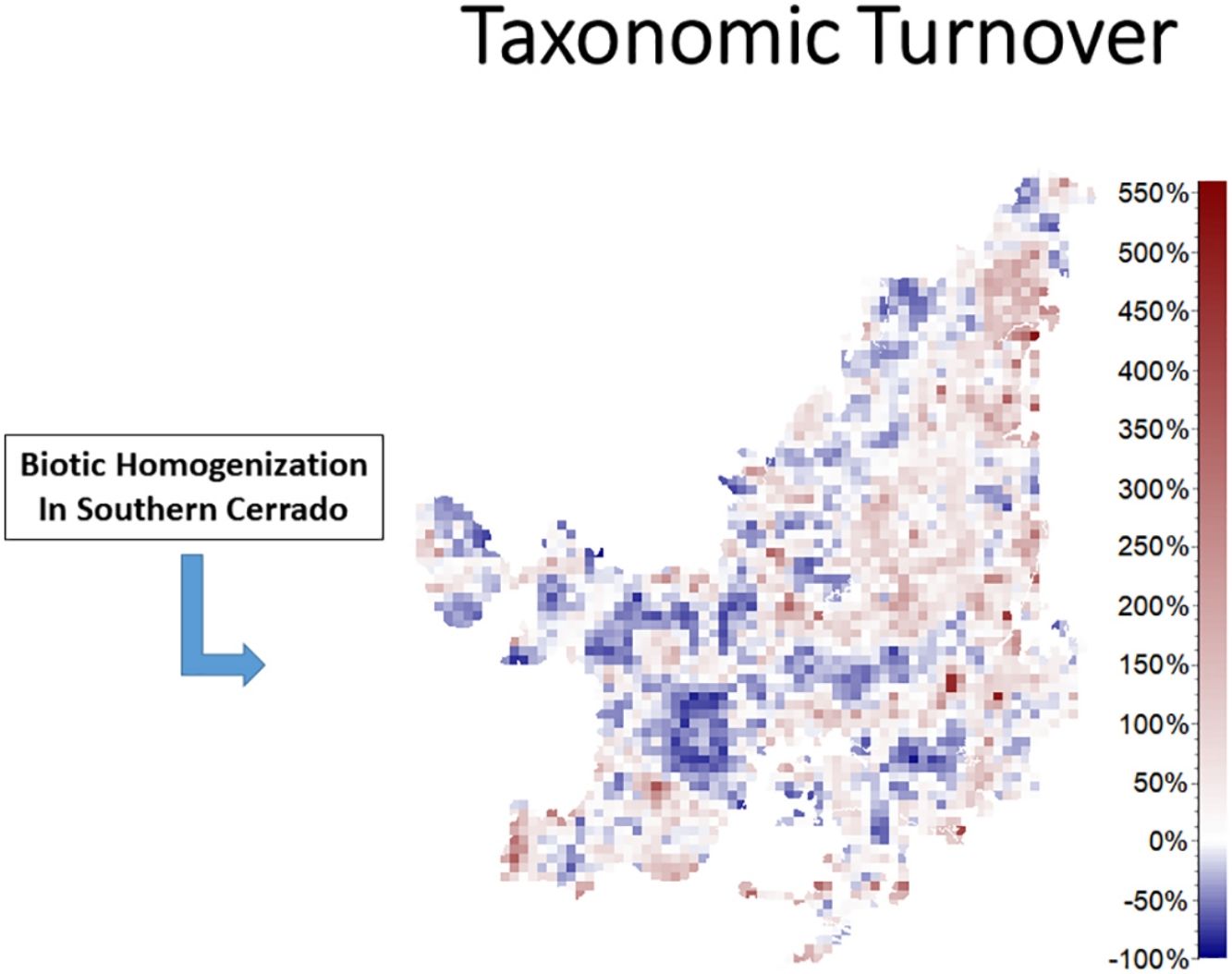
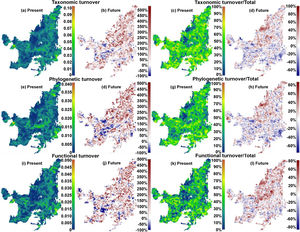
We also found regions with a decrease (indicating biotic homogenization) and an increase in spatial beta diversity of Cerrado mammals. Reductions in spatial taxonomic (Fig. 2a–d), phylogenetic (Fig. 2e–h) and functional (Fig. 2i–l) turnover were most concentrated in cells located at the Southern Cerrado (blue pixels in Fig. 2). This indicates that mammalian communities living mostly in this region will become more taxonomically, phylogenetically, and functionally homogenized in the coming years. Contrarily, several areas (mostly in Northern Cerrado) presented an increasing in taxonomic, phylogenetic, and functional turnover in the future. Thus, these areas are likely to receive species taxonomically, phylogenetically, and functionally different to those occurring in nearby areas. In this sense, we can expect to observe biotic heterogenization rather than homogenization in these areas.
Maps indicating the present values and the percentage of turnover (out of total beta), and the increase or decrease in both, of taxonomic (“a”, “b”, “c”, and “d”), phylogenetic (“e”, “f”, “g”, and “h”), and functional (“i”, “j”, “k” and “l”) beta diversities between present time and the year of 2070 under a scenario of intermediate concentration of greenhouse gases (RCP 6.0). (For interpretation of the references to color in the text, the reader is referred to the web version of the article.)
We found that nine species may become regionally extinct, from three IUCN categories: DD, LC, and EN (Kunsia fronto, Microakodontomys transitorius, Oecomys cleberi, Oligoryzomys rupestris, Oxymycterus delator, Phyllomys brasiliensis, Thrichomys inermis, Trinomys moojeni, Wiedomys cerradensis). Also, our models indicated that 138 species from other biomes can potentially immigrate to Cerrado in the coming years. Most of these potential immigrants (∼85%) were not considered as endangered. Notably, although our approach resulted in more regional immigrants than extinctions, we still had a general decrease of taxonomic alpha diversity, and a homogenization pattern in Southern Cerrado.
DiscussionWe found that the mammalian communities may, in general, lose species richness. However, a heterogeneous pattern of phylogenetic and functional overdispersion and clustering was observed throughout the Cerrado biome. Alongside reductions, changes in species composition will occur both temporally and spatially (Figs. 1 and 2). Specifically, the highest temporal taxonomic, phylogenetic and functional turnover will be mostly found in the Central and Northern Cerrado. Moreover, we observed that several mammalian communities from the lower part of Cerrado are going to become more taxonomically, phylogenetically and functionally homogenized. Contrarily, communities from the remaining area are mostly expected to increase in spatial beta diversity.
The richness of mammalian communities is expected to decrease in the near-future Cerrado. This result goes in line with the expected shifts in geographic ranges for several biological groups, including mammals, birds, amphibians, reptiles (Diniz-Filho et al., 2009; Lawler et al., 2009), and trees (Simon et al., 2013; Siqueira and Peterson, 2003; Zwiener et al., 2018). These studies predicted a loss in the geographic range of most of Cerrado's species due to upcoming climatic changes. In fact, there is evidence that Cerrado protected areas (PAs) are not able to keep the present levels of mammal evolutionary history and trait diversity (Carvalho et al., 2010). These authors also found that the Central and Southern Cerrado regions would need more conservation attention due to their high phylogenetic and functional diversity. However, we found that Southern Cerrado region will experience a future decrease of phylogenetic (but increase in functional) diversity (Figs. 1 and 2). Thus, we will expect that several mammalian communities to become taxonomically, phylogenetically and functionally homogenized. According to our results, more attention should be focused in the Northern Cerrado, which is today the region with the highest amount of native vegetation (Ballesteros-Mejia et al., 2018; MMA, 2011; Strassburg et al., 2017) and also where agricultural expansion will likely take place in the future (Ministério da Agricultura Pecuária e Abastecimento). Together with the expected high rates of land conversion, we found that this region may also experience a general decrease in both taxonomic and phylogenetic diversity. Therefore, homogenization is going to be strongest where human activities have already had a high impact in the ecosystem and, possibly, in the region's climate.
This homogenization could, certainly, cause several ecological and evolutionary impacts (Olden et al., 2004). For example, modifying species composition could alter community functioning, reducing the stability and resistance to environmental changes due to the narrowing of possible species specific responses, such as already observedfor insular mammal communities (Longman et al., 2018). From this perspective, evidently, the information about temporal changes in species compositions and alpha diversities represent an invaluable background that should be integrated when analysing future changes in biodiversity. This integrative approach could allow us to avoid losing or changing the composition of species, clades and species ecological traits in communities (Carvalho et al., 2010; Hidasi-Neto et al., 2015). Likewise, this is a warning that conservation actions must be urgently taken to conserve or regenerate the lower part of Cerrado, and to promote connectivity (Baguette et al., 2013) between areas where homogenization and heterogenization are expected to occur.
For example, the Serra da Bodoquena National Park is located at Southwestern Cerrado, where we found a future decrease of both taxonomic and phylogenetic alpha diversities of mammals, and an intermediate to high nestedness between present and future communities. Therefore, there will only be a taxonomic and phylogenetic “partial compensation” (see Fig. 1 in Sobral et al., 2016) of mammals in the area where the national park is located. In other words, in the future such assemblages have a great likelihood to be a poorer version of the actual fauna in terms of species and evolutionary lineages. Moreover, in the same area, there was an intermediate nestedness in the functional composition of mammals between the present and the future. However, functional diversity increased with time, indicating a functional “compensation with gain” (Sobral et al., 2016). This indicates that the potential increase in functional diversity in the future will also partially change the actual trait composition. Another example for this picture is Chapada dos Veadeiros National Park, located in Central Cerrado, which presented a future decrease in mammalian richness, and also a high turnover rate, indicating a change in the composition of species (“loss with no compensation”; Sobral et al., 2016). Also, phylogenetic and functional diversities increased with time, but their temporal turnovers were higher. This indicates that the reduced species pool in the area will have a more diverse composition of clades and species traits, but will be phylogenetically and functionally different from the species pool in the present (“gain but no compensation”; Sobral et al., 2016).
These results highlight the challenges that climate change imposes to current conservation policies and decisions. Firstly, because not only current threatened species are likely to disappear by the year 2070. In fact, interestingly, regional extinctions were not only due to endangered species disappearance, but also from the disappearance of DD and LC species (Table 1) as previously reported in other (see Nori and Loyola, 2015). Secondly, despite that our models predicted an expressive number of future immigrations that do not occur in the Cerrado nowadays, most (∼85%) of potential immigrants were from non-threatened categories. Thus, it is likely that more widely distributed species will become more common in the future (McKinney and Lockwood, 1999; Olden et al., 2004; Wilkinson, 2004).
Numbers and percentages, for each IUCN category, of potential regionally extinct and immigrant species expected in the Brazilian Cerrado for the year of 2070 under a scenario of intermediate concentration of greenhouse gases (RCP 6.0).
| Species | DD | LC | NT | VU | EN | CR |
|---|---|---|---|---|---|---|
| Extinctions | 3 (33.3%) | 2 (22.2%) | 0 | 0 | 4 (44.4%) | 0 |
| Immigrants | 17 (12.32%) | 96 (69.56%) | 4 (2.9%) | 9 (6.52%) | 7 (5.07%) | 5 (3.62%) |
There is still a lack of knowledge on the effects of climate change on whole communities. However, new methods are being developed, helping community ecologists to study classical and new hypotheses in order to shed light on ecology and conservation biology research agendas (Albouy et al., 2012; Diniz-Filho et al., 2009). Here, we showed a consistent prediction about both spatial and temporal alpha and beta diversities of mammal communities from an endangered biodiversity hotspot. These results are important because confirmed the idea, from a perspective conservation, that new and future efforts to maximize the performance of the current protected areas (PA) network have to be differently planned for specific regions depending on how the species pool size and its composition are expected to change through time (Prieto-Torres et al., 2016). In this sense, adding new areas to conserve both present and future ranges of species (as well as their taxonomic, phylogenetic and functional diversity) could represent a less costly strategy (in area and resources) than using a two-step process (i.e., representation of current ranges and afterwards addressing the consequences of climate change). Thus, we encourage future researchers to develop detailed studies about the current species’ representativeness within the PAs network (including the potential effects of future climate change herein identified) in order to identify and design priority conservation areas complementing national network across the Cerrado. Additionally, studies on the future effects of human impacts on biodiversity, such as habitat destruction or modification, will help us to have a better understanding about how urbanization may change ecological communities.
We thank Matheus S. L. Ribeiro for his insightful comments on the very first version of this study. The authors extend their gratitude to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001) for the MSc (DCJ, LMM) and PhD (JHN, FR) scholarships received. RDL and MVC are consistently supported with Productivity Grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This manuscript was improved by comments from three anonymous reviewers.