Recent studies have characterized the influence of agroecosystems on biodiversity. However, a set of components associated with the management of these areas is still neglected in landscape-level studies, especially in areas of recent agricultural intensification. The resources and conditions provided by agroecosystems to different species are highly variable in space and time, and failing to account for this variation may lead to misleading conclusions about the biodiversity status in these environments. In this perspective, we provide a conceptual overview to highlight why and which landscape components still need to be better explored to provide an adequate assessment of the agroecosystem effects on biodiversity. We used a Brazilian heterogeneous intensive-farming landscape as an example to outline the components that we believe are important for understanding biodiversity patterns in such landscapes. An in-depth description of agroecosystems can help us create better landscape-level management strategies and to design more effective green-way policies.
Agroecosystems are the most common ecosystems of the Anthropocene (DeClerck et al., 2016) and understanding how agroecosystem type (Fahrig et al., 2011) and management influence biodiversity (Vasseur et al., 2013) is of utmost importance for the conception of future green-way policies and conservation programs (Martel et al., 2017; Martin et al., 2019). Agroecosystems can be defined as ecological systems modified to produce specific goods of value to humans, such as food, fiber, and other agricultural products (Conway, 1987; Swift et al., 2004). Recently, ecological studies have been increasingly focused on the effects of agroecosystems on biodiversity within agricultural landscapes (Driscoll et al., 2013; Martel et al., 2017), which can be understood as landscapes with a mosaic of agroecosystems, human infrastructure, and possibly natural vegetation (Marshall, 2004).
However, mainly in areas of recent agricultural expansion, few studies have assessed the full suite of agricultural landscapes’ components that may influence biodiversity conservation. Agroecosystems are frequently characterized as barriers in landscapes (Ricketts, 2001), and the complete functional contribution of these areas or other non-natural land cover types has not often been characterized. Besides the compositional heterogeneity (i.e., diversity of crop cover types - Fahrig et al., 2011; Alignier et al., 2020), other essential heterogeneity components, such as configuration (i.e., shape and spatial arrangement of crops - Fahrig et al., 2011; Alignier et al., 2020) and temporal variation, should also be considered in studies of agricultural landscapes. Further complicating this issue, the social, economic, psychological, and cultural factors that are intrinsic to agricultural landscapes are usually not taken into account, as only biophysical conditions are usually considered.
Both spatial and temporal heterogeneity are essential elements of agricultural landscapes (Vasseur et al., 2013) and may affect biodiversity. The high spatio-temporal variation of the agricultural landscapes is mainly associated with the phenological cycles of the crops and differences in agricultural practices. For example, management of landscape configuration that modified edge density has increased functional biodiversity and yield-enhancing ecosystem services provided by arthropods in European agroecosystems (Martin et al., 2019). The decrease in mean-field size and an increase in crop diversity may increase landscape connectivity for short-distance dispersal plant species, facilitating plant dispersal into the field interiors (Alignier et al., 2020).
Species respond differently to environmental conditions, depending on a set of biological characteristics such as diet breadth and dispersal ability (Bommarco et al., 2010; Ewers and Didham, 2006; Martin et al., 2019). Some agroecosystems may provide resources for a variety of species (Fahrig et al., 2011) and increase landscape permeability due to their structural, compositional, and management characteristics (Ricketts, 2001; Cooney et al., 2015; Kay et al., 2016; Martel et al., 2017). For example, in Southeastern Brazil, Puma concolor (Carnivora, Felidae) and its prey use the resources provided by sugarcane to persist in a highly anthropogenic landscape (Magioli et al., 2014). The use of plantation rows in some agroecosystems facilitates the movement of some Brazilian marsupials (Prevedello and Vieira, 2010) and Australian reptiles (Kay et al., 2016).
In agricultural landscapes, the interactions among species and agroecosystems depend not only on the agroecosystem type but on the phenological crops stage, resource availability, and refuge effects (Baudry et al., 2003; Martin et al., 2019). The production system types (e.g., traditional or intensive), management practices - such as harvest and plowing periods, crop succession and rotation (Martel et al., 2017) - and agrochemical inputs also affect these interactions (Bertrand et al., 2016; Burel and Baudry, 2005; Vasseur et al., 2013).
Also, farmers’ decisions on system management to guarantee economic benefits (Kleijn et al., 2019; Martel et al., 2017) drive rapid landscape changes and may influence local biodiversity in different ways (Martel et al., 2017). Thus, the full matrix heterogeneity description and its variability through time (Baudry et al., 2003), as well as temporal variation in functional connectivity (Auffret et al., 2015; Martensen et al., 2017) and the influence of farmers’ decisions and agronomic drivers of crop patterns on biodiversity (Martel et al., 2017), should ideally all be included in landscape-level research in agricultural landscapes.
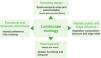
Here, we provide a conceptual overview of which landscape components yet need to be better explored to provide an effective assessment of the agroecosystems' effects on biodiversity from a landscape ecology perspective. For this, we focus on common concepts of landscape ecology, highlighting the components intrinsic to agricultural landscapes. Our inspiration for this work is a Brazilian intensive-farming landscape comprising a Long-Term Ecological Research (LTER) project called COFA-PELD (Functional Connectivity in an Agricultural Landscape). We also provide advice for a better characterization of agroecosystems in landscape ecology studies and intend to inspire research on yet little-explored issues regarding agricultural landscapes, in order to aid with the creation of specific and efficient management strategies and public policies for biodiversity conservation in agroecosystem-dominated landscapes.
Heterogeneity in agricultural landscapesLandscape heterogeneity can be conceptualized into three different components: 1) spatial (composition and configuration); 2) functional, and 3) temporal heterogeneity (Azevedo et al., 2000; Burel and Baudry, 2005; Cale and Hobbs, 1994; Fahrig et al., 2011; Li and Reynolds, 1995). Landscapes with a higher degree of heterogeneity contain a larger number of different land cover types (compositional heterogeneity) which are arranged in a complex manner (configurational heterogeneity - Fahrig et al., 2011). In agricultural landscapes, compositional crop heterogeneity refers to the diversity of crop cover types and configurational crop heterogeneity refers to the shape and arrangement of the crop fields within the landscapes (Alignier et al., 2020).
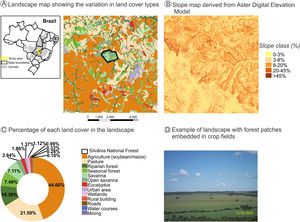
The natural and diverse arrangement of landscape biophysical characteristics - such as geomorphology, topography, soil fertility, temperature, and drainage - often create favorable natural gradients for the establishment of agroecosystem mosaics (Marshall, 2004; Vasseur et al., 2013). The natural heterogeneity in the COFA-PELD landscape (Fig. 1) is an example of how the biophysical characteristics can guide the establishment of agroecosystems, leading to a complex mosaic of agroecosystem interspersed by natural vegetation remnants. The north is predominantly occupied by pasture (Fig. 1A and C, 21.6% of cover) in steeper slope terrain (>20% slope, Fig. 1B), while the fields in the south with mild slope (<8% slope, Fig. 1B) are predominately occupied by soybean and maize plantations (Fig. 1A, B, C, and D - 44.7% of cover), which use agricultural machinery that requires mild slopes (<12% slope). Natural vegetation patch density is higher in the north than in the south. This pattern is associated with the predominance of livestock activity, with natural vegetation remnants providing thermal comfort during the cattle’s rest period.
A landscape with intensive crop farming in Silvânia, Goiás State in Central Brazil. This landscape is the focal area of the COFA-PELD (Functional connectivity in an agricultural landscape long-term ecological project) funded by the Brazilian Ministry of Science and Technology (MCTI/CNPq). The landscape has 33,400 ha and a protected area (Silvânia National Forest) in its center, with 487 ha. (A). Slope map identifies favorable natural conditions for the establishment of agricultural mosaics - high-slope areas are occupied by pasture, and low-slope areas are planted with soybean and corn due to the farming machinery (B). Landscape cover map created from Google Earth imagery to identify (A) and quantify the different cover types in the landscape (C). Example of land cover types in the landscape, with small patches and strips of riparian forest embedded in pastures and soybean/maize plantations (D). All spatial information is freely available in Harvard WorldMap platform: https://worldmap.harvard.edu/maps/peld_silvania.
Under such environmental constraints, the spatial and temporal arrangement of agroecosystems in agricultural landscapes depend essentially on landowners’ decisions. They are responsible for defining the crops and farming systems in place, and influence landscape heterogeneity patterns and quality, and their decisions are guided mostly by economic, but also social, cultural, and environmental factors (Kleijn et al., 2019; Martínez-García et al., 2013; Latawiec et al. 2017). Their decisions may configure a rapid change in the field, thus leading to short and long-term modifications in the multiple components of heterogeneity.
The long-term changes are related to the substitution of production systems, which changes the amount, size, spatial arrangement, shape, and type of the components in the mosaic, thus influencing its spatiotemporal heterogeneity. The short-term changes are related to crop rotation and harvest practices, which are not directly associated with size and shape changes, but may modify the resources in the mosaic components and thus in the functional and temporal heterogeneity. Thus, the understanding of ecological processes therein requires the quantification of these heterogeneity components from the study’s planning stage.
Spatial and functional heterogeneitySpatial heterogeneity is related to the composition, amount, size, and spatial arrangement of different land cover types, whereas functional heterogeneity refers to the quality and amount of resources that each agroecosystem can provide to a given species or ecological species profile (Azevedo et al., 2000; Cale and Hobbs, 1994; Fahrig et al., 2011; Sirami, 2016). The influence of spatial heterogeneity on biodiversity has been shown for different taxonomic groups and land cover types (e.g., Atauri & De Lucio 2001; Verberk et al. 2006; Katayama et al. 2014; Perović et al. 2015; Klingbeil & Willig 2016). However, even though the use of spatial heterogeneity as a landscape structure attribute has moved a step forward, our concern is that most studies still focus only on the composition and even on the spatial arrangement of the mosaic components, describing the agroecosystems basically by crop diversity, amount, size, and edge density (Bertrand et al., 2016; Fahrig et al., 2011).
Spatial heterogeneity per se does not describe the functional heterogeneity related to crop quality and resource availability, which modulates the effects of agroecosystems on ecological processes and species responses to distinct landscape elements (Cale and Hobbs, 1994; Fahrig et al., 2011). For example, tufted-ear marmosets Callithrix sp. may move through rubber tree plantations, rubber agroforestry, and forests, but they rest and feed mainly in forests and rarely in rubber agroforests (Ferreira et al., 2018). Therefore, rubber tree plantations, while allowing movement, provide no resting sites and feeding resources for this species, meaning that these cover types are functionally different (Ferreira et al., 2018).
Thus, spatial heterogeneity descriptors derived merely from land cover maps, with no information on ecological requirements of species, can be less effective for truthfully characterizing the mosaic quality and resource use in the landscape. In turn, functional heterogeneity may provide a better approach to measuring heterogeneity in agricultural landscapes, thus providing information on the relationship of crop type and quality to the requirements and habitat selection by different species.
However, functional heterogeneity is complex to measure because it depends on how species or functional groups can be affected by different land cover types in a landscape (Fahrig et al., 2011). Therefore, it depends on detailed information on species requirements, such as habitat selection, foraging strategies, predator avoidance, resting, and movement ability. An alternative to a species-specific approach is to use guilds or functional groups, assuming that species with similar ecological requirements will respond similarly to the different land cover types (Magioli et al., 2014; Muylaert et al., 2016; Suárez-Castro et al., 2018).
The number and type of resources offered by each matrix and the farming practices - such as management intensity and frequency of agrochemical application - can be used to measure functional heterogeneity (see Da Silva et al. 2015 for examples with mammals). Similarly, landscape functional heterogeneity for a given species, e.g., the red-cockaded woodpecker, can be estimated from the percentage of ecologically stable land cover (Azevedo et al., 2000). In addition, management practices can be associated with the stability of different crop cover types in a landscape, thus allowing the design of a functional land cover map that couples each crop cover category to different levels of stability. Therefore, multifunctional systems, such as agroforestry or shadow cocoa with low input and management, can be more stable than more intensely managed systems (i.e. Eucalyptus or Pinus plantation) for forest specialist species (see Giubbina et al. 2018). However, for species adapted to open vegetation, pastures can be more stable than sugarcane or soybean, which can also be a consequence of the low inputs and management in pastures.
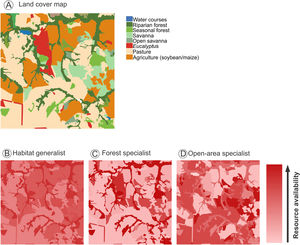
To better represent the functional heterogeneity in agricultural landscapes, we can convert the categorical land cover map (Fig. 2A) into a functional heterogeneity map, according to each species or functional group requirements such as forest specialists, habitat generalists, and open-area specialists (Fig. 2B–D). In Fig. 2, we represent the same landscape (Fig. 2A) as continuous resource availability maps (Fig. 2B, C and D), associating each land cover with species habitat requirement or preference. In this perspective, the landscape provides different amounts of resources for species with different requirements. For example, open-area and forest specialist species have opposite patterns of resource availability, since these species tend to use a specific vegetation type as preferential habitat. The habitat generalist species map has higher variation in resource availability because they tend to explore resources from other natural or non-natural vegetation types. Thus, functional landscape quantification based only on land cover maps can over- or underestimate landscape functional heterogeneity depending on the species analyzed.
A landscape scenarios with different levels of functional heterogeneity (resource availability) for species with different habitat requirements. (A) Land cover map characterizing the different land cover types in the landscape. (B), (C) and (D) are functional representations of landscape resource availability for species with different habitat requirements, namely: 1) a habitat generalist (B). 2) a forest specialist (C), and 3) a open-area specialist species (D). Warmer colors in the gradient indicate higher resource availability. For the habitat generalist, both natural environments (riparian and seasonal forest, savanna and open savanna) have high resource availability, while crops (soybean/maize) have intermediate resource availability. The forest specialists are mostly restricted to natural forest areas (riparian and seasonal forest), but may also use environments with forest-like structure such as Eucalyptus plantations. Conversely, the open-area specialist is limited to open-canopy natural areas (savanna and opened savanna) but may also use the resources provide by crops such as soybean and maize.
Other crop characteristics can also be used to assess functional heterogeneity, such as the capacity to provide food, escape from predators, or nesting sites (see Fahrig et al., 2011). Furthermore, it is essential to consider the particularities of each agroecosystem in the landscape, including variation in farming management - such as the use of agrochemicals and the harvesting - and plowing periods, which are known as hidden heterogeneity elements (Bertrand et al., 2016; Vasseur et al., 2013).
Although simple measures of spatial heterogeneity can fail to characterize landscape functionality, there are some alternatives to make these metrics more realistic when used in agricultural landscapes. The use of simple spatial metrics such as the Shannon diversity index of agricultural cover types through time can provide clues to successive changes in agroecosystems. For instance, variation in Shannon index and crop diversity (e.g., field size, crop succession rate, and changes in crop composition) along different years or seasons may indicate variation in functional heterogeneity through time (Bertrand et al., 2016; Vasseur et al., 2013). Other landscape metrics - such as contagion and interspersion - are less used, but can describe the arrangement and composition of agroecosystems at different time spans (see an example in Klingbeil & Willig, 2016).
Furthermore, landscape composition and spatial arrangement also depend on the farming system in place (Baudry et al., 2003; Fahrig et al., 2011; Puech et al., 2015). For example, whereas in traditional systems, small and diversified farming is interspersed with remaining natural vegetation patches (i.e., less crop field size implying to a higher configurational crop heterogeneity), in intensive systems, a large area with a single land use type (such as pasture, sugarcane, soybean, corn or Eucalyptus plantation) dominates the landscape (Fig. 1). The use of agrochemicals also varies between these systems, being more common in monoculture areas. Therefore, for describing the temporal variation in crop quality as resources for species, we can combine the data on agrochemicals application per hectare per month with land cover information, generating multi-temporal agrochemical maps.This can be particularly important for the advance of landscape ecotoxicology, a very promising research field that integrates toxicology, ecology, and landscape ecology at larger spatial scales than the traditional approaches (Johnson, 2002).
Characterizing the production system type at the landscape level is also important for understanding the effects of multiple production systems on biodiversity (Gabriel et al., 2010). For instance, in the COFA-PELD study region, the livestock farming system tends to devise a higher quality mosaic in the north portion of the region, compared to the south, where soybean and corn monocultures dominate the mosaic (see Fig. 1). Agroforestry systems may provide more benefits for biodiversity than conventional production systems, but these benefits can differ according to the agroforestry system type (Santos et al., 2019). In general, agroforestry systems are more stable and have similar structures compared to the habitat of many native species. Although agroforestry systems stand in contrast to production systems based on livestock, livestock production may be not homogeneous. For example, rotational grazing is a recognized practice for improving pasture management and productivity (Latawiec et al., 2017). Nevertheless, rapid changes in the abiotic environment typical to this system, such as the speedy establishment and growth of forage species, may have either positive or negative influences on the population of insects, depending on the species investigated (Martel et al., 2017; Ravetto Enri et al., 2017).
Temporal heterogeneityAlthough landscapes can be described as static, e.g. by focusing on the spatial and functional heterogeneity of crop mosaics, they are dynamic, and therefore the temporal dimension must be incorporated when understanding biodiversity responses in agriculture-dominated landscapes. The temporal heterogeneity of agroecosystems may be related to: a) abrupt changes in land use, e.g. switching between plants with different phenological cycles, such as Eucalyptus (semi-perennial) to annual crops, and b) short-term changes in land cover quality due to crop life cycle, crop rotation (using crops from different families, such as soybean, a Fabaceae, and corn, a Poaceae), succession cropping (using different crops in different seasons) or management, such as the use of herbicides and pesticides (Vasseur et al., 2013; Bertrand et al., 2016; Burel and Baudry, 2005; Gallé et al., 2018).
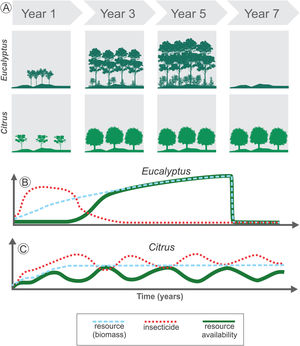
The temporal heterogeneity (i.e., short- and long-term changes into spatial heterogeneity) in agroecosystems may affect resource availability due to both variation in crop biomass due to plant life cycle, and variation in resource availability due to farming management (Fig. 3). For instance, we consider Citrus as a perennial crop and Eucalyptus as a semi-perennial crop, demonstrating the changes in resource availability and the use of insecticides as a management practice (Fig. 3). The fast-growing Eucalyptus plantations drastically change the structure of local vegetation in a short period (Fig. 3A). Thus, even with low-intensity management during the Eucalyptus life cycle, this agroecosystem has high temporal heterogeneity: in the first stage, it has a low-height bushy structure, which later evolves into a forest, and is harvested after 4-7 years in Brazil, depending on the purpose (Figs. 3A and B). On the other hand, Citrus is a perennial crop and may offer stable biomass availability during the entire cycle (Figs. 3A and C), since area replacement is motivated only by pest infestations and by market guidelines, which can be a long-term driver. However, management practices are often used during the entire crop cycle, increasing landscape temporal heterogeneity in a short-time span (Fig. 3C).
Long- and short-term changes in temporal heterogeneity in agricultural landscapes. Long-term variations are associated with land cover changes or harvest practices (A). For example, Eucalyptus plantation leads to high initial disturbance in the landscape when compared to Citrus, due to the fast growth of the trees in the initial years, increasing the resource availability for species rapidly (blue dashed line in B). After 4 to 7 years the crop is totally harvested, decreasing drastically the resources available (blue dashed line in B). Short-term changes are associated with management practices used during the crop cycle (B and C). Before harvesting, Eucalyptus may provide stable amount of resources over the years for some species, which depends on the biomass as resource (green solid line in B). This perennial crop also has an initial intense management with insecticides, followed by a decrease in their use (red dotted line in B). Thus, total resource availability depends on both the standing biomass and on insecticide use. Conversely, Citrus is also a perennial crop and may offer a stable biomass during the entire cycle (blue dashed line in C). However, in non-organic Citrus systems the continuous use of insecticides (red dotted line in C) or other management practices such as understory cleaning and pruning can lead to periodic variation in resource availability for some species (green solid line in C).
Crop rotation is a sustainable and very common farming practice that alters temporal heterogeneity, and consequently the resources available for different species (Burel and Baudry, 2005). For example, in the area of COFA-PELD (Fig. 1), soybean is grown in summer whereas other crops (maize and sorghum) are planted in the same area after soybean harvest to increase farmer’s gain and improve soil nutrients. A system like this can lead to a dynamic equilibrium when used for long time, as different species can use these crops as complementary habitats in different seasons depending on management and agrochemical frequency applications (Ricketts et al., 2006; Mandelik et al., 2012).
In contrast, abrupt changes in the cropping system, such as modifications in land cover structure from a perennial to an annual crop, such as Citrus to soybean, can result in short-term high disturbance level and loss or gain of land cover quality depending on species or functional group. Management practices may also vary throughout the year, which can affect land cover quality as complementary habitat or resource for species. For example, the replacement of non-tillage by conventional tillage and the use of pesticides may temporarily disrupt the food web, cause disturbances in the system and increase its temporal heterogeneity (Fig. 3).
It is important to note that, although natural systems are also temporally heterogeneous, these modifications are more gradual and usually caused by natural factors. For example, in highly seasonal environments forests, such as deciduous forests or hyper seasonal savannas, resource availability may change through time, but species may have evolved and adapted to cope with such temporal variability.
We can use multi-temporal land cover maps with a fine spatial resolution to consider landscape temporal heterogeneity. High spatial and temporal resolution imagery allows characterizing abrupt changes in temporal heterogeneity and short-term and cyclical changes, such as crop rotation and succession. This characterization must be in accordance with the farming calendar of the specific region under study. Satellite images have been used to evaluate the processes of degradation, restoration, and renovation of tropical pastures (Aguiar et al., 2017). The same approach based on the estimation of phenological metrics from time series images can be applied to characterize agroecosystems cycles on fine temporal scales (see examples in Prasad et al., 2015).
Moreover, Long-Term Ecological Research sites in agricultural landscapes - such as COFA-PELD - are important to provide detailed multi-temporal land cover maps to characterize landscape heterogeneity along with different cycles in a cropping system, and considering short time intervals. Accordingly, when it is not possible to generate multi-temporal maps on a fine temporal scale (weeks or months) or to acquire multi-temporal remote sensing images with a fine spatial resolution (i.e. 0.5 to 5 meters), we recommend the use of additional methods for minimizing the lack of information. For example, the history of the cropping system may be assessed by interviewing farmers or landowners, who are likely to know how the farming practices and crop types changed through time.
In summary, the intrinsic variability of agroecosystems compels a finer characterization of the production system, including the description of management practices used throughout crop growing, and to obtain biodiversity data following the entire crop cycle. It is also important to understand whether the different crop phenology stages and crop management practices can provide complementary resources for species or whether they only offer risks for species persistence in landscapes. Currently, several alternatives are available to include such information in spatial ecology analyses. For example, high-resolution imagery, such as the Google Earth images available in free Geographic Information Systems, allows the users to create maps with a fine spatial resolution (see an example of the OpenLayer plugin/QuickMapServices in QGIS). Agencies such as Planet (https://www.planet.com/markets/education-and-research/) and Digital Globe Foundation (http://foundation.digitalglobe.com/) have specific programs to freely provide remote sensing images with a high spectral, spatial, and temporal resolution to research applications.
Other alternatives are time series of vegetation indexes derived from the moderate resolution imaging spectroradiometer MODIS with a 250-m spatial resolution. This data type has been used to characterize double-cropping systems and vertical agricultural intensification (Arvor et al., 2011), and landscape heterogeneity patterns (Miranda et al., 2017). For South America, free series of MODIS images are available to download at https://www.satveg.cnptia.embrapa.br/satveg/login.html.
Particularly to Brazil, the free online platform MapBiomas (https://mapbiomas.org/) provides high-quality multi-temporal land cover maps derived from Landsat-like images classification (Souza et al., 2020). The data available on this platform are very useful for multi-temporal analyses at medium and large scales. However, these maps may not good enough to study landscape spatial and temporal heterogeneity at finer scales (e.g., plantation rows and trees as stepping stones) or for species with short-distance dispersal capacity.
Furthermore, the free platform Google Earth Engine (https://earthengine.google.com/platform/) allows the online processing of a large set of remote sensing data. The user can derive multi-temporal land cover maps, vegetation indexes, and perform other spatial analyses at different spatial and temporal scales without the necessity of a powerful computer. As presented above, researchers nowadays have plenty of options to deal better with spatio-temporal heterogeneity quantification issues.
Habitat quality and edge influenceHeterogeneity matters not only at the landscape-level or when analyzing crop mosaics. Habitats are not homogeneous in quality, structure, and resource availability. Habitat selection varies among species and is an essential factor that explains species distribution. Therefore, failing to account for habitat quality and heterogeneity within landscapes and habitat patches, or using general metrics of habitat amount - which do not differentiate between- and within-patch characteristics - can lead to erroneous interpretations about the impacts of habitat loss and fragmentation on biodiversity (Almeida-Gomes et al., 2016; Hou and Walz, 2016).
Categorical land cover maps may lead to incorrect estimation of habitat amount because patches with different structures may be classified in the same land cover class. A detailed description of variation in habitat quality and functionality for different species, using continuous and texture maps (see an example in Regolin et al., 2020) can provide a better understanding of habitat functioning in agricultural landscapes (Fischer and Lindenmayer, 2006; Ricketts et al., 2006).
The description of habitat heterogeneity is essential in conservation planning, considering that restoration of a single habitat type may not maximize species diversity (Verberk et al., 2006). For example, COFA-PELD includes several natural vegetation ecosystems, commonly found in the Brazilian Cerrado biome, ranging from grasslands to forests, including riparian and seasonally dry forests and savannas with different tree density and composition. In such landscapes, not all vegetation types are suitable for all species, and variation among neighboring patches can be substantial because different vegetation features and quality may represent abrupt variations in the landscape. The quality of matrices, such as pastures and crop plantations, also vary according to their management; for example, scattered trees in matrix areas may increase the abundance and richness of different species groups compared to open areas (Prevedello et al., 2018).
Habitat quality may also be affected by the surrounding land covers because of edge influence (Harper et al., 2005a). Edges are the transition between two environment types, which can be either natural or anthropogenic. Near the edge, quality can be lower, higher, or similar to the interior of habitat quality, which mainly depends on species sensitivity to edge effects (Ries and Sisk, 2004) and resource distribution. For instance, a species may forage in open areas but be a nest parasite of forest birds; for such species, pasture and forest offer complementary resources, and its abundance is expected to be greatest at the edge (Ries and Sisk, 2004; Ries et al. 2004).
Also, species can prefer forest edges, be very well adapted to urban areas, but avoid agriculture and pasture, as is the case of some frugivore thrush species of genus Turdus within Atlantic Forest (Silveira et al., 2016). The interface between forest and surrounding matrices (i.e., pasture or Eucalyptus plantation) shapes the spillover and phylogenetic diversity of avian assemblages within forest fragments (Barros et al., 2020a), which may have severe consequences to ecosystem services modulated by birds (Barros et al., 2020b). Moreover, dung beetle richness and abundance responses are shaped by habitat type, surrounding anthropogenic matrix, and distance (m) in relation to the edge within fragmented landscapes of São Paulo, Brazil (Martello et al., 2016).
The magnitude and depth of edge influence depend on several factors, including habitat and matrix type, and edge age (Arroyo-Rodríguez et al., 2017; Harper et al., 2005b). Edge influence also varies among species with different life history traits, for example between flying and non-flying insects, as well as between native and non-native species (Caitano et al., 2020). Edge characteristics also vary temporally due to edge regeneration, crop phenology cycle and management, and long-term changes due to the replacement of agroecosystem types (Macfadyen and Muller, 2013).
Forest patches may also affect the anthropogenic matrix by modifying microclimate conditions or increasing natural regeneration in restoration areas, a process called “forest influence” (Baker et al., 2013). These effects may be of economic relevance, providing biological control of pests near the patch edges (Macfadyen and Muller, 2013), as observed in coffee plantation areas in Brazil (see Medeiros et al., 2019) or increasing pollination services within agricultural areas surrounding natural environments (Ricketts et al., 2006).
Metrics of edge density or size may thus not be enough for characterizing edge influence in agricultural landscapes. Metrics coupling multitemporal characteristics of edges and continuous boundaries between different land cover types can be useful but have been poorly explored in landscape ecology studies (Macfadyen and Muller, 2013). Whether edges result in enhanced or decreased patch quality depends on the resource distribution and the ecological flows across the edge (Ries et al., 2004). In the presence of smooth transition areas, it may be difficult to classify these areas as either habitat or matrix, and continuous landscape classifications may be more appropriate (Hou and Walz, 2016). Finally, multiple edge effects, i.e., the interaction of two or more nearby edges, can lead to either stronger or weaker edge influence than single edges, with additive or synergistic effects, offering additional complexity (Porensky and Young, 2013), which are not accounted for when analyzing simple edge density measures.
Functional and temporal connectivityA detailed description of crop mosaic and habitat quality at different spatial and temporal scales is essential to better understanding how agricultural landscapes influence biodiversity. Likewise, the simplification of agricultural landscapes' components may affect the estimation of landscape connectivity (Martensen et al., 2012; Magioli et al., 2016). In addition to the distance between patches, both habitat and agroecosystems quality can provide different opportunities to species' movement through landscapes, both increasing or limiting movement capacity (Silveira et al., 2016; Giubbina et al., 2018). Several studies have addressed the importance of connectivity among patches within landscapes for maintaining species movement (see Tischendorf and Fahrig, 2000). Notwithstanding, few of them have proposed measuring spatio-temporal differences in permeability among matrix and habitat types to estimate functional connectivity (i.e., the degree to which the landscape facilitates or impedes organism’s movement) in agricultural landscapes - but see an empirical study with bees in Boscolo et al. (2017) and a methodological approach in Martensen et al. (2017).
Structural connectivity based on the Euclidean distance between resource patches in a binary cover map ignores species behavior. It tends to add errors in connectivity estimation due to the lack of information on species requirements and matrix permeability (Taylor et al., 2006). In agricultural landscapes, the errors associated with this measure of connectivity can be stronger, due to the diversity of matrix types and species adaptation to permeate through anthropogenic environments (Magioli et al., 2016; Boscolo et al., 2017).
More porous matrices increase functional connectivity by facilitating movement across the landscape and, thus, to access food resources (Ricketts et al., 2006; Taylor et al., 2006). In this context, even small habitat fragments under massive edge influence from the surrounding crops may serve as temporary habitat patches, which can increase landscape permeability and connectivity for some species with habitat use plasticity (Gallé et al., 2018; Hou and Walz, 2016).
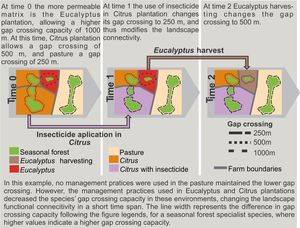
Functional connectivity can also vary with crop phenology cycle, farming system, cropping management (see an example in Box 1), and spatial configuration of crop fields. In France, landscapes with traditional milk production systems have higher connectivity than those with intensive milk production (Baudry et al., 2003). Crops planted in rows may favor reptile and small mammal movement within agricultural landscapes, thus increasing functional connectivity for some species (Kay et al., 2016; Prevedello et al., 2010); scattered trees in matrix may also improve connectivity (Manning et al. 2006). Management with intensive machinery or with a large number of workers for specific periods of the year, such as during harvesting and management of the stubble, may affect species movement and landscape use behavior. Therefore, all these farming systems and crop management are dynamic in space and time and may cause temporal variations in landscape connectivity.
Therefore, an oversimplified interpretation of farming systems may affect the estimation of landscape connectivity (Martel et al., 2017). Agricultural landscapes may have a high temporal variation in functional connectivity, and it would be inadequate to measure connectivity solely based on a single snapshot (Auffret et al., 2015; Driscoll et al., 2013; Martensen et al., 2017). In agricultural landscapes, a single snapshot can represent a unique moment of low or high landscape connectivity, whereas functional connectivity, which matters more, may have been highly variable to different organisms (Magioli et al., 2016).
Social and economic factorsStudies in agricultural landscapes should consider the general sampling design issues (Box 2), but also farmers' management choices that may affect sampling site and time interval. For instance, the replacement of a perennial crop, such as Eucalyptus plantations, by short cycle crops, such as soybean or corn, may affect sampling design or even modify the study's question. Such a decision may also affect the characterization of long-term ecological processes and the continuous collection of standardized data, essential for Long-Term Ecological Research programs. Land conflicts in areas of agricultural frontier may also be limiting for the allocation of long-term experiments. Furthermore, access to the sampling sites also is difficult during intensive periods of agroecosystem management, such as during crop harvesting, which can hamper data collection.
Educational projects to bring awareness to the farmers about the benefits of ecosystem services can strongly influence regional landscape transformation (Gabriel et al., 2010). Farmers can encourage their neighbors to adopt more sustainable practices and provide experimental sites to develop ecological researches in their lands. Additionally, farmers may also facilitate the collection of biodiversity data, as we have recently experienced in the COFA-PELD project (Lima and Bastos, 2019). In contrast, they may also impede access to areas by restricting ecological research, and hamper the use of conservation practices when they are not convinced of the benefits they will gain with the outcomes of research (Kleijn et al., 2019).
Characteristics such as land-tenure security or intensified environmental inspection actions may hamper access to private properties. The local policymakers may have an essential role in supporting ecological research, informing the farmers about the goals and benefits of the research activities. Local policies can also significantly influence the landscape design, thus changing key landscape structure attributes. Loans for specific activities can promote short-term land cover conversions, while also implying in landscape homogenization. Payment for environmental services initiatives, implementation of habitat restoration projects, and incentives to adopt multifunctional systems can help to maintain landscape heterogeneity and specific ecosystem services (Santos et al., 2019), as recently implemented in the region of COFA-PELD project. Additionally, farmers tend to be more receptive to the use of environmental practices when clear financial gains are highlighted (Kleijn et al., 2019).
Final considerations: challenges for landscape ecology studies in agricultural landscapesIncorporating detailed descriptions of the farming systems and management practices in landscape ecology analysis is essential for a more realistic understanding of agroecosystems dynamics, particularly in intensive-farming systems. In addition to forest cover (see a concept review performed by Arroyo-Rodríguez et al., 2020), we highlighted a set of additional landscape components relevant to be considered for a better understanding of how crop mosaic effects biodiversity, such as spatio-temporal and functional components. In the context of agriculture expansion, this in-depth characterization of landscape attributes, and social and economic factors may be of utmost importance for ecologically sound landscape management programs, thus leading to environmental sustainability and guaranteeing food security. However, quantifying the influence of agroecosystems on biodiversity requires more effort in experimentation with sophisticated design, both in space and time, high technology (e.g., use of satellite images with high spatial, temporal, and spectral resolution), and consequently additional funding for research on this field.
The dynamics of these areas are dependent on farmers' choices, and generalizations can be complicated since the variation of production systems matches the biophysical, economic, social, and environmental conditions of the local landscape in different parts of the globe. To future researches, the characterization of agricultural landscapes must be able to respond to questions such as 1) What kind of agroecosystems and management can be more sustainable? 2) Which type of production systems are capable of maintaining biodiversity on a local and landscape-scale? 3) During which periods of the year are these agroecosystems more biodiversity-friendly? 4) Which landscape elements matter the most for biodiversity conservation in agroecosystems? More socio-ecological studies are needed to characterize farmers' perceptions about biodiversity conservation and their intention to conserve natural ecosystems. This will be crucial to identify the main factors influencing local farmers' decisions to convert the production system or adopt new production practices. Knowledge of farmers' perception may permit the design of more efficient guidelines for biodiversity conservation at farm and landscape-scale levels.
Well-designed Long-Term Ecological Research programs that allow acquiring a set of data in the same area during years can help us to identify how biodiversity in complex heterogeneous landscapes varies in space and time for both short and long-term temporal trends. However, such studies do not offer the same opportunities to include control areas, as predesigned experiments, due to the dynamics of the agroecosystems. On the other hand, these environments offer high degree of realism because a variety of factors define the characteristics of landscapes.
We suggest that future studies should focus on making detailed descriptions of which agronomic practices in production systems can contribute to local biodiversity in multiple timeframes. Information related to short-term economic benefits is well regarded by farmers and influences them to choose new management practices. Thus, when possible, studies should also include information related to the economic gains provided by ecological farming practices. Exploring and correctly interpreting all the elements that compose agricultural landscapes and their complex interactions is a challenge that requires inter- and trans-disciplinary approaches, which can be achieved with interaction and agreement among researchers, environmental policymakers and farmers.
Conflict of Interest StatementThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author ContributionsJSS, RGC and MCR conceived the work. JSS and PD structured and wrote the manuscript. JEFO assisted with the manuscript organization and text revision. FM helped with manuscript organization and FM and JSS designed figures. ASJ, MEF, and CS-N provided data. RC and MCR led the working team, and RC is responsible for the funding. All authors read and approved the final manuscript.
This work was supported by grants to the research network PELD COFA supported by MCT/CNPq/CAPES/ (project n° 441278/2016-7) and CAPES/PROCAD (project n° 88881.068425/2014-01). JSS received a CAPES postdoctoral fellowship and a postdoctoral grant from São Paulo Research Foundation (FAPESP, process n° 2019/09713-6). PD and FM received a CAPES/PNPD fellowship, JEFO was supported by FAPESP (project n° 2014/23132-2). RGC, MCR, and MEF have continuously been supported by productivity grants from CNPq (processes n° 312045/2013-1; 312292/2016-3; 312229/2014-3), which we gratefully acknowledge. MCR was also funded by FAPESP (projects n° 2013/50421-2; 2020/01779-5) and CNPq (project n° 442147/2020-1). We also thank the Harvard University for hosting our cartographic databases, and make them available for viewing, analysis, and download, through the WorldMap platform.