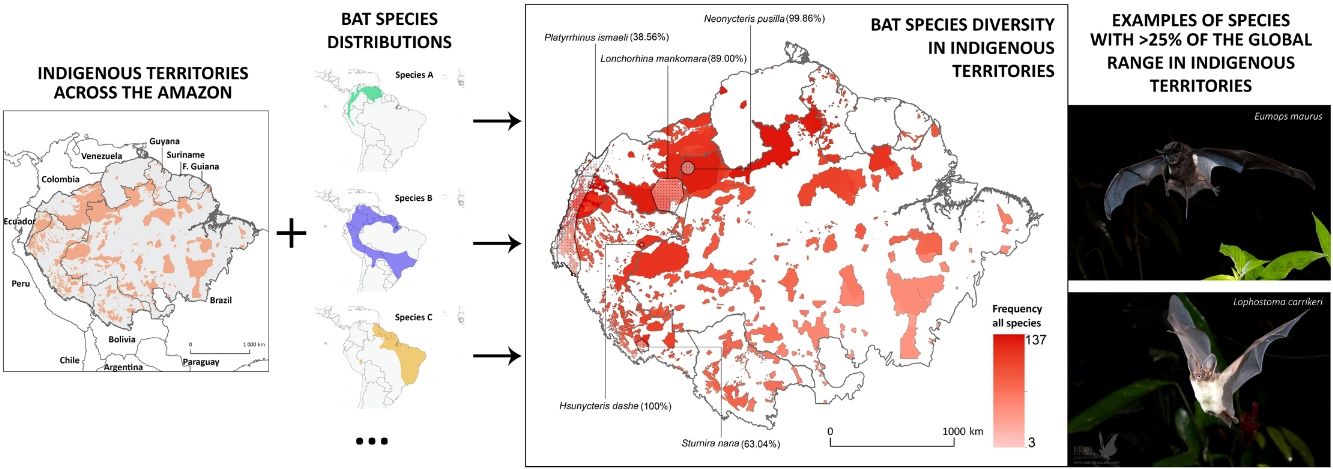
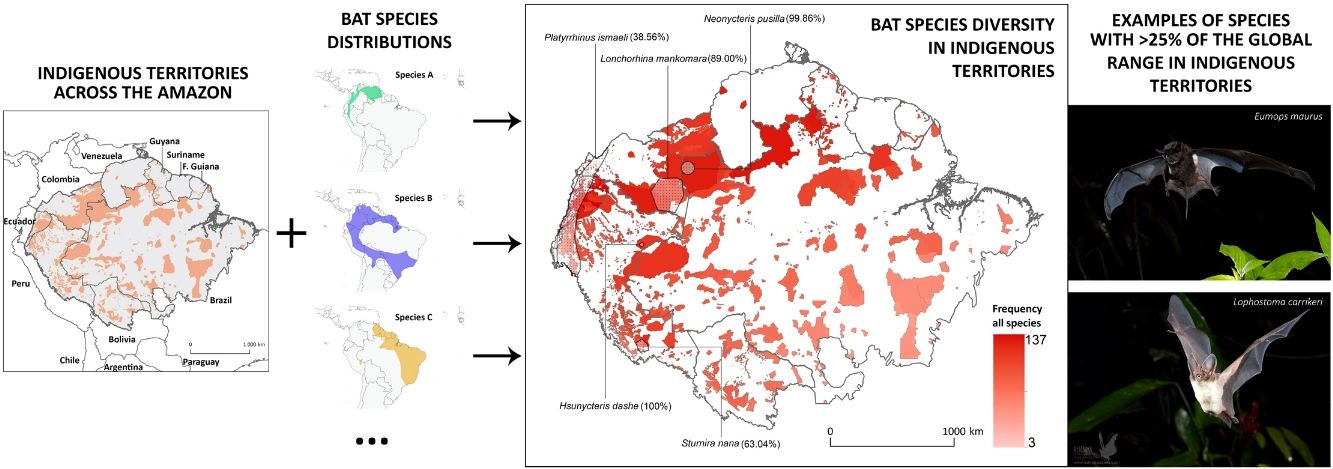
Indigenous Peoples have shaped and managed vast tracts of the Amazon rainforest for millennia. However, evaluations of how much biodiversity is governed under Indigenous stewardship are scarce. Here, we integrate geospatial data of officially recognized ITs across the Amazon biogeographic boundaries with the distribution range of >200 Amazonian bat species, to: (i) assess the potential contribution of ITs for the conservation of this species-rich mammalian group across the Amazon; (ii) investigate which ITs host the greatest number of bat species; and (iii) analyse how threatened and Data Deficient bat species are distributed within the ITs of the nine Amazonian countries. Twenty-two bat species were found to have >25% of their global distribution range within Amazonian ITs, including many forest-dependent species with restricted distribution ranges and a highly threatened or Data Deficient conservation status. Some particularly diverse ITs were found to harbour over half of the known Amazonian bat species, particularly in transboundary areas in the North-western Amazon. At the national level, the highest number of species with over 25% of their national Amazonian distribution within ITs was found in Peru (145), followed by Brazil (136), Colombia and Ecuador (both with 134). This study reveals the potential role of Indigenous Peoples in Amazonian bat conservation and emphasizes the contribution of their stewardship for maintaining the ecosystems in which some of the most rare and unique bat species are found.
A substantial proportion of the world’s biodiversity lies in areas traditionally managed, owned, used and/or occupied by Indigenous Peoples (Brondizio and Le Tourneau, 2016; Schuster et al., 2019). Recent work has estimated that Indigenous Territories (hereinafter ITs) intersect at least 40% of the world’s last remaining natural areas with very low human disturbance and around 32% of the world’s Intact Forest Landscapes (Garnett et al., 2018; Fa et al., 2020). Moreover, it is widely recognized that a significant share of the biodiversity occurring on ITs is highly dependent on Indigenous Peoples’ knowledge systems, management practices, customary institutions and cultural connections to their lands (Berkes, 1999; Jones et al., 2008; Cámara-Leret et al., 2019). Much research shows that Indigenous stewardship often encourages the sustainable management of biodiversity, despite the fact that it might not always be framed as, or explicitly focused on, environmental preservation (Schuster et al., 2019; Díaz et al., 2019; Fernández-Llamazares and Virtanen, 2020). In fact, Indigenous Peoples often manage their lands in ways that are compatible with, or actively support, biodiversity conservation (Sterling et al., 2017; Leiper et al., 2018).
With over 300 different Indigenous groups and more species of plants and animals recorded than in any other terrestrial biome in the planet, the Amazon is a global hotspot of both biological and cultural diversity (Hoorn et al., 2010; Le Tourneau, 2015). A growing body of research is showing that some of the best-conserved Amazonian habitats, including old-growth forests, have been actively shaped and managed by Indigenous Peoples over millennia (Barlow et al., 2012; Carvalho and Mustin, 2017). Human ecologists, ethnobiologists and anthropologists, among others, have documented the myriads of institutional arrangements that Amazonian Indigenous Peoples have developed to govern the management of lands and, incidentally or not, the biodiversity they harbour (Kohn, 2013; Sirén, 2017; Fernández-Llamazares et al., 2020). It has been recurrently shown that formal land titling of ITs is an effective means for buffering against deforestation across much of the Amazon biome (Nolte et al., 2013; Blackman et al., 2017; Schleicher et al., 2017).
Although Amazonian Indigenous Peoples do have a wide range of legitimate socio-political and economic aspirations that do not always align with the conservation goals of certain organizations (Kohler and Brondizio, 2016), there are numerous examples of local governance regimes (e.g., Indigenous and Community Conserved Areas) that are significantly contributing to conserve ecosystems with high species richness and ecological intactness (Le Tourneau, 2015; Schleicher et al., 2017; Fernández-Llamazares et al., 2020). For example, it has been estimated that 70.5% of the IT surface in Ecuador overlaps with Intact Forest Landscapes, with no signs of habitat fragmentation through remote sensing (Fa et al., 2020). Similarly, research has shown that Brazilian ITs generally have higher vertebrate species richness than the country’s protected areas (Schuster et al., 2019). There is increasing recognition that ITs hold many globally important conservation values, as they remain free from extensive industrial and intensive agricultural operations (Garnett et al., 2018; Fa et al., 2020). The IPBES Global Assessment concluded that the decline of nature is lower in areas managed by Indigenous Peoples than in other lands (see Díaz et al., 2019).
Since the 1980s, countries within the Amazon biome (e.g., Brazil, Bolivia, Peru) have made remarkable strides in ensuring legal recognition of ITs. As of today, we know that there are at least 2447 officially recognized ITs in the Amazon biome, covering approximately 25% of its biogeographic surface (RAISG, 2019). Yet, the extent to which Amazonian terrestrial biodiversity is governed under direct Indigenous stewardship remains an elusive question up to this date. While there have been numerous analyses of how well-represented is biodiversity in Amazonian protected areas (e.g., Oliveira et al., 2017; Fonseca and Venticinque, 2018; Frederico et al., 2018), we still do not know how much of the region’s biodiversity is harboured in ITs (see Schuster et al., 2019 for an exception focusing specifically on Brazilian ITs).
In this article, we use geospatial analytical methods to explore the potential role of ITs in conserving the Amazon’s bat diversity. We focus on bats because they are the second most diverse mammalian order (Burgin et al., 2018) and the Amazon biome is home to one tenth of the world’s known species (López-Baucells et al., 2016), with over 100 species living sympatrically in some localities (Rex et al., 2008). Additionally, Amazonian bats are not only taxonomically and phylogenetically diverse, but also ecologically diverse, as they occupy a wide range of trophic niches (Kunz and Fenton, 2005). Given that a large proportion of Amazonian ITs overlap Intact Forest Landscapes (Fa et al., 2020; Fernández-Llamazares et al., 2020), and considering that some of the most threatened bat species are forest specialists highly dependent on old-growth forests (Medellín et al., 2008; Rocha et al., 2017, 2018), we expect that ITs have an important role to play in Amazonian bat conservation.
In the context of growing concerns over bat conservation in the Neotropics (Frick et al., 2019), we used expert-revised IUCN distribution maps to analyse whether the distribution ranges of 223 bat species intersect with officially recognized ITs, thus estimating the potential contribution of Indigenous stewardship for the conservation of this species-rich mammalian group across the Amazon biome. More specifically, we: (i) assess overlaps between bat ranges and ITs at the biome-wide scale; (ii) examine patterns of overlap between bat ranges and ITs for each Amazonian country; and (iii) identify geographical patterns of bat distribution ranges within ITs. We finally discuss possible tools and pathways that can maximize the contributions of Indigenous Peoples to the conservation and monitoring of Amazonian bats.
Materials and methodsGeographic and species distribution range datasetsThe spatial overlap between ITs and bats across the entire Amazon biome was assessed based on a cross-sectional comparative analysis of two main spatial datasets (i.e., ITs and bat species distribution range data), which were analysed both under the biogeographic boundaries of Amazonia and for each specific country.
The extent and distribution of the ITs were obtained from the Rede Amazônica de Informação Socioambiental Georreferenciada (RAISG, 2019). Since this data has been publicly accessible for many years (with the consent of Indigenous Peoples), we do not foresee any ethical problems in including and presenting the geographical boundaries of the ITs in this article. On the contrary, we believe this might encourage researchers to further recognize the conservation values of these territories and increase the likelihood of engaging Indigenous Peoples in land and ecosystem management. Blank areas in our maps do not necessarily indicate an absence of Indigenous Peoples or their lands, but rather areas for which officially recognized Indigenous land tenure cannot be inferred based on publicly available geospatial data sourced from RAISG. As such, our analyses only include ITs that have been officially recognized following the standard categorization of RAISG (i.e. titled, homologated, demarcated and/or approved by decree).
Bat species distribution ranges were sourced from the IUCN Red List of Threatened Species distribution maps (IUCN, 2018). We considered a total of 223 species that had at least part of their distribution range within the Amazonian biome. All distributions were carefully reviewed and validated by three bat researchers with extensive expertise in Neotropical bat studies (A. L.-B., P. M. V. and R. R.) and, when needed, corrected using ArcGIS v. 10.3.1 (ESRI, 2013). All modifications were based on new species descriptions or taxonomical re-assessments, as well as on several bat distribution range expansions recently published in scientific literature (e.g., Mantilla-Meluk and Montenegro, 2016; López-Baucells et al., 2014, 2018; Velazco et al., 2017). This dataset is available from author R. R. upon request.
Geospatial data for each of the Amazonian country surface areas were sourced from the Global Administrative Areas spatial database (GADM, 2016). The extent of the Amazon biome was determined following its standard biogeographic definition, as defined and provided by RAISG (see a comprehensive description in RAISG, 2017). We stress that while RAISG mostly uses a hydrographic definition of the Amazon (including territories up to the watershed of the headwaters of Amazonian rivers), in this paper we restrict our analysis to the biogeographic boundaries of the Amazon, based on the extent of the Amazon biome (see Eva and Huber, 2005 for further details). We note that some of the figures presented in this paper might vary from the ones reported by RAISG, due to the differences in the definitions used.
Geospatial analysesFor each bat species, we calculated the proportion of the distribution range that overlaps with ITs based on the spatial intersection of the different geographical datasets. We first compiled bat species distribution ranges (validated and corrected) for a total of 223 species into a single map, which we overlapped with the geographic distribution of ITs. The spatial overlay operation was carried out using the geo-algorithm “intersect” in ArcGIS v. 10.3.1 (ESRI, 2013), which enables the calculation of the surface of overlap between ITs and bat distribution ranges.
The first part of the analysis was performed at the biome-wide scale. Considering that around 25% of all the Amazon biome is covered by officially recognized ITs, we adopted this threshold (i.e., 25%) to identify bat species with a substantial section of their distribution range intercepting with ITs. As such, we identified those species with more than 25% of their global distribution range within Amazonian ITs across the entire biome. Additionally, for each of these selected species (those with >25% of their distribution overlapping with Amazonian ITs), we also calculated the percentage of its global distribution within the Amazon section of each of the nine countries (e.g., percentage within Peruvian Amazonia).
Furthermore, the effect of the ITs’ size, their average altitude and latitudinal differences on total bat diversity was modelled using a generalised linear model with Gaussian distribution. All three fixed factors were centred and scaled and effects plots were created using the allEffects function from the 'effects' package in R (Fox, 2003; Fox and Weisberg, 2019). We also created two histograms describing the rarity distribution of bat species across ITs and the distribution of richness in rare bat species across all ITs (see Supplementary Materials for further details). In the context of this study, we defined as rare those species occurring across less than 10% of the ITs of the Amazon biome.
The second part of the analysis was performed at the national scale. The rationale for evaluating national-level conservation values of ITs is that many conservation plans and targets are designed on the sole basis of species distribution range data at the national level (e.g., Montesino-Pouzols et al., 2014; Dallimer and Strange, 2015). As such, we calculated for each species the overlap between the national distribution and the national IT cover within Amazonia. This allowed us to identify those species with more than 25% of their national distribution range within Amazonian ITs in each of the nine countries analysed.
Based on their IUCN Red List status, bat species were classified as Threatened (i.e., species listed as Vulnerable, Endangered and Critically Endangered), Data Deficient (hereinafter DD) or Not Evaluated (hereinafter NE). We then calculated the total number of bat species within each category for each IT and each of the nine considered countries. In order to analyse and visualize how the proportion of Threatened vs DD species are distributed across the Amazon, we also created a bivariate map showing both types of conservation status (see Fig. S1 in the Supplementary Materials). We classified ITs into three classes using quantile classification – based on the distribution of number of threatened species. For Threatened species, we classified ITs as Low (one species), Medium (two species) and High (three to five species). For DD species, we classified ITs as Low (zero to three species), Medium (four to five species) and High (six to 11 species). We identified megadiverse ITs as those ITs with the highest number of bats with overlapping distribution ranges (i.e., first quartile, 103–137 species). Additionally, all bats were also classified into different functional guilds based on Kalko (1998), using the type of diet (i.e., insectivorous, frugivorous, nectarivorous, sanguivorous or carnivorous) and diet strategy (i.e., aerial and gleaning) as the separation criteria.
All the geospatial analyses were carried out in ArcGIS v. 10.3.1 (ESRI, 2013) under the World Cylindrical Equal Area coordinate system.
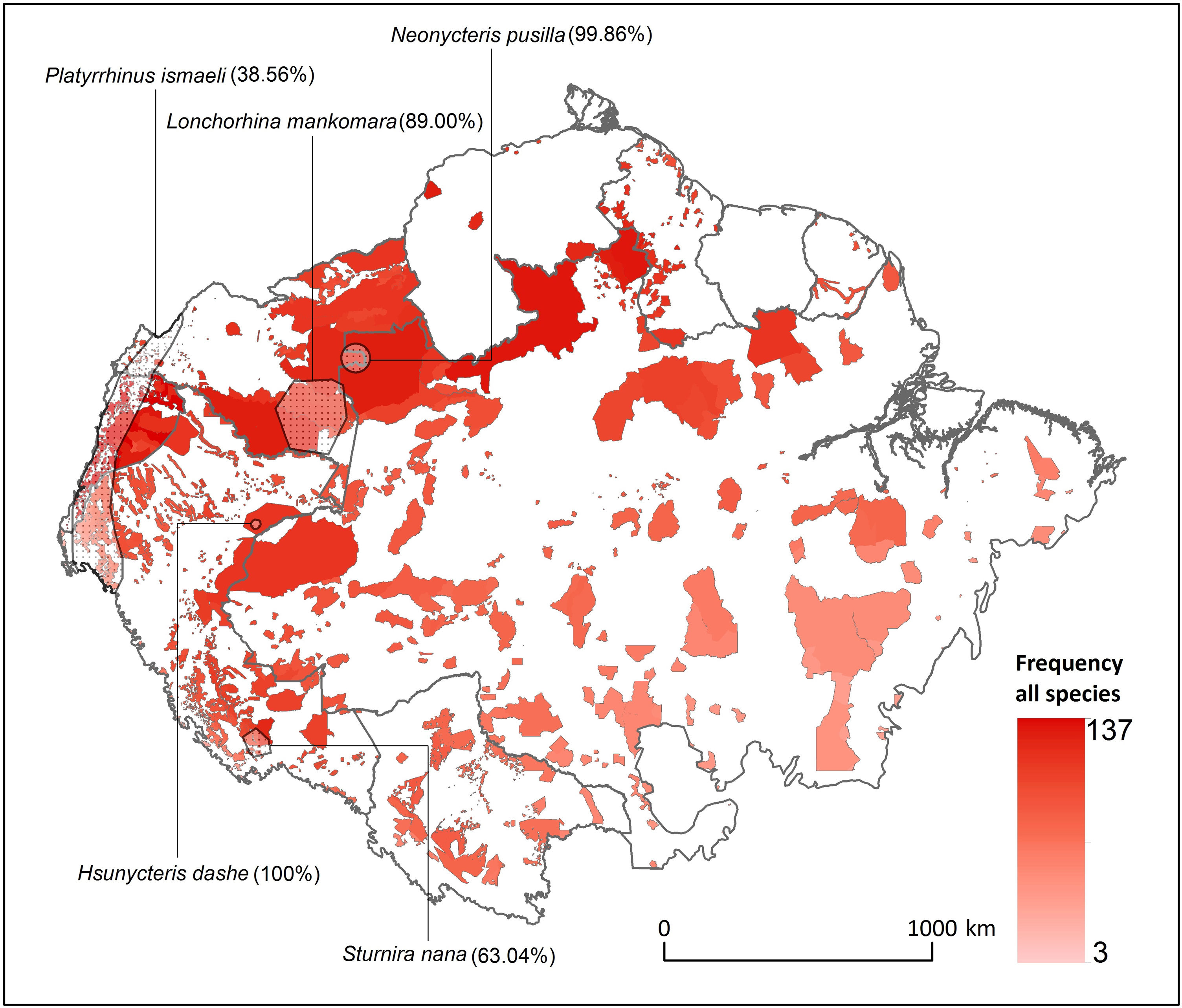
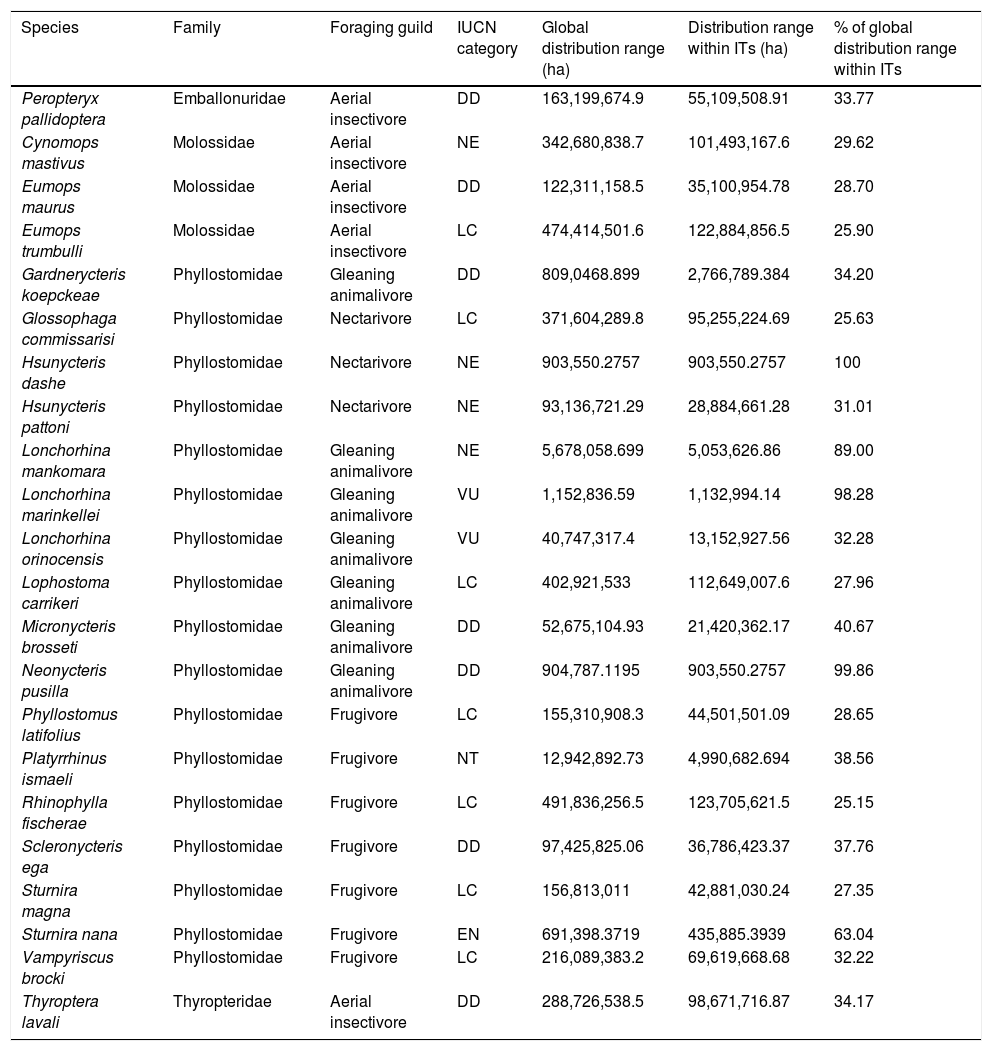
ResultsOverlap between bat ranges and ITs across the Amazon biomeWe found a total of 22 species from four different families and 17 genera that had more than 25% of their global distribution ranges overlapping with ITs (Table 1). Of these, overlapping proportions oscillated between 100% for Dashe’s nectar-feeding bat Hsunycteris dashe and 28.6% for the Carriker's round-eared bat Lophostoma carrikeri (see Fig. 1 for examples of these distribution ranges). Three of the species with over 25% of their global distribution ranges overlapping with ITs are classified as Threatened (i.e., one as Endangered and two as Vulnerable), seven are classified as DD species and four have not yet been evaluated. The remaining seven are currently listed as either Least Concern or Near Threatened (Table 1).
Bat species with over 25% of their global known distribution range located within Indigenous Territories (ITs) across the Amazon Basin.
| Species | Family | Foraging guild | IUCN category | Global distribution range (ha) | Distribution range within ITs (ha) | % of global distribution range within ITs |
|---|---|---|---|---|---|---|
| Peropteryx pallidoptera | Emballonuridae | Aerial insectivore | DD | 163,199,674.9 | 55,109,508.91 | 33.77 |
| Cynomops mastivus | Molossidae | Aerial insectivore | NE | 342,680,838.7 | 101,493,167.6 | 29.62 |
| Eumops maurus | Molossidae | Aerial insectivore | DD | 122,311,158.5 | 35,100,954.78 | 28.70 |
| Eumops trumbulli | Molossidae | Aerial insectivore | LC | 474,414,501.6 | 122,884,856.5 | 25.90 |
| Gardnerycteris koepckeae | Phyllostomidae | Gleaning animalivore | DD | 809,0468.899 | 2,766,789.384 | 34.20 |
| Glossophaga commissarisi | Phyllostomidae | Nectarivore | LC | 371,604,289.8 | 95,255,224.69 | 25.63 |
| Hsunycteris dashe | Phyllostomidae | Nectarivore | NE | 903,550.2757 | 903,550.2757 | 100 |
| Hsunycteris pattoni | Phyllostomidae | Nectarivore | NE | 93,136,721.29 | 28,884,661.28 | 31.01 |
| Lonchorhina mankomara | Phyllostomidae | Gleaning animalivore | NE | 5,678,058.699 | 5,053,626.86 | 89.00 |
| Lonchorhina marinkellei | Phyllostomidae | Gleaning animalivore | VU | 1,152,836.59 | 1,132,994.14 | 98.28 |
| Lonchorhina orinocensis | Phyllostomidae | Gleaning animalivore | VU | 40,747,317.4 | 13,152,927.56 | 32.28 |
| Lophostoma carrikeri | Phyllostomidae | Gleaning animalivore | LC | 402,921,533 | 112,649,007.6 | 27.96 |
| Micronycteris brosseti | Phyllostomidae | Gleaning animalivore | DD | 52,675,104.93 | 21,420,362.17 | 40.67 |
| Neonycteris pusilla | Phyllostomidae | Gleaning animalivore | DD | 904,787.1195 | 903,550.2757 | 99.86 |
| Phyllostomus latifolius | Phyllostomidae | Frugivore | LC | 155,310,908.3 | 44,501,501.09 | 28.65 |
| Platyrrhinus ismaeli | Phyllostomidae | Frugivore | NT | 12,942,892.73 | 4,990,682.694 | 38.56 |
| Rhinophylla fischerae | Phyllostomidae | Frugivore | LC | 491,836,256.5 | 123,705,621.5 | 25.15 |
| Scleronycteris ega | Phyllostomidae | Frugivore | DD | 97,425,825.06 | 36,786,423.37 | 37.76 |
| Sturnira magna | Phyllostomidae | Frugivore | LC | 156,813,011 | 42,881,030.24 | 27.35 |
| Sturnira nana | Phyllostomidae | Frugivore | EN | 691,398.3719 | 435,885.3939 | 63.04 |
| Vampyriscus brocki | Phyllostomidae | Frugivore | LC | 216,089,383.2 | 69,619,668.68 | 32.22 |
| Thyroptera lavali | Thyropteridae | Aerial insectivore | DD | 288,726,538.5 | 98,671,716.87 | 34.17 |
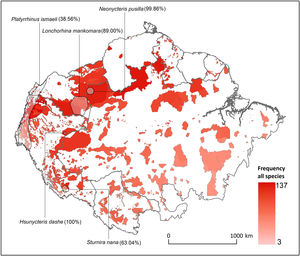
Distribution of Amazonian Indigenous Territories (ITs) classified according to the total number of species occurring within their boundaries. Shadowed areas in red correspond to officially recognized ITs. Distribution ranges of some bat species with >25% of overlap with ITs have been added to illustrate some of our results.
Gleaning animalivores (seven species) and frugivores (six species) of the family Phyllostomidae were the most represented of the bat species with over 25% of their distribution range overlapping with ITs (Table 1). Aerial insectivorous bats on the other hand were only represented by three species of molossids, the Guianan bonneted bat Eumops maurus, the Colombian bonneted bat Eumops trumbulli and the recently described Cynomops mastivus (Moras et al., 2016), one species of emballonurid, the Pale-winged dog-like bat Peropteryx pallidoptera, and the LaVal’s disk-winged bat Thyroptera lavali (family Thyropteridae). Some species such as Dashe’s nectar-feeding bat Hsunycteris dashe are known from only one type locality, which is inside of the IT Comunidad Nativa Matsés in northeastern Peru (Velazco et al., 2017).
Some ITs were found to harbour up to 137 different bat species (i.e., more than 50% of all the Amazonian bat species; Fig. 1). The 22 species with more than 25% of their global distribution within Amazonian ITs are predominantly present in Colombia (19 species), Brazil (18) and Peru (17), with up to 13 species being distributed across the three countries (Table S1 in the Supplementary Materials). In fact, some species had between 70 and 100% of their Amazonian distribution within a single country (e.g. Scleronycteris ega or Phyllostomus latifolius in Brazil, the three Lonchorhina spp in Colombia, or both Sturnira nana and Hsunycteris dashe in Peru).
Finally, our results also show that a large fraction of the bat species analysed (i.e., 32%) are in fact rare (occurring across less than 10% of the ITs of the Amazon biome), and that those territories richer in total number of species are also ranking high in terms of number of rare species (see Figs. S2 and S3 in the Supplementary Materials).
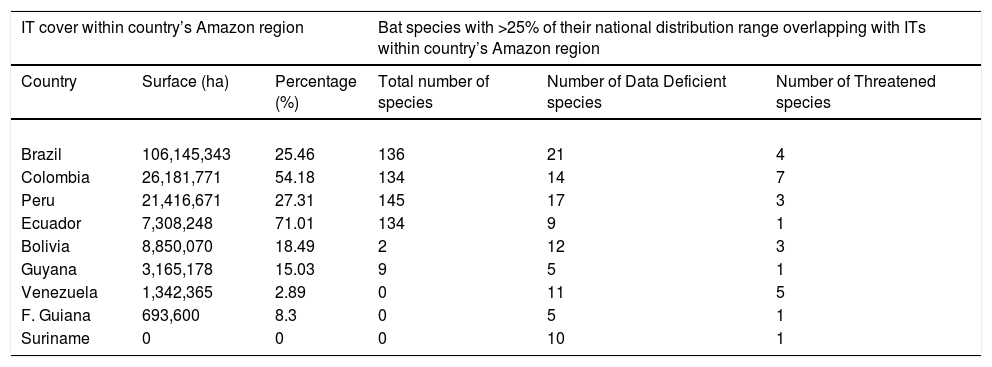
Patterns of overlap between bat ranges and ITs at the national levelThe number of species with more than 25% of their distribution range overlapping with ITs was consistently higher when conducting analyses at the national level (Table 2). Peru was the country with the highest number of bat species with over 25% of their national Amazonian distribution range overlapping with ITs (145), closely followed by Brazil (136), Colombia (134) and Ecuador (134). In contrast, the other five Amazonian countries (i.e., Bolivia, Guyana, French Guiana, Suriname and Venezuela) had fewer than 10 species with more than 25% of their Amazonian distribution range within ITs (Table 2). Here it is important to note that countries with the highest number of bat species on ITs (i.e., Peru, Brazil, Colombia and Ecuador) correspond to those with a highest percentage of officially recognized IT land cover (i.e., ranging from 25 to 55%), whereas countries with a lowest number of bat species overlapping with ITs match those with lower officially recognized IT land cover (i.e., 0–20%). At the national level, the number of Threatened and DD species with more than 25% of their national distribution range overlapping with ITs varied respectively between one (Ecuador, Guyana, French Guiana and Suriname), seven (Colombia), five (French Guiana) and twenty-one (Brazil).
Summary of the geographical overlap analyses between the cover of Indigenous Territories (ITs) and the ranges of bats across the nine countries that share the Amazonian biome. The distribution of land surface area covered by ITs (officially recognized) is in accordance to RAISG 2019.
| IT cover within country’s Amazon region | Bat species with >25% of their national distribution range overlapping with ITs within country’s Amazon region | ||||
|---|---|---|---|---|---|
| Country | Surface (ha) | Percentage (%) | Total number of species | Number of Data Deficient species | Number of Threatened species |
| Brazil | 106,145,343 | 25.46 | 136 | 21 | 4 |
| Colombia | 26,181,771 | 54.18 | 134 | 14 | 7 |
| Peru | 21,416,671 | 27.31 | 145 | 17 | 3 |
| Ecuador | 7,308,248 | 71.01 | 134 | 9 | 1 |
| Bolivia | 8,850,070 | 18.49 | 2 | 12 | 3 |
| Guyana | 3,165,178 | 15.03 | 9 | 5 | 1 |
| Venezuela | 1,342,365 | 2.89 | 0 | 11 | 5 |
| F. Guiana | 693,600 | 8.3 | 0 | 5 | 1 |
| Suriname | 0 | 0 | 0 | 10 | 1 |
At the biome-wide scale, most of the ITs that harbour the highest levels of bat diversity are found in the North-Northwest region of the Amazon, with ITs becoming gradually poorer following a latitudinal gradient towards the South (Fig. 2). Moreover, transboundary areas in the North-Northwest Amazon count with the highest density of ITs, as well as the ITs with the largest areas. Most of the species that were identified as having more than 25% of their global distribution ranges within ITs were generally found in the transboundary areas between Brazil, Colombia and Peru, in the region around Três Fronteiras and close to the Putumayo River (Fig. 2). Some of the ITs with the highest levels of bat diversity correspond to the Alto Río Negro Indigenous Territory (Brazil), the Yanomami Indigenous Territory (Brazil), the Raposa Serra do Sol (Brazil) and the Resguardo Predio Putumayo (Colombia).
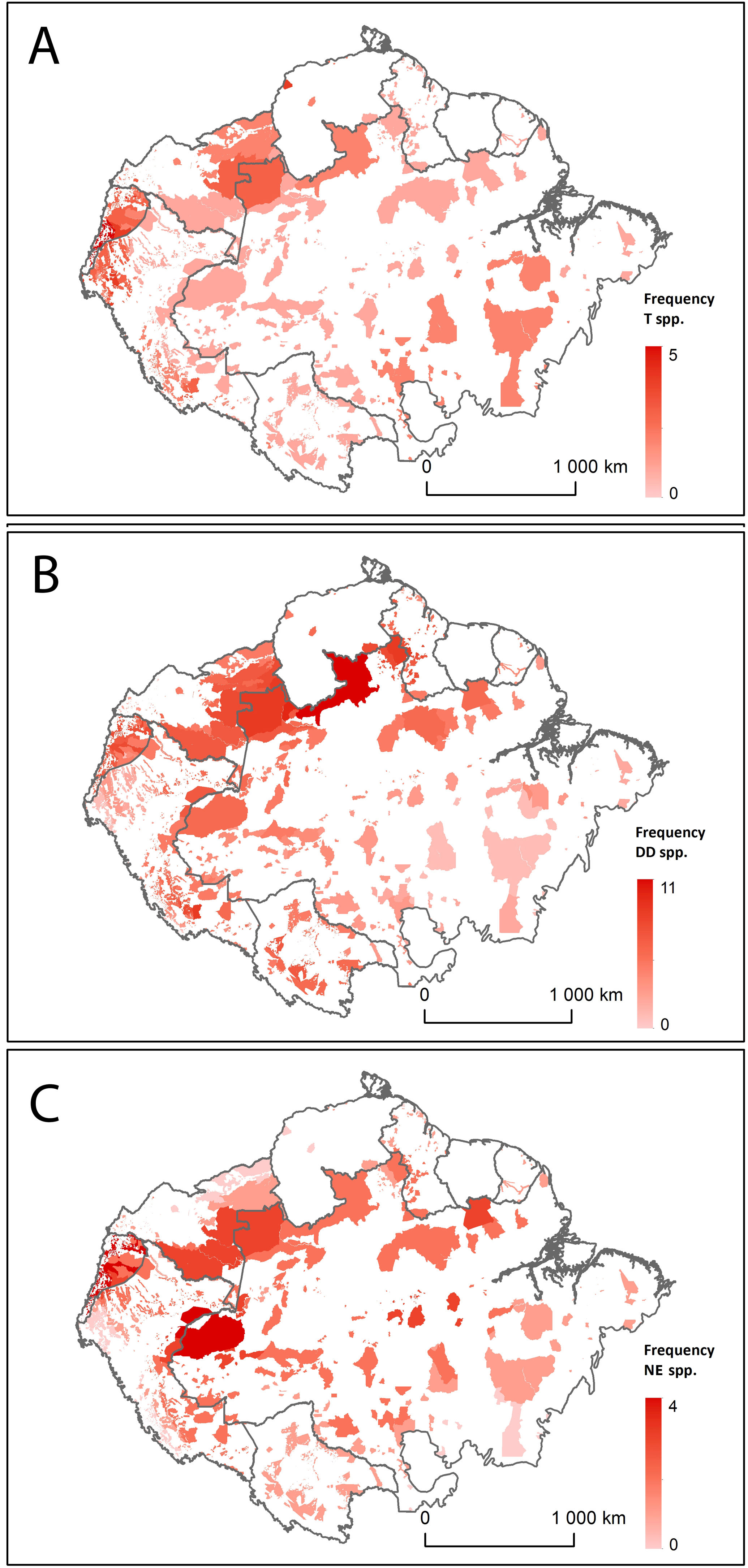
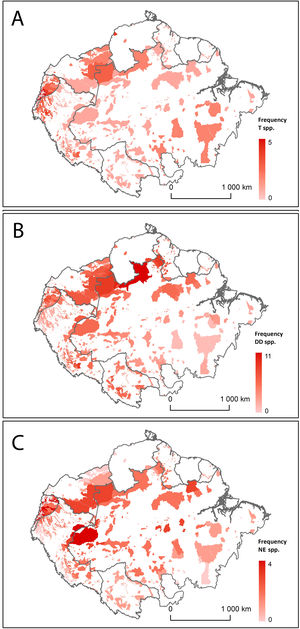
Distribution of Amazonian Indigenous Territories classified according to A) the number of Threatened (T) bat species; B) the number of Data Deficient (DD) bat species; and C) the number of Not Evaluated (NE) bat species occurring within their boundaries. Shadowed areas in red correspond to recognised Indigenous Territories (ITs).
We also found that ITs at higher altitude had lower bat species richness than those in the lowlands (b = −0.068 ± 0.002; z = 28.59; p < 0.0001; see Fig. S4 in the Supplementary Materials). In addition, bat species richness in ITs was found to increase towards northern latitudes (b = 0.025 ± 0.002; z = 11.46; p < 0.0001; see Fig. S5 in the Supplementary Materials). However, IT size did not have any significant effect on bat species richness (b = 0.002 ± 0.002; z = 1.17; p = 0.242; see Table S2 and Fig. S6 in the Supplementary Materials).
The analysis of geospatial patterns for specific conservation categories showed clear differences between the distribution of Threatened, DD and NE species. The first ones have a relatively even distribution range across all Amazonian ITs, although they are remarkably prevalent in the ITs in Southeast Brazilian Amazonia. In contrast, DD and NE species tend to be more abundant in ITs within the North-western Amazon (Fig. 2). ITs located in two transboundary areas at Colombia-Brazil and Colombia-Ecuador frontiers harbour both high numbers of threatened and DD bat species (Fig. 2).
DiscussionOur work shows the potential role that ITs could play in conserving Amazonian bat diversity. We found that 22 Amazonian bat species had over one quarter of their known global distribution ranges overlapping with ITs. Even if this only represents around 10% of all the bat species surveyed, most of these species are forest-dependent, have small distribution ranges and a threatened or DD conservation status. Because most of these species are rarely captured in bat surveys, their natural history is still poorly documented (e.g. Eumops maurus is known for fewer than a dozen localities; López-Baucells et al., 2018). Yet, the evidence from the current literature on how species traits’ influence the persistence of Amazonian bats in modified landscapes (Farneda et al., 2015; Fraixedas Núñez et al., 2019) suggests that many of these species are highly associated with relatively undisturbed old-growth forests. This includes species such as the Least Big-eared Bat Neonycteris pusilla (99.86% in ITs), or the Marinkelle's sword-nosed bat Lonchorhina marinkellei (98.28%). For a number of species, such as Carriker's Round-eared Bat Lophostoma carrikeri (27.96%) and Brock's yellow-eared bat Vampyriscus brocki (32.22%), this assessment is supported by quantitative analysis of habitat specificity (see Rocha et al., 2018 for further details).
We also found that a considerable share of Amazonian bat species (up to 145, in the case of Peru) have more than 25% of their national distribution ranges within Amazonian ITs. Considering that most biodiversity conservation plans are designed at the national level (Montesino-Pouzols et al., 2014), the role of ITs in national bat conservation strategies could be therefore very significant, particularly in Brazil, Colombia, Peru and Ecuador. Additionally, megadiverse ITs in transboundary areas between Brazil, Colombia and Peru were found to be high priority conservation areas for Amazonian bats.
We believe that the patterns observed in this study can be linked to: (i) spatial covariation between IT location and high habitat suitability for bats; and (ii) long-term effects of Indigenous stewardship on bat habitat quality. On the first point, median elevation and closer proximity to streams have been reported to increase the likelihood of occurrence for isolated Indigenous societies in the Amazon (Kesler and Walker, 2015). Along these lines, Carrasco-Rueda and Loiselle (2019) recently highlighted that riparian forest strips provide important resources for bats in Peruvian Amazonia. The fact that many Indigenous communities are settled in the proximity of rivers and streams, mostly in lowlands, could therefore explain at least some of the patterns observed. Similarly, we have also shown that average elevation and latitude are important environmental correlates of bat richness across ITs in the whole Amazon biome (see Figs. S2 and S3 in the Supplementary Materials). Following a commonly observed pattern, the diversity of Neotropical bats tends to decline with increasing altitude (Carvalho et al., 2019) and decreasing latitude (Ramos Pereira and Palmeirim, 2013; Arita et al., 2014).
On the second point, extensive research has already highlighted that Indigenous communities have shaped forests’ structure and composition over millennia through practices such as understorey clearance, domestication, fire management, drainage and soil modification, to cite just a few (see Levis et al., 2017, 2018; Roberts et al., 2017). All these practices might have increased landscape heterogeneity and created highly suitable micro-habitats for bats, although this needs to be confirmed by on-the-ground field-based research. A recent study has shown that ITs represent around 45% of all the remaining wilderness areas in the Amazon, but account for less than 15% of all the forest loss occurring within these lands (Fernández-Llamazares et al., 2020). As species richness of Amazonian bats increases with landscape-scale cover of old-growth forest (Rocha et al., 2017), it is thus plausible that many bat species might benefit from the relatively undisturbed forest habitats retained in the region’s ITs, which total an area of three times the surface of Germany (see Fernández-Llamazares et al., 2020). Advanced geospatial analyses based on satellite data have shown that deforestation levels are generally lower in ITs than in other lands (e.g., Nolte et al., 2013; Schleicher et al., 2017). This is evidenced throughout the southern rim of the Amazon, where today ITs represent the only islands of biological and cultural diversity in the larger landscape (Le Tourneau, 2015). However, the natural values of the habitats harboured in ITs, and their potential for bat conservation, are only starting to be uncovered.
Half of the 22 bat species that had over 25% of their distribution range within ITs across the Amazon biome are either DD or NE. These figures are reasonably higher at national levels (e.g., 21 DD bat species in Brazilian Amazonia). Research shows that NE and DD species are very likely to be under immediate threat (e.g., Bland et al., 2015; Jarić et al., 2016; Welch and Beaulieu, 2018), but the pervasive lack of data on population status and trends hinders efforts to prioritize conservation action (Frick et al., 2019). Although knowledge on Amazonian bat diversity is steadily increasing (e.g., Reis et al., 2006; Pacheco et al., 2008; López-Baucells et al., 2014, 2018), available information on the occurrence and distribution of bat species in the Amazon is still scarce, heterogeneous, and scattered (Aguiar and Machado, 2005; Frick et al., 2019). For example, it is estimated that robust bat data is available for less than 25% of the Brazilian Amazon (Bernard et al., 2011).
This work pinpoints at several priority ITs in terms of bat diversity where research efforts could be focused, should Indigenous communities and their legitimate political organizations choose to allow, and/or engage in, these efforts. An important finding from this work is that bat research and conservation is particularly important in transboundary ITs. At least 4589 km of the Brazilian border in the Amazon intersects transboundary wilderness areas (see Fernández-Llamazares et al., 2020). All along the Brazilian borders, ITs are critical for maintaining large, contiguous and well-conserved forest ecosystems, particularly across the Western Amazon (Le Tourneau, 2015; Rull et al., 2016). Due to their substantial topographic complexity, these transnational areas harbor exceptional levels of endemism (e.g., Kessler, 2002). In this paper we have shown that some of these transboundary areas are dominated by species-rich ITs of great conservation importance, given that some of these ITs harbour the whole estimated global distribution of some highly threatened bat species. Yet, ITs along borderlands tend to be tenure-insecure, and due to their limited federal oversight, they are particularly vulnerable to land encroachment and pressures such as logging and mining (Salisbury et al., 2011). Stronger transboundary cooperation is critical to safeguard the natural and cultural values of these lands from intensive development (Pringle, 2014; Walker et al., 2014). Additionally, the consolidation of trans-boundary Amazonian policy initiatives, such as the Guiana Shield Initiative, the Madre de Dios, Acre and Pando Initiative, or the Andes-Amazon-Atlantic Biocultural Corridor seems particularly important for bat conservation.
Finally, we believe that Indigenous Peoples could potentially play a critical role in filling bat data gaps and information deficits in large parts of the Amazon, as well as in refining our understanding not only of the conservation status and population trends of many NE and DD species, but also gradients of bat species richness, areas of endemism and largely unknown distribution range discontinuities. Scholars are increasingly acknowledging the importance of establishing partnerships for knowledge co-production between scientists, practitioners and Indigenous Peoples to improve and enrich the knowledge basis that underpins conservation policy and practice (Tengö et al., 2014; Sterling et al., 2017). There are many examples of how collaboration between Indigenous Peoples and researchers has furthered our understanding of species ecological distribution ranges, baselines and trends (Mistry and Berardi, 2016; Skroblin et al., 2019), including IPE’s landmark program on participatory biodiversity monitoring in Amazonian conservation units (IPE, 2019).
Complementing conventional science-based monitoring methods (e.g., bioacoustics) with Indigenous observations and knowledge can help to monitor local biodiversity in more efficient ways than science alone (see also Kutz and Tomaselli, 2019; Ward-Fear et al., 2019). For example, bat species that are observable while roosting can be monitored by directly counting them in specific locations that might be known by the local communities (Frick et al., 2019). Furthermore, providing opportunities for capacity development (e.g., participatory monitoring through roost counts or acoustic surveys) for Indigenous communities can also be desirable, but only when conducted within a collaborative framework (Danielsen et al., 2007; Ban et al., 2018). Filling bat data gaps across the Amazon will require an investment in building capacities of new generations of bat experts (Bernard et al., 2011) and we believe that Indigenous knowledge-holders could play a crucial role in this endeavour. As a case in point, the three specimens of Hsunycteris dashe that allowed the identification and description of the species were collected at diurnal roosts discovered by Matsés Indigenous Peoples in Peruvian Amazonia (Velazco et al., 2017). Therefore, when appropriate and desired by Indigenous Peoples, scientists could collaborate with Indigenous knowledge-holders in the monitoring of bats across the Amazon biome.
Study limitationsSampling biasesIt is important to note that the numbers highlighted in this study are most likely conservative estimates. While we only used officially recognized ITs for our analysis, we know that many Indigenous lands remain unrecognized to this day. We therefore consider that the amount of biodiversity that de facto depends on Indigenous stewardship across the Amazon should be reasonably higher. Moreover, we know that bat research within Amazonian ITs has been meagre at best (e.g., Bernard et al., 2011; Delgado‐Jaramillo et al., 2020), especially for some guilds such as the aerial insectivorous bats, for which available information is rather poor in almost all the Amazon. The fact that research permits to sample bats –as well as other taxa– in many Amazonian countries do not include authorization to enter ITs (e.g., Bolivia, Brazil) adds to this challenge. The stringent legislation controlling access and activities within ITs has been pinpointed as a potential barrier to carry out conservation-related research in these areas, given the increased bureaucratic load entailed (Guedes dos Santos et al., 2015).
IUCN distribution mapsWe acknowledge that the use of IUCN distribution maps has some limitations. IUCN distribution maps represent the known or inferred limits of a species’ distribution range as a minimum convex polygon shape. Therefore, the polygon represents those areas where certain bat species might occur, although this does not mean that the distribution of the species is even within the whole area, or that it does not expand beyond it. It is important to note that these distribution polygons are, in practice, positioned somewhere between the extent of occurrence and the true area of occupancy of the species (see Rondinini et al., 2006 and Gaston and Fuller, 2009). Even after our careful, expert revision of all the IUCN distribution maps, we note that our study findings should be treated with caution.
While some authors highlight that IUCN distribution data possibly underestimate the extent of occurrence and overestimate the true area of occupancy (see Montesino-Pouzols et al., 2014), others have observed that these data do not appear to be subject to high omission errors (Venter et al., 2014). For amphibians, for instance, 95% of the known occurrence of 4500 amphibian species fall within or immediately adjacent to their mapped distribution (Ficetola et al., 2014). Moreover, these maps have been shown to be relatively robust to commission errors (i.e., the species is mapped as present in locations where it is in fact not present). Venter et al. (2014) simulated commission errors in the IUCN data to see how it would influence their results, and they actually found out that their results were robust to randomly simulated commission errors on the maps.
Nonetheless, despite recognised data gaps and limited data accuracy for certain range-restricted species (particularly in some remote areas), the IUCN Red List of Threatened Species represents insofar the most frequently used and updated dataset on the distribution range of vertebrate species and their conservation status (Schipper and Chanson, 2008; Le Saout et al., 2013; Montesino-Pouzols et al., 2014). They have been extensively used in conservation research, particularly in global-level overviews at coarse-scale resolution (e.g., Strassburg et al., 2012; Davidson and Dulvy, 2017). In fact, their use is ubiquitous in analyses on the effectiveness of protected areas in representing biodiversity (Rodrigues et al., 2004; González-Maya et al., 2015; Klein et al., 2015) and incipient in studies assessing the biodiversity harboured in ITs (see Schuster et al., 2019). As such, it constitutes the most tenable geospatial resource to carry out exploratory analyses at coarse-scale resolution on the intersections between bat distribution ranges and ITs (see also Conenna et al., 2017), in the very same way in which they have been used in studies on protected area effectiveness (Jenkins et al., 2015; Klein et al., 2015).
Species distribution modelsFuture studies on this vein could benefit from the use of species distribution models (SDM), predictive modelling, and/or species’ area of habitat data (e.g., Rondinini et al., 2011; Santini et al., 2019; Brooks et al., 2019). For instance, Delgado‐Jaramillo et al. (2020) have modelled the distribution of several Amazonian bat species using Maxent. Although we have opted not to use predictive modelling due to the scarce number of records of several of our target species, we emphasise that for some better known Amazonian bat species, predictive models could be used to identify the potential overlap of their distribution and ITs. Such an approach would greatly benefit from a curated database of bat distribution across the Amazon (see Muylaert et al., 2017 for an example of such a database for the Atlantic Forests of South America). As for SDMs, it is important to note that their limitations are substantial when applied to microendemics or to small numbers of occurrences, and when accurate corresponding covariates are not available (Wisz et al., 2008; Synes and Osborne, 2011; van Proosdij et al., 2015).
ConclusionsThis study presents new evidence on the potential role of ITs in safeguarding the biodiversity of the largest and most diverse rainforest on our planet. At a time when parties to the Convention on Biological Diversity prepare the post-2020 Biodiversity Framework, we hope that this manuscript can add to the discourse on the role of Indigenous Peoples in conservation, and serve as a lodestone for other similar studies assessing the conservation potential of Indigenous lands.
Evaluating the contributions of Amazonian Indigenous Peoples to biodiversity conservation is perhaps timelier than ever, given that nature managed by Indigenous Peoples is under increasing pressure (Begotti and Peres, 2019; Romero-Muñoz et al., 2019). Current socio-political trends across the entire Amazon have put Indigenous Peoples’ millennia-long forest stewardship under assault, with many ITs being opened up to mining, agro-business, logging, infrastructure development, and oil and gas operations (Finer et al., 2015; Fernández-Llamazares et al., 2018). We believe that answering this question is central to the implementation of several global conservation agreements (Garnett et al., 2018).
Our results reveal the important contributions of stewardship by Indigenous Peoples in governing the territories where some of the Amazon’s most unique, rarest and unknown bat species are found. We have documented that the future of substantial numbers of bat species in Amazonian countries (notably Brazil, Colombia, Peru and Ecuador) will largely depend on whether Indigenous Peoples’ choose to safeguard them or not. A corollary of this is that we need to devise more effective mechanisms to collaborate with Indigenous Peoples in the monitoring, management and conservation of these species. Advancing global systems of rights, responsibilities and mechanisms to support Indigenous Peoples’ contributions to conservation research and practice is paramount if the remaining tracts of healthy and thriving Amazonian ecosystems and the biodiversity they harbour are to remain intact from the expansion of commodity frontiers and resource extraction.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work has been supported by the Academy of Finland (grant agreement nr. 311176) and the Kone Foundation. We acknowledge the Rede Amazônica de Informação Socioambiental Georreferenciada (RAISG) for publicly sharing geospatial data on the distribution of Indigenous Peoples’ lands across the entire Amazon. Additional funding was provided by the Portuguese Foundation for Science and Technology to A.L.-B. (PD/BD/52597/2014) and by ARDITI – Madeira’s Regional Agency for the Development of Research, Technology and Innovation to R.R. (grant M1420-09-5369-FSE-000002).