Changes in animal population dynamics and community composition following species (re)introduction may have unanticipated consequences for a variety of downstream ecosystem processes, including infectious disease transmission. Due the lessons learned from ongoing projects, we present a novel approach on how to anticipate, monitor, and mitigate the vampire bats and rabies in rewilding projects. We pinpoint a series of precautions and the need for long-term monitoring of vampire bats and rabies responses to rewilding projects and highlighted the importance of multidisciplinary teams of scientist and managers focusing on prevention educational program of rabies risk transmitted by bats. In addition, monitoring the relative abundance of vampire bats, considering reproductive control by sterilization and oral vaccines that autonomously transfer among bats would reduce the probability, size and duration of rabies outbreaks. The rewilding assessment framework presented here responds to calls to better integrate the science and practice of rewilding and will help conservation practitioners and researchers to develop effective message framing strategies that minimize bats emerging infectious diseases and support biodiversity and its associated ecosystem services.
The urgent need to reverse anthropogenic impacts on ecosystems and biodiversity is one of the biggest challenges for modern society (Svenning et al., 2016; Perino et al., 2019; Moreno-Mateos et al., 2020). Discussions on post-2020 biodiversity strategies by the signatory countries of the Convention on Biological Diversity are currently being initiated, and the United Nations General Assembly has recently declared 2021–2030 the “decade of ecosystem restoration” (United Nations, 2019). Due the designation, policy- and decision-makers will push animal restoration topics to the forefront of discussions about how to reach post-2021 biodiversity goals, especially because restoration projects could provide a buffer to species extinction (Galetti et al., 2017) and restore plant–animal interactions and ecological processes impaired by defaunation (Pires, 2017; Genes et al., 2019; Mittelman et al., 2020). However, changes in animal population dynamics and community composition following species (re)introduction may have unanticipated consequences for a variety of downstream ecosystem processes, including food web structure (Lovari et al., 2014), predator-prey systems (Bovendorp and Galetti, 2007) and infectious disease emergence (Lafferty and Gerber, 2002). This highlights the need to develop frameworks to anticipate, monitor, and mitigate the unintended ecosystem consequences of ‘re-wilding’ projects.
In recent years, bats have received growing attention as reservoirs of pathogens linked to emerging zoonotic diseases that cause significant human and animal morbidity and mortality. Key examples include Ebola, Nipah and potentially the ongoing COVID-19 pandemic, given that viral strains closely related to SARS-CoV-2 have been detected in bats (Letko et al., 2020). In the Neotropics, the most notorious bat-transmitted pathogen is rabies virus (Rhabdoviridae, Lyssavirus), which causes a nearly universally lethal infection in all mammals (including humans) and is maintained by a wide variety of bat species (Streicker et al., 2010; Fisher et al., 2018). Among the three species of vampire bats (Desmodus rotundus, Diaemus youngi, and Diphylla ecaudata) that rely on blood as their food source, D. rotundus is the only known reservoir for rabies. D. rotundus frequently bites domestic and wild animals and more rarely humans during feeding (Bobrowiec et al., 2015; Galetti et al., 2016; Gnocchi and Srbek-Araujo, 2017; Zórtea et al., 2018; Gonçalves et al., 2020), creating opportunities for the transmission of rabies virus and potentially other saliva-borne viruses (Bergner et al., 2020).
Official guidelines for managing rabies transmitted by vampire bats involve pre-exposure vaccination of livestock, post-exposure vaccination of humans bitten by vampire bats (and more rarely pre-exposure human vaccination) and bat population control (Benavides et al., 2020a; Recuenco, 2020). Although bat culls sometimes involve vigilante action such as burning or destruction of bat roosts, governments actively discourage these non-specific actions in favor of topical, anticoagulant poisons (“vampiricides”) which spread intra-specifically by vampire bat grooming. Similar poisons can be applied to the wounds of bitten livestock, and ingested when bats return to feed (Thompson et al., 1972). Policies for vampire bat monitoring and initiating culls are country-specific and implemented by Ministries of Health and/or Ministries of Agriculture. In general, culls are reactive to reports of increased vampire bat bites on livestock or detection of rabies cases via passive surveillance systems. While culling effectively reduces vampire bat bites, some studies suggest that significant mitigation of the burden of rabies in humans and domestic livestock would require implementing culls over impractically large geographical areas (Streicker et al., 2012; Blackwood et al., 2013; Bakker et al., 2019). Other proposed strategies for controlling vampire bat populations involve modified farming practices that reduce the accessibility to bats (e.g., artificial lighting, protected corrals, altering the composition and locations of herds) or hormonal reproductive control of bats, though the latter is in early research stages (Benavides et al., 2020a).
D. rotundus has specialized on blood of medium to large-bodied mammal species and demonstrates strong plasticity of foraging strategies due to changes in local prey availability across regions (Table 1). Due to the proliferation of livestock production in much of the Neotropics and its relative reliability compared to native wildlife, livestock are generally preferred prey (Voigt and Kelm, 2006; Gonçalves et al., 2017; Bohmann et al., 2018). However, dietary shifts to humans and wildlife have also been reported following changes in local prey abundance. For example, in Brazil, conversion of pasture into plantation agriculture forced vampire bats switch from the formerly abundant livestock animals to wild animals (Galetti et al., 2016). In Belize, removal of livestock increased D. rotundus feeding on human beings (McCarthy, 1989) and removal of wildlife by hunting was speculated to increase human rabies risk in Peru (Stoner-Duncan et al., 2014). In the context of rewilding, released species may be at particular risk since candidate areas for restoration tend to have low availability of medium to large-bodied wild prey due to historical human disturbance (Galetti et al., 2017; Fernandez et al., 2017). In addition, rewilding projects typically rely on captive born animals (Fernandez et al., 2017) that have diminished functional traits associated with adaptation to captivity, including exposure to predators (Jule et al., 2008), which could heighten vulnerability to vampire bats.
Prey species observed bitten by vampire bats in the Neotropical area.
| Prey | Common name | Country | Bioregion | Ref |
|---|---|---|---|---|
| Reptile | ||||
| Elaphe flavirufa | Yellow-red Rat Snake | Mexico | Mesoamerica | 1 |
| Mammals | ||||
| Callicebus aureipalatii | Madidi Titi Monkey | Peru | Amazon | 2 |
| Coendu sp. | Porcupine | Mexico | Mesoamerica | 3 |
| Dasypus sp. | armadillo | Mexico | Mesoamerica | 3 |
| Hydrochaeris hydrochaeris | Capybara | Brazil, Venezuela | Atlantic forest,Illanos | 4,5,6,7 |
| Mazama americana | Red Brocket | Brazil | Atlantic forest,Pantanal | 8,9 |
| Odocoileus virginianus | White-tailed Deer | Mexico | Mesoamerica | 10 |
| Otaria flavescens | Southern Sea Lion | Peru, Chile | Coast, Island | 2,11,12 |
| Pecari tajacu | Collared Peccary | Brazil | Atlantic forest | 8 |
| Priodontes maximus | Giant Armadillo | Brazil | Atlantic forest | 8 |
| Saimiri boliviensis | Squirrel Monkey | Peru | Amazon | 2 |
| Sturnira liliuma | Yellow-shouldered Bat | Argentina | Dry forest | 13 |
| Sus scrofa | Wild Boar | Brazil, Mexico | Atlantic forest,Pantanal,Mesoamerica | 9,14 |
| Tapirus terrestris | Lowland Tapir | Peru, Brazil | Amazon,Atlantic forest,Pantanal | 2,8,9 |
| Tayassu pecari | White-lipped Peccary | Peru | Amazon | 2 |
| Sciurus sp. | Squirel | Mexico | Mesoamerica | 16 |
| Birds | ||||
| Cormorants | sea birds | Chile | Island | 12 |
| Pelicans | sea birds | Chile | Island | 12 |
| Spheniscus humboldti | Humboldt Penguin | Chile | Island | 17 |
1-Villa and Lopez-Forment, 1966; 2-Streicker and Allgeier, 2016; 3-Greenhall et al., 1983; 4-Gonçalves et al., 2020; 5-Azcarate, 1980; 6-Ibanez, 1981; 7-Carranza, 1982; 8-Zortéa et al., 2018; 9-Galetti et al., 2016; 10-Sánchez-Cordero et al., 2011; 11-Catenazzi and Donnelly, 2008; 12-Mann, 1951; 13-Lord et al., 1973; 14-Hernández-Pérez et al., 2019; 15-Gnocchi and Srbek-Araujo, 2017; 16-Greenhall, 1972; 17-Luna-Jorquera and Culik, 1995.
Recently, an unprecedented study in Brazil showed changes in vampire bat feeding following a rewilding project (Gonçalves et al., 2020). Specifically, in a land-bridge island where 100 individuals of 15 non-volant mammal species were introduced with the intent of ‘‘restoring’’ the local fauna, D. rotundus fed primarily on introduced capybaras (Gonçalves et al., 2020). Thirty-six years following restoration, the relative abundance of common vampire bats was higher on the islands than the mainland nearby due to the increased prey availability. The restoration further transformed the land-bridge island into a high-risk area for rabies transmission as three introduced capybaras were confirmed to have died from bat transmitted rabies (Gonçalves et al., 2020).
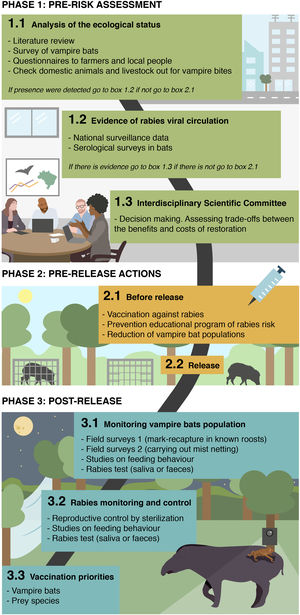
Due the lessons learned from ongoing rewilding projects combined with public health risk if rabies infections from (re)introduced species lead to human exposures, we present a novel approach on how to prevent and control vampire bats and rabies in rewilding projects (Fig. 1). While we highlight vampire bats as a focal example, we seek to establish a generalizable rewilding-monitoring framework that can anticipate and detect how indirect processes may alter the outcomes of restoration projects.
Phase 1: pre-risk assessmentThe first step of the framework should assess the presence and infection status of natural reservoir hosts in the focal area. In the case of vampire bats, presence could be verified by field surveys or questionnaires to local people about bat bites on domestic animals (a strong indicator of D. rotundus presence). If vampire bats are confirmed present, viral circulation could be assessed using serological surveys in wild bats to detect antibodies from recent exposures (Meza et al., 2020). Since naïve domestic animals are increasingly recognized to sometimes survive and mount detectable antibody responses to rabies virus exposures, serological surveys of livestock reported to be un-vaccinated by their owners could similarly indicate viral circulation (Benavides et al., 2020b). Finally, recent records of rabies incidence in humans, domestic or wild animals from national surveillance systems could provide further evidence of rabies circulation. If vampire bats are present and there is evidence of viral circulation, multidisciplinary teams of scientists and managers should develop plans that assess the benefits and costs of the rewilding project, both in terms of the success of restoration (particularly how added rabies mortality would affect the initial establishment of rare/threatened species) and potential public health risks arising from human exposures to rabies cases in restored wildlife.
Phase 2: pre-release actionsReduction of vampire bat populations may be considered prior to release by a multidisciplinary teams of scientist that have to evaluate the ethical issues on both, to mitigate effects on re-introduced prey (blood loss, rabies transmission) and on other bat species which may compete with D. rotundus for roost space. In much of its range, D. rotundus populations are controlled using topical, anticoagulant poisons, which are either applied directly to captured bats and spread by grooming or can be applied to livestock to be ingested when bats feed (Johnson et al., 2014). Although social disruption of culls is predicted to increase rabies transmission in endemic areas (Streicker et al., 2012; Blackwood et al., 2013), culls carried out in rabies free populations may reduce the likelihood of viral incursions (Bakker et al., 2019). Reproductive control by sterilization (Serrano et al., 2007) is less explored alternative. Although the time lag between sterilization campaigns and diminished population sizes makes this tool ineffective at the pre-release stage, it could be a valuable long-term strategy to manage vampire bat population sizes (see phase 3).
Presumably, if vampires are present and rabies is detected, all mammals (> 1 kg) should be vaccinated against rabies before release. Under this phase, prevention educational program of rabies risk transmitted by bats should begin by a multi-disciplinary task force, included public and animal health services and universities, focusing on local people living in the destined site and those actually working on the rewilding project.
Phase 3: post-releaseManagement of vampire bat populationsMonitoring presence and/or abundance of vampire bat is particularly challenging in the context of rewilding. Surveys of bat bites are unlikely to be appropriate since livestock will no longer be abundant following most habitat restoration projects and analogous monitoring of more elusive wildlife is not practical. Another indirect technique, acoustic monitoring, has limited utility due to the ‘soft’ echolocation of vampire bats (Rodríguez-San Pedro and Allendes, 2017). Consequently, labor-intensive field surveys may be the only option to monitor increases in vampire bat populations following restoration. Past studies have measured relative abundance using mark-recapture in known roosts or by carrying out mist netting away from roosts and potential prey sources (Delpietro et al., 1992; Streicker et al., 2012). In conjunction with capture efforts, studies on feeding behaviour [e.g. genetic analysis of blood meals (Bohmann et al., 2018) feces (Bobrowiec et al., 2015), camera trap (Galetti et al., 2016; Zortéa et al., 2018) and stable isotopes (Streicker and Allgeier, 2016; Gonçalves et al., 2020)] could monitor if introduced species (and which) are being exploited as a blood source in order to guide longer term vaccination priorities.
Rabies monitoring and controlMonitoring for rabies in the bats themselves can indicate viral circulation, but has limitations from a long term management perspective. The most commonly used antibody assays of rabies exposure necessarily lag behind transmission. Moreover, other bats (e.g. insectivores) may also have rabies and could cause seroconversion in D. rotundus; necessitating caution in interpretation of serological data. Monitoring by killing bats and testing their brains for rabies antigen is also unlikely to be productive since the prevalence of rabies is usually so low in randomly sampled bats (<1%). PCR based testing in saliva or feces might be considered, especially if field-based testing becomes available since several teams have shown that RNA can be detectable more readily than previously expected (Bergner et al., 2020; Begeman et al., 2020), but cost limits this approach to occasional evaluations rather than long term monitoring.
Since the long term persistence of rabies seems to be a spatial process (e.g., waves of infection that move across the landscape or metapopulation dynamics), cases in livestock or humans reported through existing national surveillance systems in areas near rewilding projects could function as an early warning system. Since rabies is an OIE notifiable disease, such surveillance systems are well established and routinely used, providing a wealth of data on the distribution of rabies. Moreover, genetic or antigenic typing of rabies positive animals could provide clear evidence of vampire bat rabies (as opposed to variants associated with other bats or terrestrial wildlife) circulation in or in close proximity to restoration projects (Fooks and Jackson, 2020). In most countries, analogous rabies diagnostic testing of suspicious wildlife is not routinely carried out in the absence of human exposure. Such testing might have greater priority in the context of restoration projects since restoration actions themselves are likely to reduce the presence of livetock available for rabies detection via existing passive surveillance systems. Whether relying on reports from domesticated or wild animals, communication between restoration managers and the epidemiologists and laboratory diagnosticians in rabies control programs will be vital.
Rabies circulation in vampire bats might also be incidentally detected through reports of unusual bat activity (e.g., daytime flight or discovery of moribund or dead bats) by the local public. Educational programs in the pre-release phase might emphasize the importance of reporting such events, to whom reports should be directed, and instructions for safe handling of moribund bats. Educational materials should convey not only the risk of human rabies from bats but also conservation messages about the broad ecological benefits of bats (e.g., seed dipersal, pollination).
If evidence of rabies circulation is detected, interventions could either target restored wildlife or the bats themselves. Non-bat species could be considered for vaccination based on observations of vampire bat bites or analysis of vampire bat diets in conjunction with an evaluation of the species-specific impacts of rabies losses for rewilding aims. Vaccination of bats may be effective, particularly given tentative evidence that vaccines that administered to bats that are already infected may stop them from transmitting (Cárdenas-Canales et al., 2020). The new and promising bat-to-bat transfer and ingestion oral vaccine (Bakker et al., 2019) may reduce costs and increase population level coverage. The vaccine is transferred among individuals through contacts due high rates of social grooming and, even at low but achievable levels of vaccine application, can control vampire bat rabies and could be applied either before or during outbreaks (Bakker et al., 2019).
ConclusionWe pinpoint a series of precautions and the need for long-term monitoring of vampire bats and rabies responses to rewilding projects. We highlighted the importance of multidisciplinary teams of scientist and managers, including national surveillance systems, focusing on prevention educational program of rabies risk transmitted by bats in rewilding projects. In addition, monitoring the relative abundance of vampire bats, considering reproductive control by sterilization and oral vaccines that autonomously transfer among bats would reduce the probability, size and duration of rabies outbreaks. The rewilding assessment framework presented here responds to calls to better integrate the science and practice of rewilding (Nogués-Bravo et al., 2016) and also the urgent need for long-term studying of bat-transmitted pathogen in the Neotropical area as the region is considered a geographic hotspots of “missing bat zoonoses” (Olival et al., 2017). Although there are challenges remaining, we believe that the implementation and further development of our monitoring framework will help catalyse a positive and ambitious vision for rewilding. Furthermore, the application of this framework provides guidance for practitioners, funders and decision-makers to incorporate or demand a multifaceted perspective for rewilding and, simultaneously, incentivize conservation initiatives to go beyond the recovery of species and habitats and include ecosystem function and processes. The framework is applicable to management a variety of bat-transmitted pathogens in restoration projects that aims to promote beneficial interactions between society and nature, range from conservation translocation to all variation of rewilding (Corlett, 2016).
Conflict of interestThe authors declare that there is no conflict of interest.
F.G. and M.G. was supported by (FAPESP) São Paulo Research Foundation (Grant 2017/24252-0, 2019/00648-7). D.G.S. was supported by a Wellcome Trust Senior Research Fellowship (Grant: 217221/Z/19/Z).