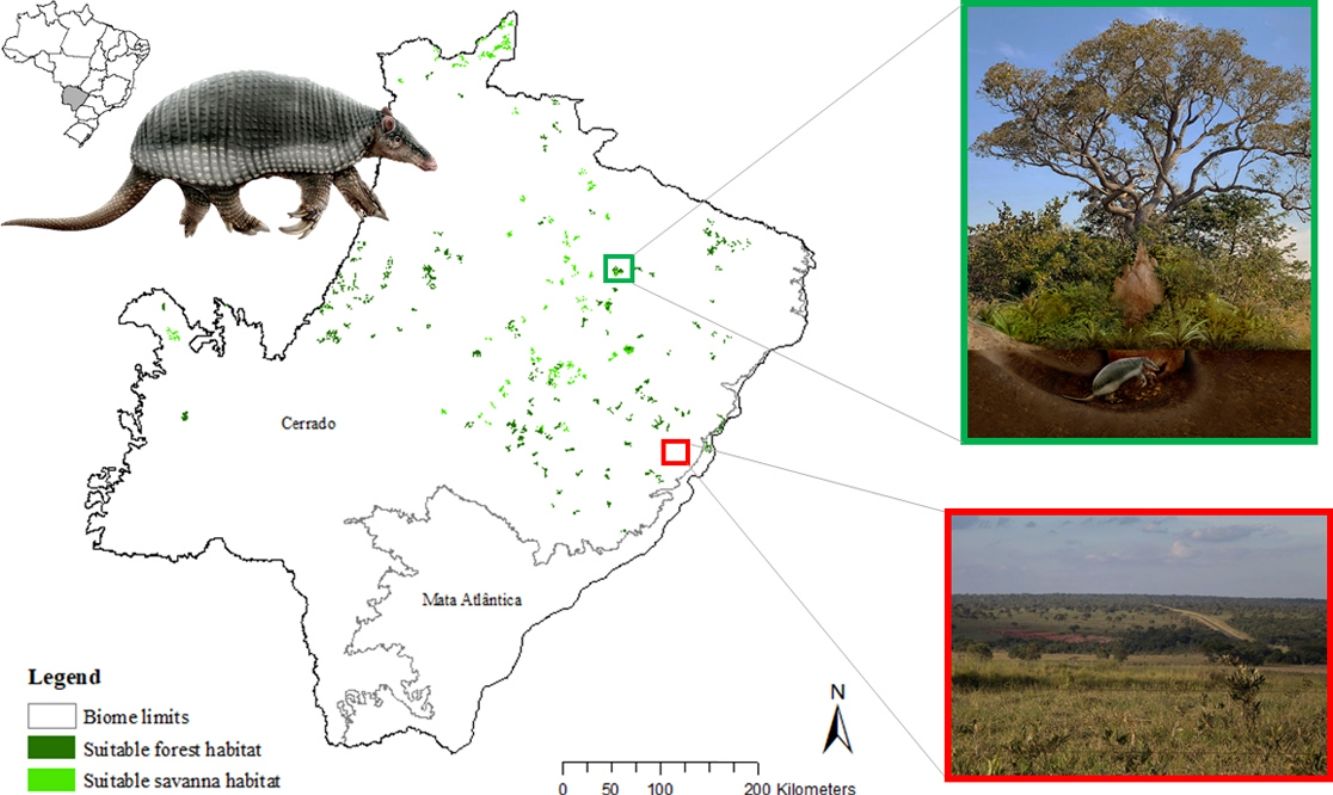
The potential distribution of a cryptic, naturally rare large mammal, the giant armadillo Priodontes maximus, was explored in fragments of Cerrado and Atlantic Forest in the state of Mato Grosso do Sul (259,641 km2) Brazil. Watersheds (N = 344) throughout the state were surveyed for evidence of the species with 164 confirmed presences and 180 absences. A total of 144 independent presence points were used to model the current potential distribution of the species using the Maxent algorithm with 30 possible explanatory variables. Suitable and unsuitable areas, average patch size of suitable habitats and conversion rate of habitat from 1985 to 2018 were also calculated. Results highlight the severe fragmentation level of the suitable areas left for the giant armadillos, with suitable habitat patches (forest and savanna) surrounded by an agricultural matrix. Furthermore, only four suitable patches are larger than 100 km2 (132.63 ± 32.04 km2). Due to the species low population growth rate it is possible that individuals recorded are part of declining or functionally extinct populations. Further studies on the density and population trends of giant armadillos within this fragmented landscape will be key to understanding the potential long-term persistence of giant armadillos in the degraded Cerrado of MS.
Larger mammal species face a relatively higher extinction risk under habitat loss and fragmentation scenarios. This is due to their low population growth rates, extensive home ranges and typically lower densities (Cardillo et al., 2005; Ripple et al., 2017; Hirt et al., 2018). Furthermore, extinction risk is negatively related to vertebrate geographic range size (Ripple et al., 2017). Hence, to enable the development of efficient conservation strategies, we need to understand species distributions and the habitat requirements necessary to maintain individuals and viable populations within these regions. This information is especially critical for the conservation of elusive species living in highly altered biomes, given that vertebrate population declines are often related to large-scale land conversion (Fritz et al., 2009).
The giant armadillo Priodontes maximus Kerr, 1792 (Mammalia: Cingulata) is the largest living species of Cingulata, with adults measuring up to 1.5 m and weighing up to 60 kg (Carter et al., 2016; Desbiez et al., 2019). The species is believed to have low population growth rate as Desbiez et al. (2020a) reported a litter size of one, prolonged parental care, and an inter-birth interval of three years, while Luba et al. (2020) estimated age of sexual maturity between seven or eight years old. Therefore, the loss of a single individual can have significant impacts on populations (Desbiez et al., 2020a; Luba et al., 2020). This rare large sized myrmecophagous species has extensive home ranges, is solitary, nocturnal, and has fossorial habits, spending all day in its deep characteristic excavations, easily going unnoticed by the local people (Eisenberg and Redford, 1999; Silveira et al., 2009; Desbiez and Kluyber, 2013; Desbiez et al., 2020b, unpublish. data). Their excavations are often the only evidence of their presence in an area and have been the focus of several studies (Carter, 1983; Anacleto, 1997; Ceresoli and Fernandez-Duque, 2012; Porfírio et al., 2012; Desbiez and Kluyber, 2013).
This cryptic species is naturally rare, but widely distributed throughout 11 countries in South America in habitats ranging from tropical forest to open savanna (Smith, 2007; Abba and Superina, 2010). The species is threatened by habitat loss and fragmentation, hunting activities, road collisions, and even suspected illegal traffic (Anacleto et al., 2014; Chiarello et al., 2015; Carter et al., 2016; Banhos et al., 2020). The species is currently classified as “Vulnerable” (A2cd), by the International Union for the Conservation of Nature and Natural Resources (IUCN) Red List of Threatened Species (Anacleto et al., 2014) and by the Brazilian Institute for Biodiversity Conservation (ICMBio; Chiarello et al., 2015).
In Brazil, this species can be found in the Amazon, Atlantic Forest, Cerrado and Pantanal ecoregions (Fonseca et al., 1996). In the Atlantic Forest, the species is almost extinct due to habitat loss and hunting (Srbek-Araujo et al., 2009; Fontes et al., 2020). Only 11.7% of the Atlantic forest original cover remains, and its largest remnants are concentrated in the Southeastern region of Brazil (De Rezende et al., 2015). Nevertheless, Massocato and Desbiez (2017) have shown that giant armadillos can persist in protected fragments in the transition zone between the Cerrado and Atlantic forest biomes of the state of Mato Grosso do Sul (MS), Midwest Brazil. In addition, the Cerrado, which is an important habitat for the species (Zimbres et al., 2013), has as little as 19.8% native undisturbed areas and over 50% of its land altered by pasture or cash crops (Klink and Machado, 2005; Strassburg et al., 2017; Green et al., 2019). In the state of Mato Grosso do Sul, there are currently 58,459 km2 of native Cerrado remaining in the entire state, which represents only 16% of the total area (Reynolds et al., 2016). However, these remaining areas of native Cerrado vegetation are highly fragmented and persist predominantly in small patches, with an average patch size of 9.05 ha ± 0.7 (Reyonlds et al., 2016). In the neighboring Pantanal, Desbiez et al. (2020b) report that giant armadillos require on average 2518 ha of almost exclusive use of forest and savannah habitat, with males having larger home ranges (5109 ha) than females (1998 ha). Therefore, the species would most likely require several fragments of native habitat to survive, which means it would need to cross higher-risk altered habitats. During a 14-year period, Lemos et al. (2020) gathered 54 records of giant armadillos within human-modified landscapes in the Cerrado of Central Brazil, showing that the species can somehow persist in highly altered and fragmented landscapes.
The savannas of Central Brazil are key for the conversation of giant armadillos (Zimbres et al., 2012). Hence, identifying suitable areas for giant armadillos in the state of Mato Grosso do Sul is key for its conservation in this region and in the country. Furthermore, the species has been selected as one of the five indicator mammal species for the ecological and economic zoning of the state, which can potentially be used for the creation of protected areas (Sugai et al., 2014). To influence public policies however, a precise distribution map of this rare elusive species must be made available to authorities. However, obtaining presence points for elusive, low-density mammals can be challenging, especially when resources are scarce and integrated management actions are urgent.
Here, we explore the potential distribution of the giant armadillo and the suitability of the remaining fragments of Cerrado and Atlantic Forest in the state of Mato Grosso do Sul, which are highly impacted by cash crop agriculture (Eucalyptus, soybean, sugar cane) and cattle ranching activities. We evaluate the model results to shed light into the long-term conservation perspectives for the species in this region.
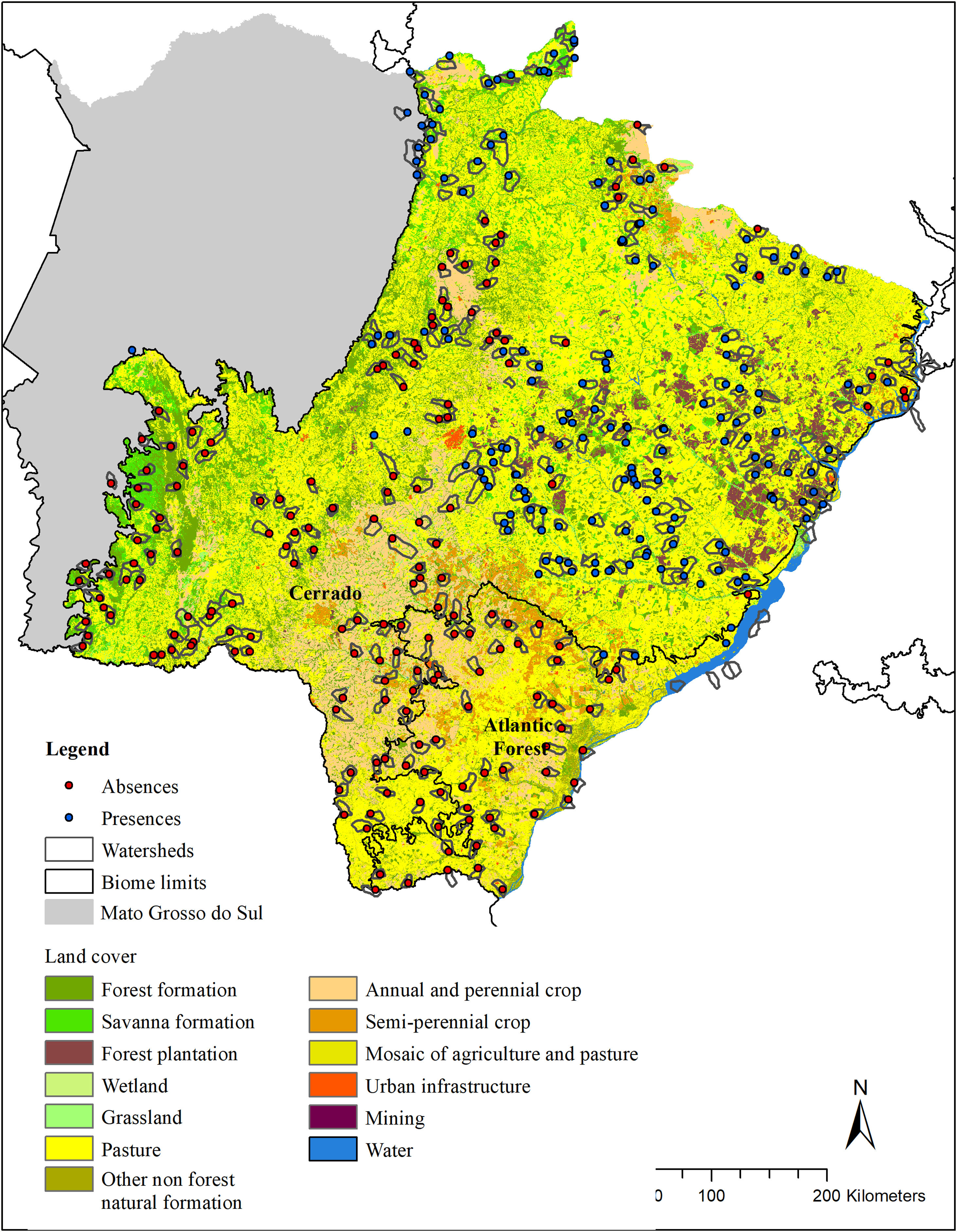
Material and methodsStudy areaThe geographical space (g-space; 259,641 km2) for modeling included the Cerrado and Atlantic Forest biomes (IBGE, 2019) of the state of Mato Grosso do Sul (MS), Brazil (Fig. 1).
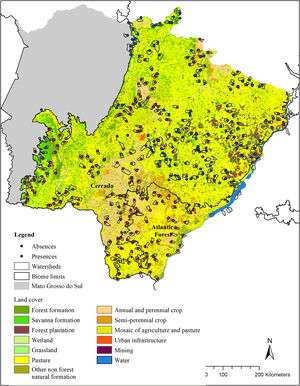
Land cover (Mapbiomas, v. 4.1) in the modeled area (g-space) of Cerrado and Atlantic forest of the state of Mato Grosso do Sul, Brazil. Watersheds surveyed for the presence of giant armadillos (Priodontes maximus) are represented by polygons. Points represent confirmed presences (blue) and absences (red).
We randomly sorted 37 municipalities (22 in the Cerrado and 15 in the Atlantic Forest) out of the 85 that make up the MS state (54 in the Cerrado and 31 in the Atlantic Forest). This sample number was chosen in order to guarantee the representation of the variable conditions found within the MS state, while ensuring the feasibility of the time consuming fieldwork. Within each sorted municipality, we mapped all the watersheds, and then selected the ones to be surveyed in the municipality. Watershed were chosen as sampling units because they are discrete landscape features that share particular ecological features. Watersheds were selected according to three criteria: I) their size, II) amount of native vegetation and III) geographic location. For the first criteria, we selected watersheds between 5000 ha and 9000 ha (7000 ± 2000 ha), because the average size of the study region’s watersheds were 7000 ha. Standardizing the watershed size made comparisons between our sample units easier and enabled the survey of the entire watershed in a matter of days. For criteria II, we used the land cover classification provided by MapBiomas and merged all native vegetation classes into one class named “native vegetation”. Watersheds were selected to sample a gradient of native vegetation cover, ranging from as little as 5% to as much as 100% cover per watershed, when possible. We therefore ensured that we had a wide range of native vegetation cover amongst watersheds surveyed. Finally, for criteria III, we selected watersheds that were, at least, 7 km apart from each other, to avoid spatial autocorrelation. The number of watersheds visited in each municipality varied according to its size, but averaged 9, ranging from 1 to 34.
Giant armadillo presence pointsThe presence of the giant armadillo at each watershed was confirmed through indirect evidences such as their unmistakable burrows or feeding excavations that can remain obvious for months after being initially made (Desbiez and Kluyber, 2013). To search for giant armadillo evidence in each watershed, we started by visiting the largest fragment of native vegetation within it, as we assumed it was the most likely place to find evidence of the species presence. If no evidence was found, we searched the next largest fragment in the watershed and so forth until all native vegetation fragments in the watershed had been surveyed. If we found any evidence of the species presence, we took the GPS coordinates of it and the species was considered present in that watershed and we moved our searches to the next watershed. Searches were conducted by a team of at least two people. In each watershed, searches for evidence of the species presence in native vegetation fragments could last between a few hours to a few days. If no evidence of giant armadillo was found in any native vegetation fragment, we then considered that the species was absent, and the watershed was classified as a confirmed absence point. The coordinate of the center of the largest native vegetation fragment was used to record the confirmed absence point. Between July 2015 and April 2017, 344 watersheds were visited (Fig. 1), with 164 confirmed presences and 180 absences.
To reduce spatial autocorrelation due to unbalanced sampling effort, we used the ‘Spatially Rarefy Occurrence Data for SDMs’ from SDMtoolbox package (Brown et al., 2017) in ArcGIS (v. 10.3) with a distance of 6 km (approximately two times the maximum daily displacement of the species; Desbiez et al., 2020b). This resulted in 144 independent presence points for modeling (Supplementary materials 1, Table S1).
Explanatory variablesWe selected 30 possible explanatory variables to model the potential distribution of this fossorial mammal: 19 Bioclimatic variables from the Worldclim database (Fick and Hijmans, 2017), global digital elevation model (DEM) from USGS (Gesch et al., 1999), Elevation and Slope Exposure from SRTM imagery (Jarvis et al., 2008), Land Cover from MapBiomas (Project MapBiomas, 2020), depth to Bedrock (R horizon) up to 200 cm and Sand content (50–2000 µm) mass fraction in % from Soil Grids (Hengl et al., 2017), Distance from Rivers from Agência Nacional de Águas (ANA) (http://metadados.ana.gov.br/geonetwork/srv/pt/main.home), fraction of green vegetation cover (Forest Cover) and fraction of absorbed photosynthetically active radiation (FAPAR) from Copernicus (Sánchez et al., 2015), Terrain Ruggedness (Sappington et al., 2007), Shannon Diversity of enhanced vegetation index (EVI) from EarthEnv (Tuanmu and Jetz, 2015) (see Supplementary Material 1, Table S2, for details). We used all variables in 30 arc-seconds (approx. 1 km) of spatial resolution. We used Pearson’s correlation to identify collinearity between pairs of variables. For any combination of covariates with a correlation coefficient r > |0.7|, we retained the one considered to have greater ecological relevance to the species. The final variables selected for modelling included: Bedrock, Sand, Terrain Ruggedness, Forest Cover, Land Cover, Shannon Diversity, DEM, Distance from Rivers, Annual Mean Temperature (Bio 1), Mean Diurnal Temperature (Bio 2), Isothermality (which is equal to Mean Diurnal Temperature Range/Temperature Annual Range; Bio 3), Temperature Annual Range (Bio 7), Annual Precipitation (Bio 12), Precipitation of Driest Month (Bio 14) and Precipitation of Warmest Quarter (Bio 18).
Modeling proceduresWe used the Maxent algorithm (Phillips et al., 2006; Phillips and Dudík, 2008; Phillips et al., 2017a,b) to model the current giant armadillo potential distribution (SDM). We defined the following parameters in the Maxent software (version 3.4.1): bootstrap method of replicates (n = 10), 30% of random points to test, 10,000 points of background, random seed, convergence threshold of 10−5, 500 maximum iterations and Cloglog output format. We evaluated the AUC (mean ± standard deviation) of average final model, the relative contributions (%) of the explanatory variables and the response curves of variables that most affected the Maxent prediction. We also examined the sensitivity, i.e., true positive fraction (true positive/(true positive + false negative); Pearson, 2007) using the 144 unique independent records of species presence.
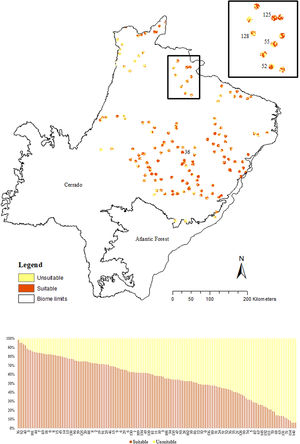
We used the 10 percentile training presence Cloglog threshold from Maxent output (=0.2636) to cut and transform the probabilistic model into a binary map discriminating suitable (1) and unsuitable (0) areas for the giant armadillo. This threshold was chosen among others by the species specialists and in the modelled area as it was the best one to represent the potential species distribution. Then, we calculated the percentage of confirmed absences from field surveys in areas predicted as suitable by the SDM.
To understand the conservation potential for the species in the state we calculated the percentage of suitable area in the study region and the percentage of suitable area that was within strictly protected areas (MMA SNUC, 2000), as well as highly suitable area (25% top percentile, i.e. pixel ≥0.68 of species probability presence). To characterize habitat suitability around known presence points and make inferences regarding population persistence on those areas, we also calculated the percentage of suitable and unsuitable areas predicted by the SDM in a buffer of a 4km-radius from each presence point. This area was defined based on the median home range size of male giant armadillos in the Pantanal (∼5000 ha; Desbiez et al., 2020b).
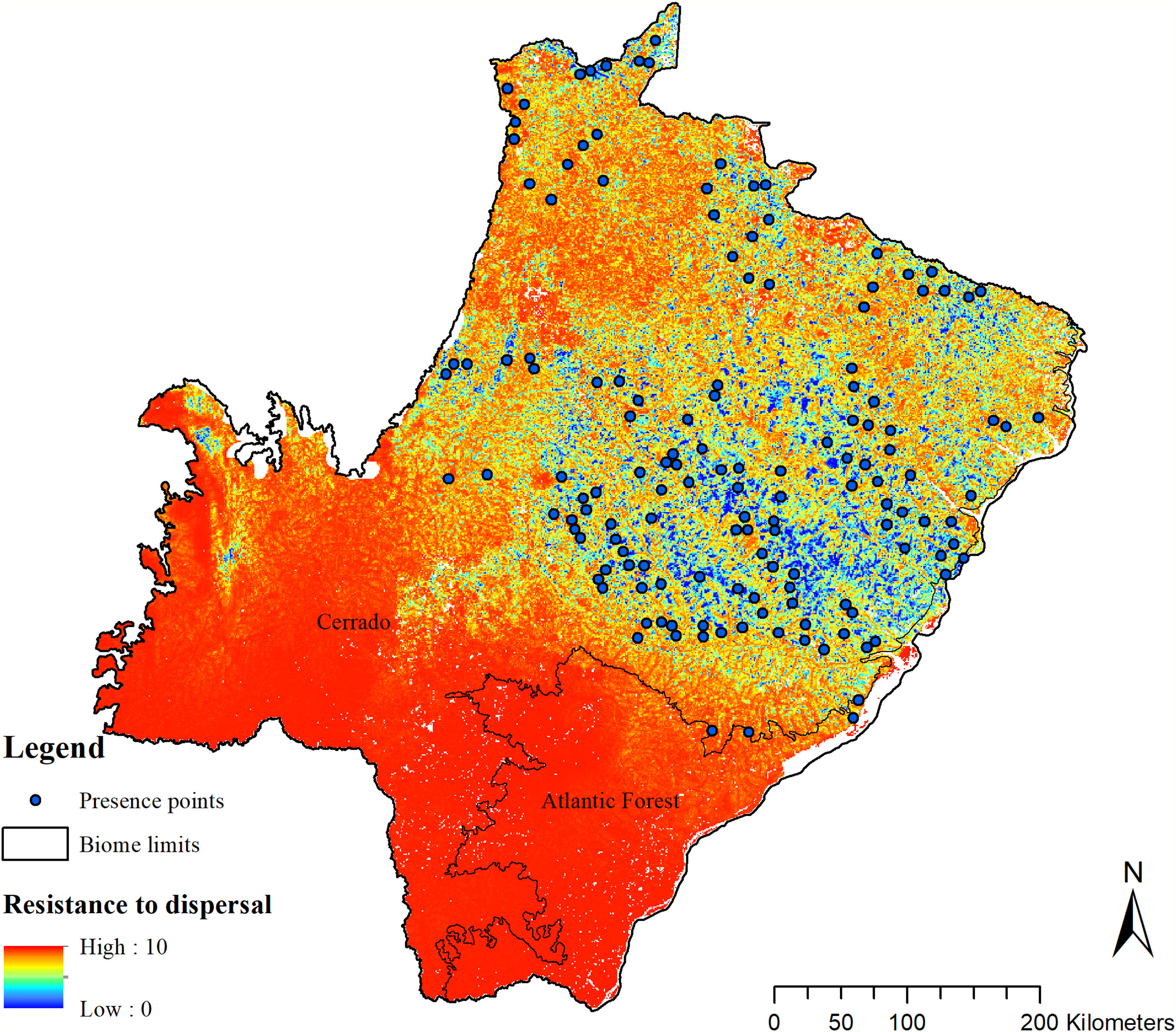
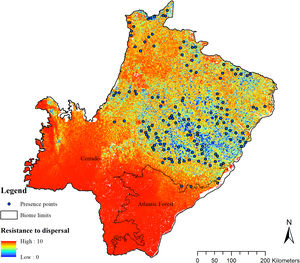
To enable the evaluation of the cost for the species dispersal through the landscape, we generated a friction surface (i.e., a raster that depicts the ease of dispersal from each locality through the landscape; Fig. 4). The friction surface was created by inverting the values of the SDM. Hence, areas of high suitability were converted into areas of low dispersal cost (SDMtoolbox package, Brown et al., 2017).
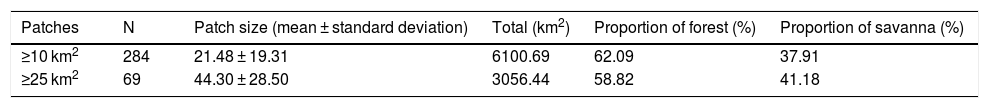
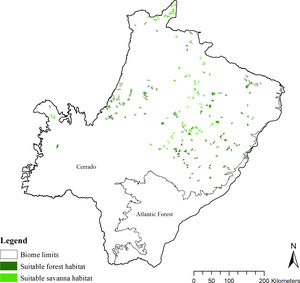
To characterize the remnant distribution and potential conservation status of giant armadillos in the region, we calculated the average patch size of suitable habitats for the species (forests and savannas; Desbiez et al., 2020c). For this analysis we considered only patches that were larger than ≥25 km2 (home-range in the Pantanal biome, Desbiez et al., 2020), but also included suitable patches ≥10 km2 as they could serve as stepping stones between larger patches. In addition, we calculated the conversion rate of habitat from 1985 to 2018 (Project MapBiomas, 2020, version 4.1) for the whole g-space and in the suitable area predicted by the model. We considered the maximum timeframe (=33 years) of habitat conversion available by Mapbiomas as it is closer to the length of time (three generations) the IUCN Red List bases their analysis upon (IUCN, 2019). For Giant armadillos three generations are estimated at 33 years (Luba et al., 2020)
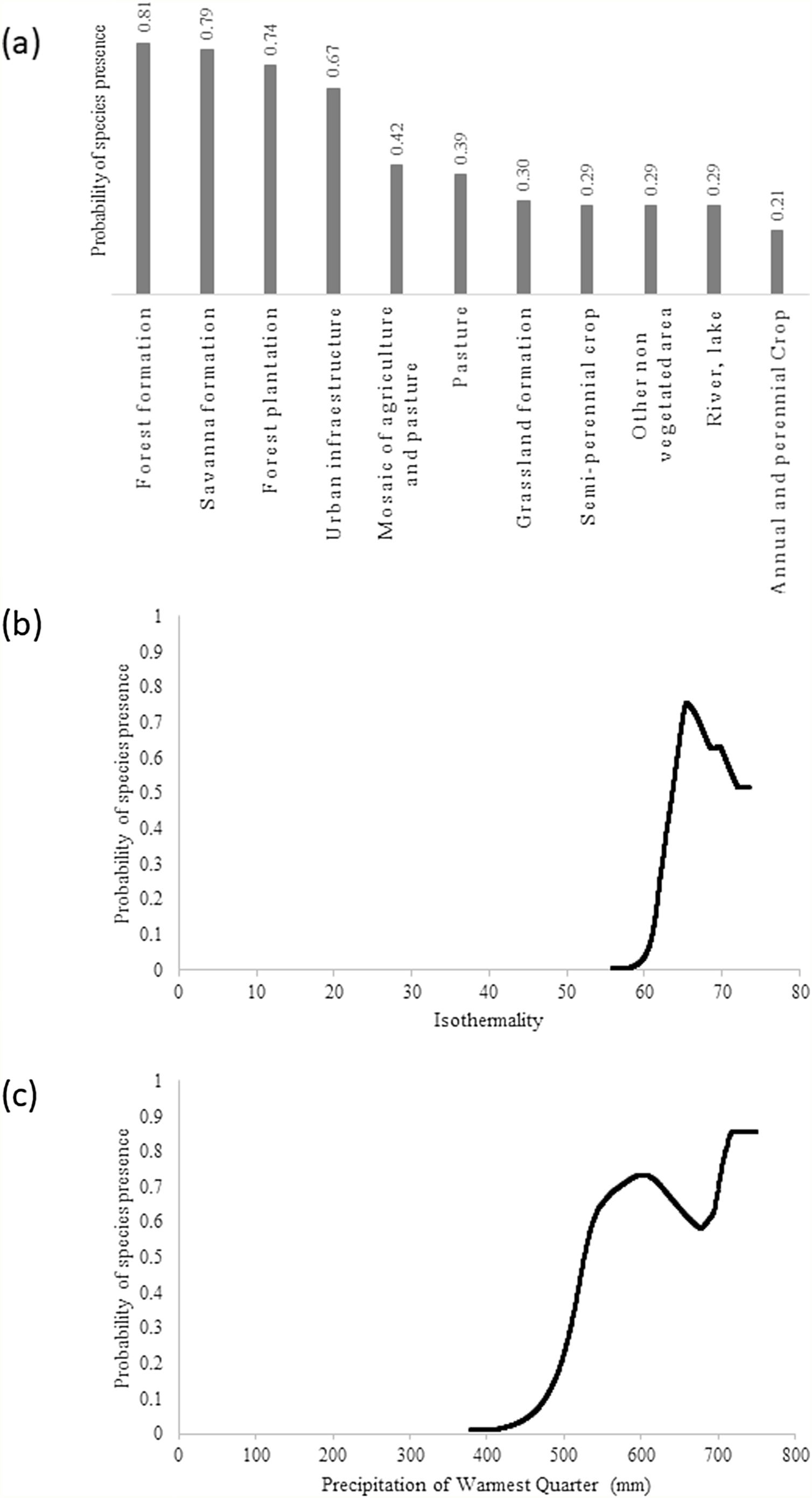
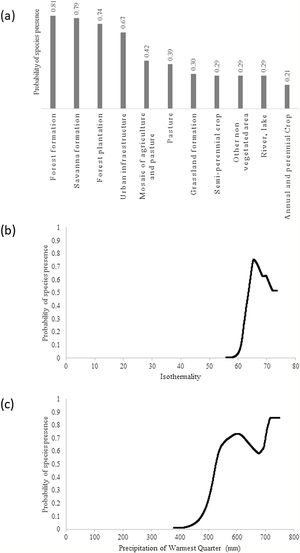
ResultsThe giant armadillo potential distribution model (SDM) in the MS had a good performance (AUC = 0.844 ± 0.026, 20.47% of omission, p ≤ 0) and a high sensitivity (86.11%). Only 8.14% of the confirmed absences were predicted in suitable areas. The most important explanatory variables that explain the species distribution were Land Cover (20.75%), Isothermality (Bio3; 17.88%) and Precipitation of Warmest Quarter (BIO18; 16.30%). According to this model, the highest probability of species presence was associated with forest formation, with Land Cover explaining 20.75% of the model prediction. In addition, the species probability was associated to Isothermality (BIO3) with values ranging from 56 to 66, being 17.88% of the model prediction explained by this predictor. Isothermality is defined by the relationship between Mean Diurnal Range and Temperature Annual Range, and is a quantification of how large the day-to-night temperature oscillation is in comparison to the summer-to-winter oscillation (Hijmans et al., 2005). For the study area, values (56–66) indicate that the diurnal temperature range is about half of the annual temperature range. Finally, the species probability was associated with the Precipitation of Warmest Quarter (BIO18), with presence within values ranging from 520 and 750 mm of precipitation, being 16.30% of the model prediction explained by this predictor (Fig. 2).
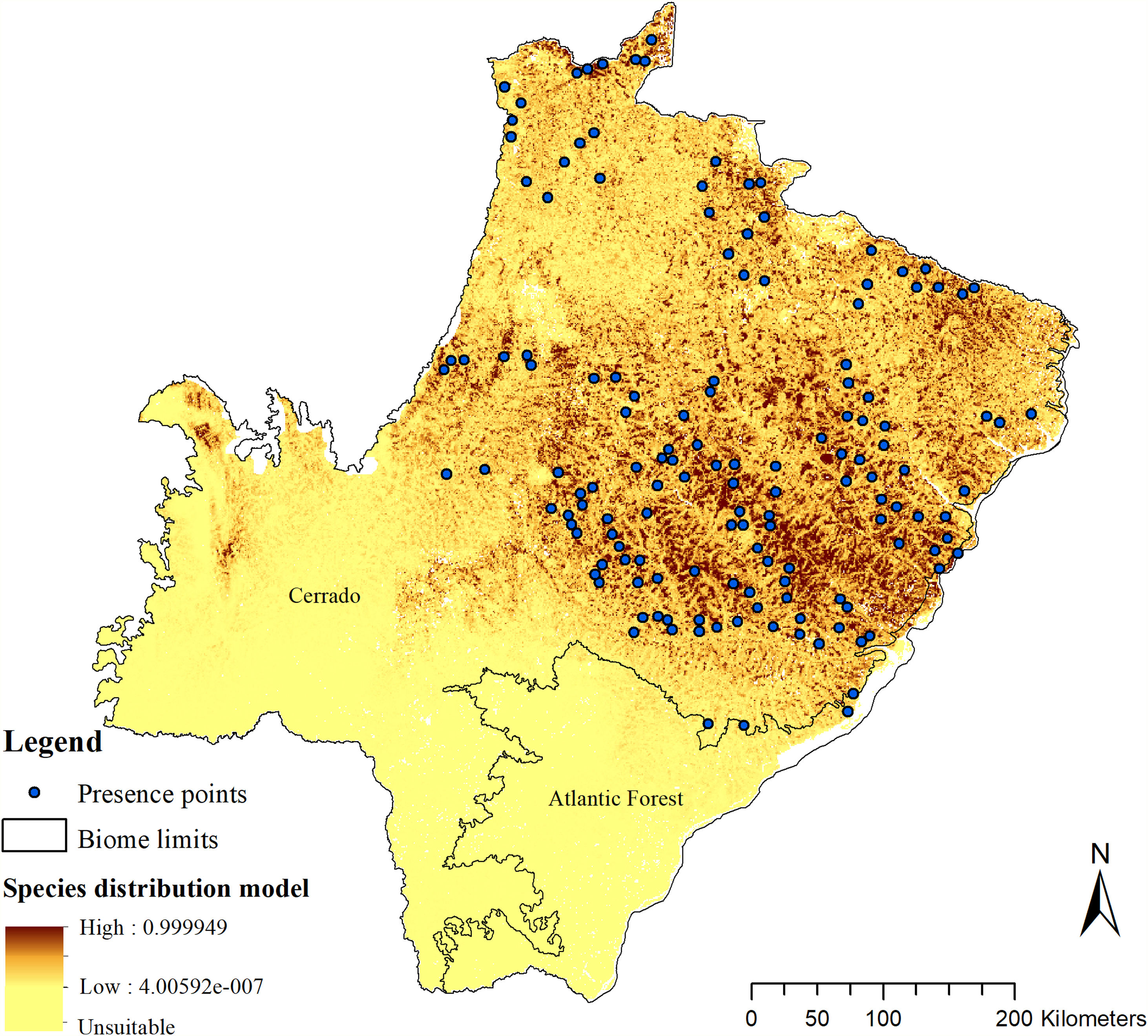
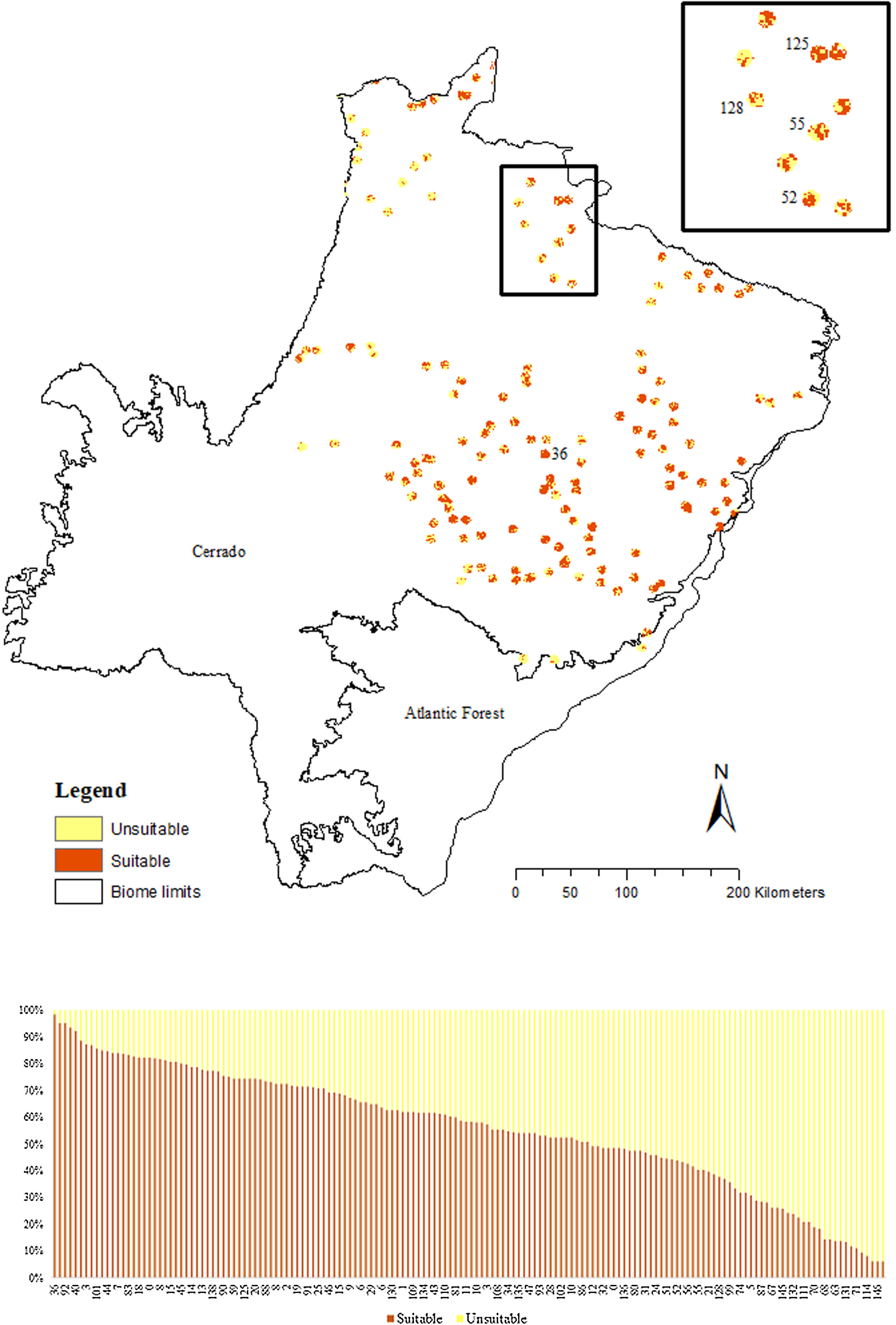
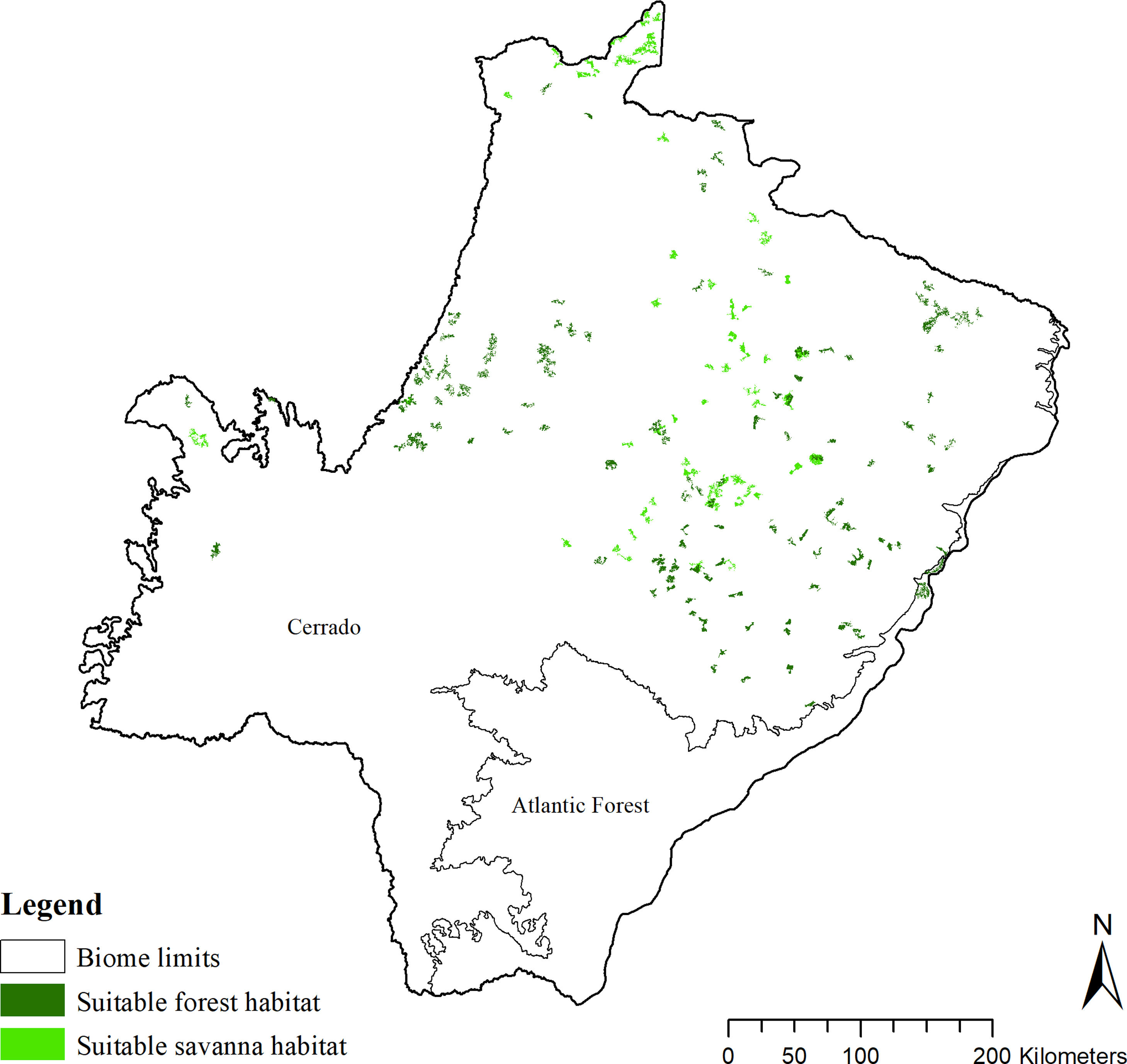
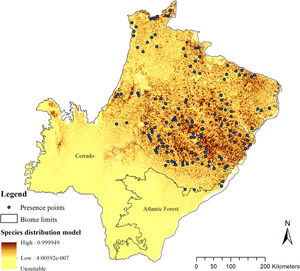
The SDM predicted the species to be distributed over a suitable area of 64,374 km2 (24.79% of the g-space) (Fig. 3), of which 10,595 km2 (only 4.08% of the g-space) are currently highly suitable for the species but only 367 km2 (0.14% of the g-space) is located in Brazilian Protect Areas (strictly protected). The suitable area encompassed 50.11% of pasture, 20.26% of forest formation, 10.07% of savanna, 7.64% of a mosaic of agriculture and pasture, 6.58% of forest plantation, and other small areas of crops and grasslands. These results highlight the severe fragmentation level of the suitable areas left for the species, confirmed by the high resistance to dispersal (Fig. 4), with suitable habitat patches (forest and savanna) surrounded by an agricultural matrix (mainly pasture). However, most of the confirmed presence points encompasses high amounts of unsuitable areas within a 4km-radius (Fig. 5) and only 24.68% of the points were surrounded by more than 75% of suitable areas.
Suitable habitat patches (≥10 km2) summed up only 6100.69 km2 (9.48% of suitable area) (Table 1). Furthermore, only four suitable patches are larger than 100 km2 (132.63 ± 32.04 km2) and the largest continuous patch of suitable habitat is 172.12 km2 (13.73% of forest and 86.27% of savanna). The conversion rate of habitat for giant armadillo between 2015 and 2018 was around 20% in the g-space and the proportion of habitat loss was higher than the proportion of habitat recovered for both g-space and suitable area extent (Table 2; Supplementary material, Fig. S1).
Characteristics of patches of suitable habitat (forest and savanna) for the giant armadillo Priodontes maximus in the state of Mato Grosso do Sul, Brazil.
| Patches | N | Patch size (mean ± standard deviation) | Total (km2) | Proportion of forest (%) | Proportion of savanna (%) |
|---|---|---|---|---|---|
| ≥10 km2 | 284 | 21.48 ± 19.31 | 6100.69 | 62.09 | 37.91 |
| ≥25 km2 | 69 | 44.30 ± 28.50 | 3056.44 | 58.82 | 41.18 |
Suitable habitat (forest and savannah) in the state of Mato Grosso do Sul (Brazil) that were lost, recovered or remained unchanged between 1985 and 2018 within the study region (G-space) and within areas classified as suitable for giant armadillos Priodontes maximus by the species distribution model (SDM). Areas are presented in squared kilometers and percentages are relative to the total amount of suitable habitat (forest and savannah) in the state.
| Habitat loss | Habitat recovered | Habitat that remained unchanged | |
|---|---|---|---|
| Suitable area | 14,962 (26.54%) | 2544 (4.51%) | 13,955 (24.75%) |
| G-space | 48,559 (21.35%) | 8121 (3.57%) | 43,425 (19.10%) |
The giant distribution model was satisfactory, with high sensitivity (∼85% of true positive fraction) and small omission and commission errors. The probability of species presence was best explained by the presence of forest and savanna formations, which corroborates the results from habitat selection studies in the neighboring Pantanal (Desbiez et al., 2020c). In the eastern part of the state of MS, the species presence has also been associated with native vegetation, with 89% of presence records associated to this landscape feature, only 11% to Eucalyptus plantations, and no records on pastureland (Esteves et al., 2018). In Central Brazil, although the species has been reported to occur in highly human-modified Cerrado landscapes, the species has been recorded mainly in forest and savannah areas as well (>80% of records; Lemos et al., 2020). In the surroundings of a Cerrado protected area in Goiás state (Emas National Park), giant armadillos are also dependent on the presence of conserved forest remnants outside of protected areas for their persistence (Vynne et al., 2011). Forest and savannah areas are the primarily selected landscape features by giant armadillos in heterogeneous well-conserved landscapes, regardless to age class, sex or activity being performed. In addition, these landscape features play a key role in the species reproduction and survival, especially in their early life stages, being crucial for the species conservation (Desbiez et al., 2020c).
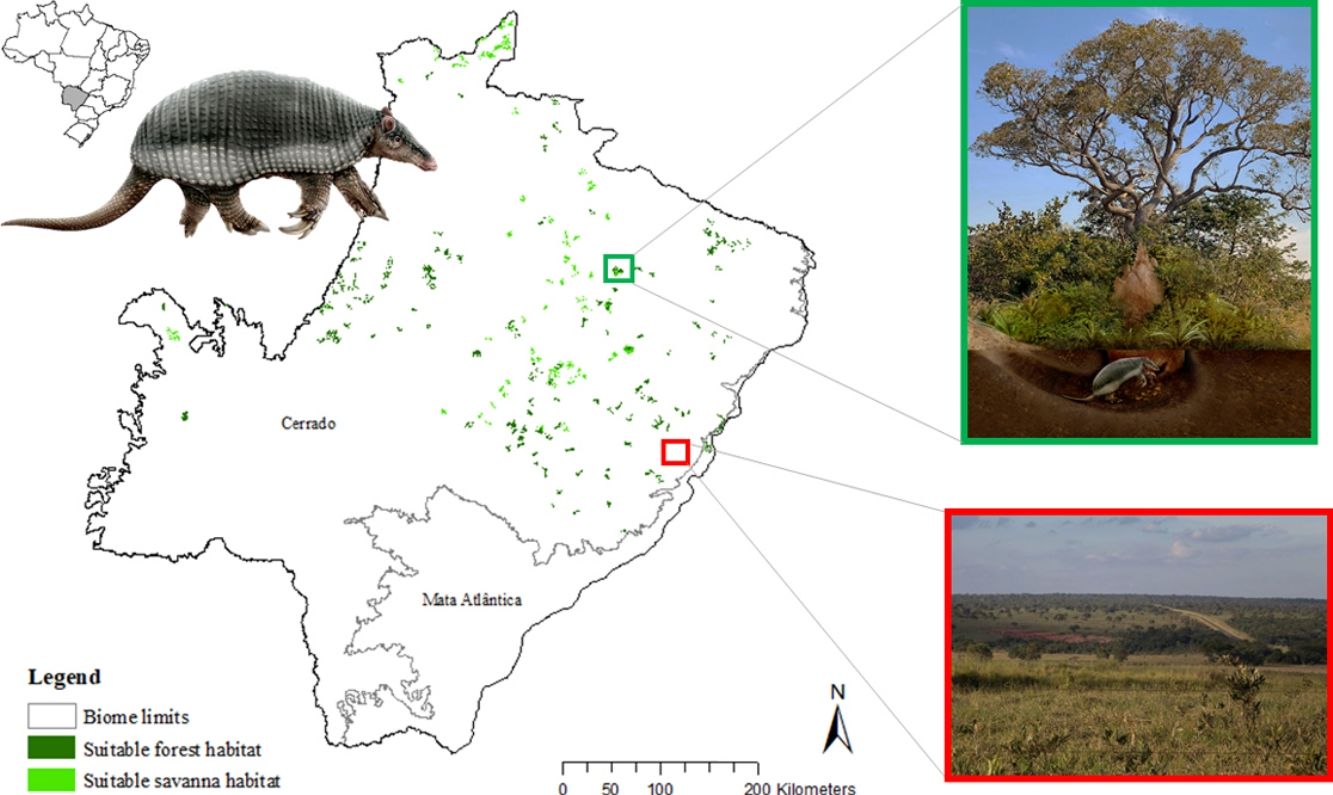
The model revealed the critical situation of the species distribution in the state, which is mainly composed of small and fragmented suitable patches. The largest habitat remnant for the species is only 172.12 km2, an area that has the potential to harbor less than seven giant armadillos, if the species follows the space use patterns observed in the Pantanal (Desbiez et al., 2020b). Furthermore, the current study reports that there are only 69 patches of suitable habitats (≥25 km2) for the species, averaging 44.30 ± 28.50 ha in size. Therefore, in the fragmented landscape of the Cerrado of MS, giant armadillos would probably need to occupy several fragments to meet their resource requirements. However, most of these small suitable patches are surrounded by an agricultural matrix, with predominance of pasturelands. Furthermore, most of the current known presences of giant armadillo are in locations surrounded by unsuitable patches, which will expose the species to some level of vulnerability. Only one confirmed presence is completely surrounded (∼98%) by suitable habitat patches (Fig. 5). Considering that in the neighboring Pantanal, an adult giant armadillo has a median home range of 2500 ha and a daily displacement of 1.6 km (Desbiez et al., 2020b), it is reasonable to suppose that giant armadillos inhabiting the landscape in MS spend most of their daily activity in unsuitable habitats. Hence, giant armadillos would have to travel through altered, less suitable, habitats, which present high resistance for dispersal, as revealed by our friction surface model (Fig. 4), increasing their potential contact with anthropogenic threats such as roads, chemicals, people, dogs, among others (Anacleto et al., 2014; Fletcher et al., 2019). This should be characterized as a significant threat to the species, as the roads of the Cerrado of Mato Grosso do Sul have recorded the highest number of vehicle collisions with giant armadillos in the country (Banhos et al., 2020).
Fragmentation may also influence social interactions, e.g. encounter rate of reproductive mates, which, in turn, can affect population abundance, distribution and viability (Banks et al., 2007). In the well-conserved Pantanal, adult giant armadillos present very low home range overlap (4%) and adult females present exclusive home ranges among themselves. Hence, males display an exploratory behavior, ranging over sites that are up to 20 km away from their core activity areas, most likely to increase their odds of encountering receptive females (Desbiez et al., 2020b). Fragmentation and resource limitation might increase even further the area needed by each individual to perform their daily activities and find resources (Ims et al., 1993). If giant armadillos present a similar spatial behavior – maintaining exclusive home ranges – outside the Pantanal, the larger area requirements in these degraded areas might reduce even further the opportunities for giant armadillos to encounter receptive mates due to physical displacement limitations (Ims et al., 1993; Jetz et al., 2004; Desbiez et al., 2020b). Nevertheless, mammals have been reported to change their movement patterns in areas modified for human activities (Tucker et al., 2018) and hence, further studies are required to understand extent to which giant armadillo movements are affected by anthropogenic effects on the structure and composition of landscapes and resource changes (Fig. 6).
Giant armadillos occupy large home ranges and travel long distances (Desbiez et al., 2020b). The excavations and burrows documented in this study can only prove that at least one individual was present in each presence area. Giant armadillos are believed to have low population growth rates, reach sexual maturity late (7–8 years old), but to potentially live over 20 years (Desbiez et al., 2020a; Luba et al., 2020). Because deforestation in the State of MS is fairly recent (less than 35 years; Silva et al., 2011; Reynolds et al., 2016), and giant armadillos are long lived species, it is possible that the individuals recorded to survive in these remnants are part of populations in decline, or even functionally extinct. Although areas of higher environmental suitability are believed to enable the persistence of healthy animal populations (Soberón, 2007; Lunghi et al., 2018), these may be too small and fragmented to support a viable population. Further studies on the density and population trends of giant armadillos within this fragmented landscape will be key to understanding what our species distribution model estimates actually mean for the population demography and the long-term persistence of giant armadillos in the degraded Cerrado of MS.
ConclusionWe explored the potential distribution of the giant armadillo and the suitability of the remaining fragments of Cerrado and Atlantic Forest in the state of Mato Grosso do Sul, Brazil, through a very labor-intensive study where the presence or absence of giant armadillos was confirmed through signs such as excavations or burrows. We highlight the severe fragmentation level of the suitable areas left for the giant armadillos. Due to the results of this study and the low population growth rate of giant armadillos, we question whether evidence recorded in the field is of already functionally extinct populations or even just isolated individuals. Furthermore, this pattern of small fragmented and isolated suitable habitats remaining in Mato Grosso do Sul raises concern for the remaining Cerrado population, which face similar fragmentation scenarios. While in the Amazon and Pantanal biomes the giant armadillo population seems to face less of an eminent risk of extinction due to the large expanses of contiguous habitat, the species is nearly extinct in the Atlantic Forest, currently remaining in only one protected area (Fontes et al., 2020). Unless a major effort is made to restore habitat connectivity and conserve remaining native habitat fragments, the future of giant armadillos, a species who plays such an important role in the ecological community as an ecosystem engineer is at stake., in the Cerrado. The Cerrado biome therefore needs to be a priority for conservation actions so that the species does not follow the trends already documented in the Atlantic Forest.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We would like to thank the over 1300 private landowners who provided us access to their land to survey for giant armadillo locations and the local communities that shared their knowledge with us. This study is part of the Giant Armadillo Conservation Program, which benefited from multiple grants, mostly from Zoos in North America and Europe, listed at www.giantarmadillo.org. Funds for this particular study include the Whitley Fund for Nature, National Geographic Society/Waitt Grants Program [grant number W379-15] and an AZA Conservation Grants Fund. We would like to thank the many volunteers who participated in the fieldwork, especially Gabriel Massocato, Yuri Ribeiro, Mariana Catapani and many others. Katia Ferraz would like to acknowledge the National Council of Technological and Scientific Development (CNPq) for the productivity fellowship [grant number 308503/2014-7].