Forest fragmentation, a result of deforestation, not only decreases the amount of habitat available for wildlife, but also increases the isolation of the remaining fragments and the area of edges surrounding them. Also, deforestation often leads to the creation of a dynamic regenerating matrix where cleared land is subsequently abandoned. Here we examine the effects of fragmentation and landscape change on the nutritional condition of Amazonian rainforest birds at the Biological Dynamics of Forest Fragments Project, near Manaus, Brazil. We analyzed ptilochronology-based measurements of feather growth rate in 12 species living in fragments within a dynamic landscape over 21 years. Ptilochronology serves as an index of nutritional condition by revealing energy available for maintenance over 1–2 weeks while the feather is grown, allowing intraspecific comparison across treatments. Feather growth rate decreased in fragments surrounded by young second-growth borders but increased as fragment size and age of adjacent second-growth vegetation increased. Results from this simple, yet informative, measure of nutritional condition reveal physiological impacts of land cover change, including the response of birds to changes occurring at both local and landscape levels. Our results highlight the importance of looking beyond presence/absence data to describe fragmentation effects, and support the value of landscape-scale approaches for the conservation of tropical forest biodiversity.
Habitat loss and degradation rank among the most significant threats to global biodiversity (Barlow et al., 2016; Foley et al., 2005). Habitat loss often leads to fragmentation, which not only decreases the amount of habitat available for wild species, but at the same time increases the isolation of the remaining fragments, as well as the amount of edges around them (Kupfer et al., 2006; Lindenmayer and Fischer, 2006). Although evidence suggests the effects of fragmentation per se are not always as negative as previously thought (Fahrig, 2017), long-term experiments of habitat fragmentation demonstrate that reduced area, increased isolation, and increased proportion of edge habitat all have important negative implications on biodiversity and ecological processes (Haddad et al., 2015).
Following fragmentation, normal abiotic conditions are significantly altered, especially at or near edges (Haddad et al., 2015; Harper et al., 2005; Lindenmayer and Fischer, 2006; Lovejoy et al., 1986; Murcia, 1995). Physical conditions near edges are typically hotter and drier than in forest interiors, which can affect forest structure, alter food resources and favor non-forest species (Haddad et al., 2015; Lovejoy et al., 1986; Murcia, 1995; Pfeifer et al., 2017; Saunders et al., 1991). Similarly, invasive species, predators, and brood parasites can all be attracted to edges, altering species interactions (Murcia, 1995; Saunders et al., 1991; Stratford and Robinson, 2005). The size of a fragment is also an important feature affecting the establishment of territories and significantly reducing the number of individuals that can share a single fragment (Johnson et al., 2011; Stratford and Robinson, 2005). Additionally, as the area of a given fragment decreases, so does the amount of core habitat that is unaffected by the surrounding environment (Gascon et al., 2000; Haddad et al., 2015; Kupfer et al., 2006). Fragment shape, position in the landscape, and connectivity to other fragments also play an important role affecting within-fragment dynamics, highlighting the importance of landscape-scale approaches to understanding the effects of habitat fragmentation (Haddad et al., 2015; Laurance, 2008).
Consequently, even when birds are able to persist in fragments they may still suffer from alterations that reduce habitat quality (Barlow et al., 2016). Because of this, forest fragments are often lacking sensitive species that cannot tolerate area or edge effects (Stratford and Stouffer, 1999). Nonetheless, certain species can persist in fragments, although they may be affected in subtle but important ways that can ultimately affect their long-term fitness and survival (Fahrig, 1997). For this reason, it is important to move beyond simply noting presence or absence of a particular species in fragments in favor of revealing processes involved in long-term persistence, including breeding success, survival, and physical condition (Johnson, 2007).
Feathers serve a variety of vital functions in the lives of birds; they are essential for body insulation, flight performance, and social communication (Stettenheim, 1976). Because of their importance, maintaining a complete, functioning set of feathers is crucial. When feathers are lost or damaged, replacements are rapidly grown, and natural selection is thought to favor the expenditure of energy and nutrients for high-quality feathers (Dawson et al., 2000). However, feathers are costly structures, made up of more than 90% protein which, depending on the species, can be up to 12% of an individual's body mass (Murphy, 1996). Therefore, both the rate of feather growth and the amount of material put into each feather can be adjusted to compensate for reduced resources or energetic demands of other activities (Grubb, 1989, 2006; Murphy et al., 1988).
Feathers are composed of alternating dark and light growth bars, with a pair of dark-light bars corresponding to a 24-hour period of feather growth (Michener and Michener, 1938). The measurement of growth bars, a technique known as ptilochronology (Grubb, 1989), has been used as an indirect measure of the nutritional condition of birds during the period of feather growth. Under good conditions, birds grow their feathers faster, which results in wider growth bars; under adverse conditions, feather growth can be reduced, resulting in narrower growth bars (Grubb, 1989, 2006). Consequently, the width of growth bars on feathers gives a day-by-day record of the nutritional regime under which a bird has lived, providing information about the bird's nutritional condition and the quality of its habitat. Since the discovery of ptilochronology as a biomarker, the technique has been applied to various ecological questions, generally giving satisfactory results as a metric of habitat quality (Brown et al., 2002; Grubb, 2006; Strong and Sherry, 2000; Talloen et al., 2008; Vangestel and Lens, 2011; Yosef and Grubb, 1992).
The effects of habitat fragmentation on the nutritional condition of birds were revealed by ptilochronology in two common species at the Biological Dynamics of Forest Fragments Project (BDFFP), the Wedge-billed Woodcreeper (Glyphorhynchus spirurus) and the White-crowned Manakin (Dixiphia pipra). Stratford and Stouffer (2001) showed that although these birds might not have significant long-term changes in their abundance, they may still suffer the physiological consequences related to fragment size. A few years later, Stouffer et al. (2006) highlighted how landscape change, especially second-growth regeneration along edges and in the matrix, positively affected bird abundance and recolonization of previously isolated fragments. Understory birds at the same site also increasingly used fragment edges as second-growth vegetation developed over 12–30 years, although with considerable differences among bird guilds (Powell et al., 2013).
In light of the important effects of the landscape on processes occurring inside the fragments, we wanted to understand the role of edge and matrix second-growth vegetation on the nutritional condition of birds in forest fragments. We measured feather growth rate in 12 species of birds living in fragments within a dynamic landscape over 21 years at the BDFFP. We expected to find lower nutritional condition in birds from smaller fragments compared to larger fragments and continuous forest. We also expected to see reduced nutritional condition in fragments surrounded by young vegetation at their edges (hereafter border), or in the matrix (hereafter second-growth), but we hypothesized that negative effect to be lost as this vegetation matured.
MethodsStudy areaThe study was conducted at the Biological Dynamics of Forest Fragments Project (BDFFP) study area, in central Amazonia, approximately 80km north of Manaus, Brazil. The BDFFP is the world's largest-scale and longest running study of habitat fragmentation. The current landscape consists of a mix of eleven forest fragments of different sizes (five 1ha, four 10ha, and two 100ha), surrounded by second growth forest at different stages of regeneration, embedded in a primary terra firme forest that extents for hundreds of kilometers to the north, east, and west (Gascon and Bierregaard, 2001). Forest fragments were isolated in the early 1980s (except one isolated by 1991), and vegetation around them has been periodically cut to maintain fragment isolation. The study area corresponds to a lowland tropical moist forest (50–100m elevation), with temperatures ranging from 19 to 39°C, and total annual rainfall from about 1900 to 2500mm (Gascon and Bierregaard, 2001; Laurance, 2001). Precipitation is common in every month, however there is a distinct dry season between June and October (Gascon and Bierregaard, 2001; Laurance, 2001). Day length remains generally constant throughout the year, with the difference in day length between the longest and shortest days of the year being approximately 18min (De Oliveira and Mori, 1999). Canopy height is about 35–40m, with emergent trees sometimes exceeding 50m (Gascon and Bierregaard, 2001).
Bird sampling and feather collectionBirds were captured using mist nets (NEBBA type ATX, 36-mm mesh, 12×2m), with the bottom of the nets at ground level. The number of nets varied depending on fragment size. In 1-ha fragments, one line of 8 end-to-end mist nets was used. In the 10-ha fragments, one line of 16 nets was used. Three lines of 16 nets were used in the 100-ha fragments, and four lines of 16 nets were used at the continuous forest sites. Also, three or four sets of 4 mist nets were used along the borders at each of the fragments. Each net lane was sampled for a day at the time, usually at no less than one month intervals, from 0600 to 1400h.
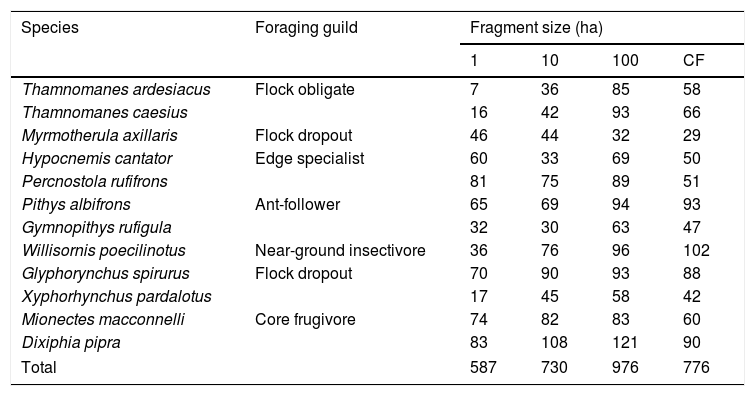
The outermost right rectrix (R6) was collected from each banded bird and placed in individual paper envelopes. Feathers that were still growing were pulled if a minimum of ten consecutive growth bars were visible. Feather samples come from this systematic sampling of all fragments that has taken place in discrete time intervals: 1991–1992, 2000–2001, and 2007–2012. We included feathers from 12 species that belong to six different foraging guilds and differ in their sensitivity to fragmentation and landscape change, based on previous research at the BDFFP (Table 1). Sample size limited the species we could analyze; we only included species with feathers collected in every fragment size and time interval.
Feather sample sizes by species and fragment size. Foraging guild assignments followed Powell et al. (2013) (CF=continuous forest).
| Species | Foraging guild | Fragment size (ha) | |||
|---|---|---|---|---|---|
| 1 | 10 | 100 | CF | ||
| Thamnomanes ardesiacus | Flock obligate | 7 | 36 | 85 | 58 |
| Thamnomanes caesius | 16 | 42 | 93 | 66 | |
| Myrmotherula axillaris | Flock dropout | 46 | 44 | 32 | 29 |
| Hypocnemis cantator | Edge specialist | 60 | 33 | 69 | 50 |
| Percnostola rufifrons | 81 | 75 | 89 | 51 | |
| Pithys albifrons | Ant-follower | 65 | 69 | 94 | 93 |
| Gymnopithys rufigula | 32 | 30 | 63 | 47 | |
| Willisornis poecilinotus | Near-ground insectivore | 36 | 76 | 96 | 102 |
| Glyphorynchus spirurus | Flock dropout | 70 | 90 | 93 | 88 |
| Xyphorhynchus pardalotus | 17 | 45 | 58 | 42 | |
| Mionectes macconnelli | Core frugivore | 74 | 82 | 83 | 60 |
| Dixiphia pipra | 83 | 108 | 121 | 90 | |
| Total | 587 | 730 | 976 | 776 | |
Following Grubb (1989), we calculated the daily growth rate (in mm/day) in each R6 feather collected by marking, on an index card, the length of feather occupied by ten growth bars. After removing the feather from the card, we measured the total length using a digital caliper (0.01mm resolution) and calculated mean daily growth rate for the feather by dividing the total length by ten. To increase the accuracy of this measurement, we repeated the process three times per feather; the final estimated daily growth rate is an average of the three measurements. In order to increase accuracy, all feather measurements were done by A. Hernández-Palma. It is important to mention that contrary to Grubb's original proposed method (Grubb, 1989), our study was not limited to induced (regrown) feathers only. To maximize sample size, we included all feathers available from each target species, regardless of whether it was original or induced.
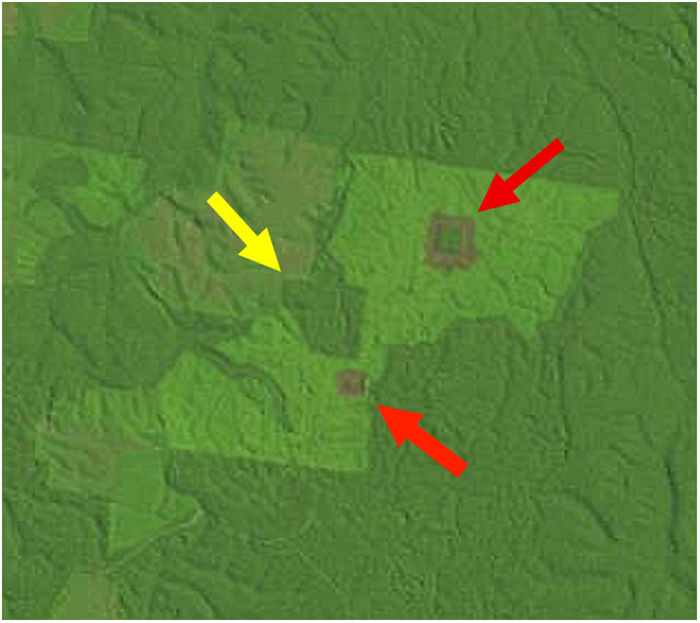
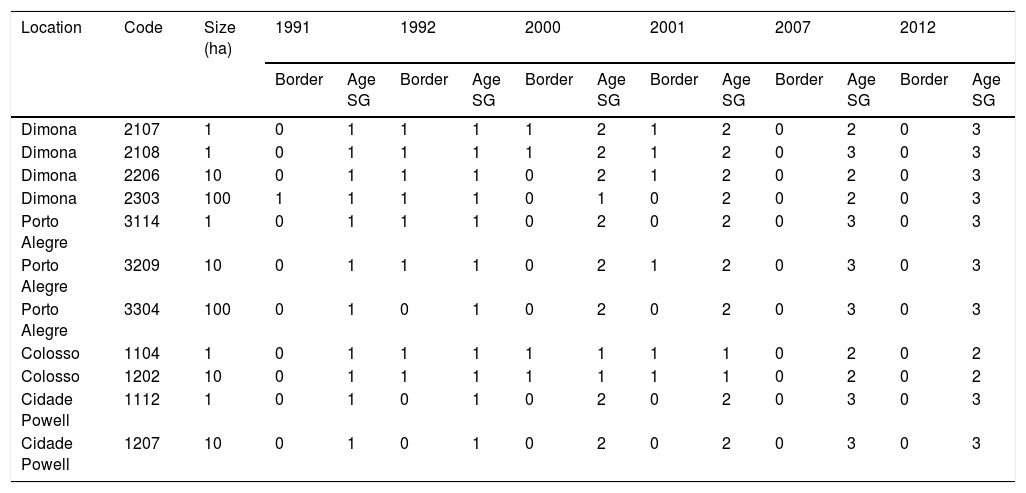
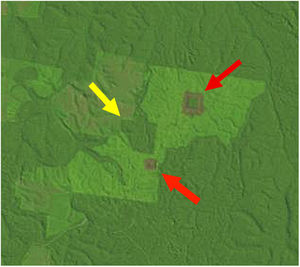
Landscape dataWe used historical records at the BDFFP (G. Ferraz, unpublished data) to reconstruct the isolation/reisolation and land-use history of each fragment. We also inspected LANDSAT images of the study area to confirm data from the historical records. In these images, ages of vegetation are distinguished by shades of color: bare ground and very young vegetation appear in shades of red, whereas older vegetation appears in shades of green, with old-growth forests having the darkest shades (Fig. 1). Based on information from the records and images, we categorized borders as either young (less than 2 years after cut) or mature (more than 2 years). Using this same information, we determined the age of second growth forest in the matrix around the fragments. Because we did not have feathers for all second-growth and fragment size combinations, we transformed the age of second growth to a categorical variable, with categories as follows: 1=0–10 years, 2=11–20 years, 3=21–30 years, and 4≥31 years (continuous forest). A summary of the isolation/reisolation history of each fragment is shown in Table 2.
A LANDSAT image from the BDFFP study area in July 1992 showing fragments of different sizes and vegetation of different ages at Fazenda Porto Alegre. The largest fragment (100-ha, yellow arrow) is surrounded by both a mature border and matrix, whereas the smaller two fragments (1- and 10-ha, red arrows) are surrounded by young borders and a mature matrix (from http://glovis.usgs.gov).
Isolation history of forest fragments at the BDFFP. Code refers to the reserve code assigned in the project and used in other publications (see Lovejoy et al., 1986 for detailed descriptions of the fragments and Stratford and Stouffer, 1999 for a map with the codes). For border status, 1 corresponds to young border and 0 to a mature border. For second-growth age categories see the methods section.
| Location | Code | Size (ha) | 1991 | 1992 | 2000 | 2001 | 2007 | 2012 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Border | Age SG | Border | Age SG | Border | Age SG | Border | Age SG | Border | Age SG | Border | Age SG | |||
| Dimona | 2107 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 0 | 2 | 0 | 3 |
| Dimona | 2108 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 0 | 3 | 0 | 3 |
| Dimona | 2206 | 10 | 0 | 1 | 1 | 1 | 0 | 2 | 1 | 2 | 0 | 2 | 0 | 3 |
| Dimona | 2303 | 100 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 3 |
| Porto Alegre | 3114 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 2 | 0 | 3 | 0 | 3 |
| Porto Alegre | 3209 | 10 | 0 | 1 | 1 | 1 | 0 | 2 | 1 | 2 | 0 | 3 | 0 | 3 |
| Porto Alegre | 3304 | 100 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 3 | 0 | 3 |
| Colosso | 1104 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 0 | 2 |
| Colosso | 1202 | 10 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 0 | 2 |
| Cidade Powell | 1112 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 3 | 0 | 3 |
| Cidade Powell | 1207 | 10 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 3 | 0 | 3 |
We used an information-theoretic framework to model feather growth rate as a function of landscape characteristics with data from all species combined. Since we interested in evaluating the effect of second-growth vegetation in edges and the matrix on the nutritional condition of birds living inside the fragments, our variable set was composed of fragment size, border status, and age of second growth forest in the matrix, all transformed into categorical values. Our full model included feather growth rate as the response variable, fragment size, border status, and age of second growth forest as fixed effects, and species as random effects to control for the differences among them. We evaluated all possible subset combinations of the landscape variables and selected the best model using the AIC criterion corrected for small sample size (AICc), from which we estimated parameters and confidence intervals. All analyses were performed in program R (R Core Team, 2017) using the MuMIn package (Barton, 2016).
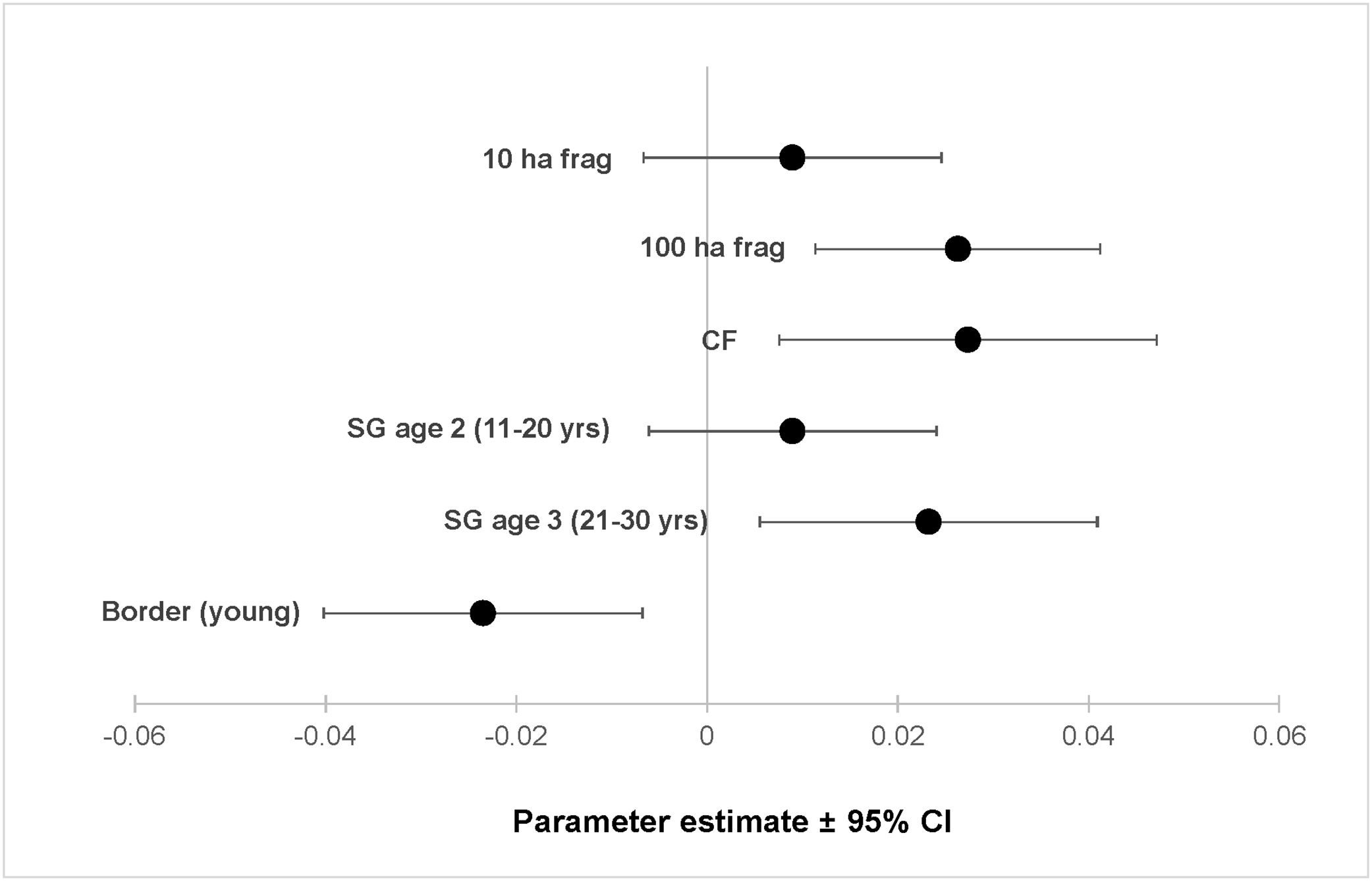
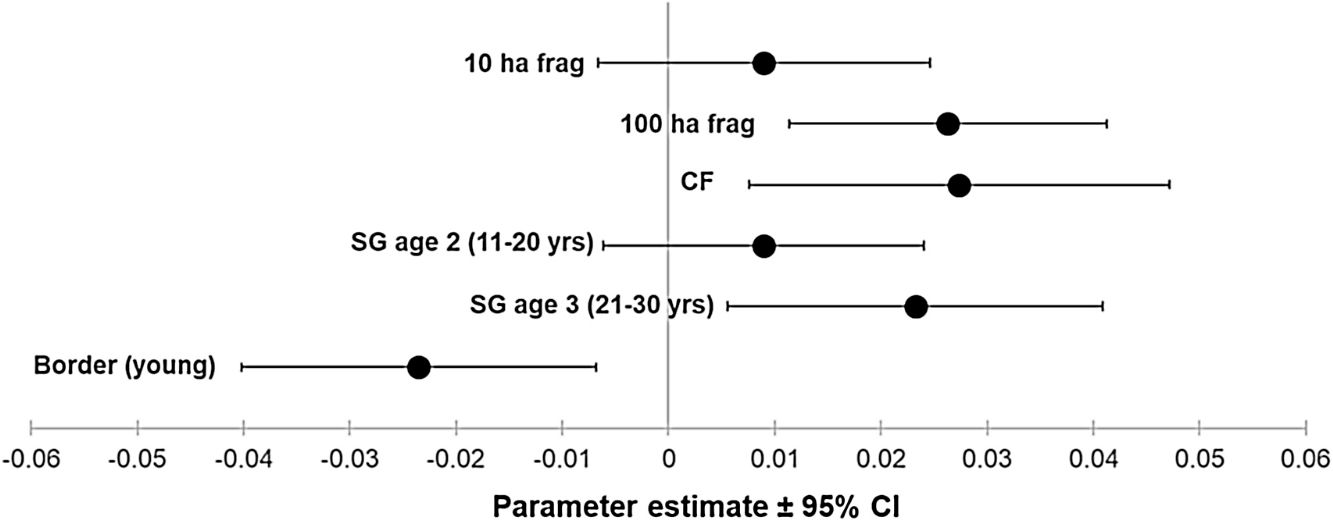
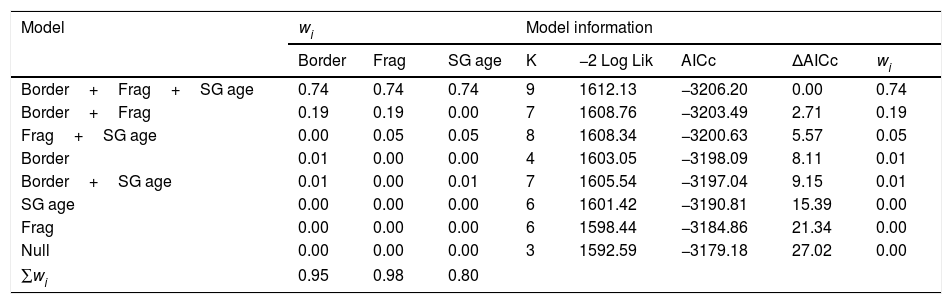
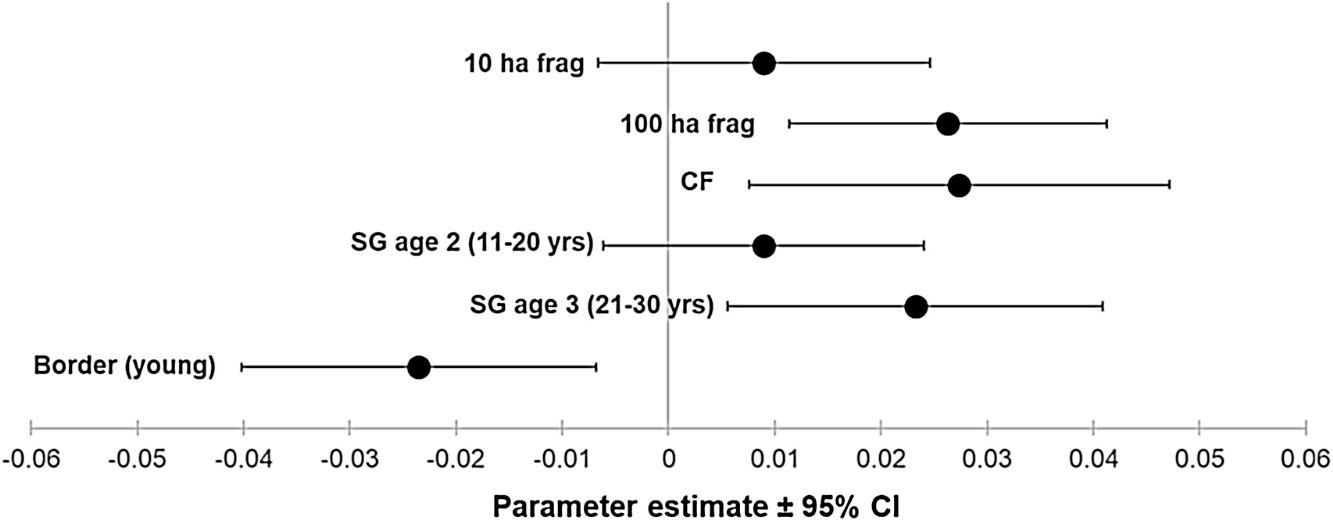
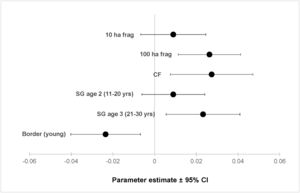
ResultsOur sample size consisted of 3069 feathers, with sample sizes ranging from n=402 for Dixiphia pipra, to n=151 for Myrmotherula axillaris (Table 1). Based on Akaike weights calculated for each variable, fragment size was the most important variable explaining feather growth rate, followed by border status and second-growth age, respectively (Table 3). The best model explaining variation in feather growth rate was the full model (Table 3). Parameter estimates for this model indicate that the presence of a young border around the fragments had a negative effect on feather growth rate, whereas increasing fragment size and age of second-growth in the matrix positively affected feather growth rate (Fig. 2). According to the parameter estimates, young borders around the fragments decreased feather growth rate by an average of 1.36% each day. On the other hand, increasing fragment size from 1ha to 100ha and continuous forest increased feather growth rate between 0.52 and 1.58% per day. Similarly, an increase in the age of second-growth in the matrix from 0 to 30 years increased daily feather growth rate up to 1.34%.
Complete list of Akaike's information criterion (AICc) model selection results for feather growth rate as a function of landscape characteristics at the Biological Dynamics of Forest Fragments Project, 1991–2012.
| Model | wi | Model information | ||||||
|---|---|---|---|---|---|---|---|---|
| Border | Frag | SG age | K | −2 Log Lik | AICc | ΔAICc | wi | |
| Border+Frag+SG age | 0.74 | 0.74 | 0.74 | 9 | 1612.13 | −3206.20 | 0.00 | 0.74 |
| Border+Frag | 0.19 | 0.19 | 0.00 | 7 | 1608.76 | −3203.49 | 2.71 | 0.19 |
| Frag+SG age | 0.00 | 0.05 | 0.05 | 8 | 1608.34 | −3200.63 | 5.57 | 0.05 |
| Border | 0.01 | 0.00 | 0.00 | 4 | 1603.05 | −3198.09 | 8.11 | 0.01 |
| Border+SG age | 0.01 | 0.00 | 0.01 | 7 | 1605.54 | −3197.04 | 9.15 | 0.01 |
| SG age | 0.00 | 0.00 | 0.00 | 6 | 1601.42 | −3190.81 | 15.39 | 0.00 |
| Frag | 0.00 | 0.00 | 0.00 | 6 | 1598.44 | −3184.86 | 21.34 | 0.00 |
| Null | 0.00 | 0.00 | 0.00 | 3 | 1592.59 | −3179.18 | 27.02 | 0.00 |
| ∑wi | 0.95 | 0.98 | 0.80 | |||||
Parameter estimates and 95% confidence intervals for best fit model predicting feather growth rate as a function of landscape variables in Amazonian understory bird species at the Biological Dynamics of Forest Fragments Project. Parameter estimates are shown as differences with respect to the first category in each variable (Frag 1ha, SG age 1, and border mature).
We analyzed feather growth rate as a measure of nutritional condition in 12 understory bird species in Amazonian rainforests fragments within a dynamic landscape, during a period of 21 years at the BDFFP. Following our expectations, feather growth rate of birds living inside forest fragments responded to changes in fragment size, as well as to changes occurring in the overall landscape. For instance, growth rate was lower in feathers collected in fragments surrounded by young borders, suggesting a reduction in the nutritional condition of the birds living in those fragments. In contrast, as fragment size increased and the second-growth vegetation in the matrix matured, feather growth rate increased, suggesting an improvement in the nutritional condition of the birds.
Parallel effects of these same landscape characteristics on bird abundance and movement have been revealed by previous research at our study site (Powell et al., 2013; Stouffer and Bierregaard, 1995; Stouffer and Bierregaard, 2007; Stouffer et al., 2006). Our results extend previous results to confirm the important influence of the landscape on not just abundance, but also condition of birds in fragments. Consequences of processes occurring both within and beyond fragments highlight the importance of landscape-scale approaches for conservation of tropical forest biodiversity. However, it is important to remember that the species included in this analysis are not the most sensitive species at our study site. Because of sample size limitations, the species in this study are ones that regularly occur in fragments, therefore our results are not to be generalized to more sensitive species that are probably affected by fragmentation and landscape change in different ways. For example, we did not have adequate sample sizes to include any terrestrial insectivores, a guild with narrow microhabitat preferences and large space needs that is generally absent from fragments until second growth is much more developed (Stouffer et al., 2009; Stratford and Stouffer, 2015).
In our analysis, fragment size appeared as the most important variable explaining feather growth rate, with a positive influence on the nutritional condition of the birds. The importance of fragment size has been long established at the BDFFP, and it may be related to the more sedentary life style, lower dispersal abilities, and large area requirements for establishing territories that many tropical bird species have, compared to birds from temperate latitudes (Stratford and Robinson, 2005). Although fragments at our study area only range from 1 to 100ha, the pervasive area effects have been evidenced in their abundance, extinction rates, and community composition at the BDFFP (Stouffer and Bierregaard, 1995; Stouffer et al., 2009; Stratford and Stouffer, 1999).
Still, these same studies in which the importance of fragment size has been revealed have also exposed the importance of considering other landscape characteristics when studying the effects of forest fragmentation. In line with our predictions, our model results showed that the presence of a young border around the fragments significantly decreased the nutritional condition of the birds, as measured by feather growth rate. In 2006, Stouffer and collaborators found that border age was one of the most important variables explaining capture rates of understory birds in fragments, being almost as important as fragment size itself. Furthermore, when removing the effect of fragment size by analyzing 1 and 10ha fragments only, the pervasive effect of the border vegetation was even more evident (Stouffer and Bierregaard, 2007). Powell et al. (2013) studied movement of birds between the interface of primary and second-growth forests and found that border age was an important variable for all the guilds studied, and that except for non-forest species, an increase of age in the border vegetation was associated with an increase in capture rates. Building on these results, and adding information on the nutritional condition of birds living in forest fragments, our results suggest that border vegetation influences not only abundance and movements in and out of the fragments, but also the physical condition of the birds dwelling in these fragments.
Edges are important, dynamic features of fragments, and their effects are pervasive especially in smaller fragments in which the amount of habitat not affected by edge effects is almost nonexistent (Gascon et al., 2000). With deforestation and logging creating more than 32,000km of new forest edge in the Brazilian Amazon every year (Broadbent et al., 2008), studying the consequences of edge regeneration on within-fragment processes is essential. Recently created edges are abrupt and alter not only physical conditions, but the way species distribute, behave and interact with each other along edges (Murcia, 1995; Saunders et al., 1991). However, when edges are allowed to regenerate, their detrimental effects become less severe, and they act as a buffer that protect the interior of forest fragments (Gascon et al., 2001; Lovejoy et al., 1986; Saunders et al., 1991). Nevertheless, the ability of edges to regenerate and serve this protective function depends on the quality of the matrix, more specifically the land use intensity and history of matrix vegetation. This dependence creates a feedback mechanism in which low-intensity land uses in the matrix allow edge regeneration, which buffers the forest interior from detrimental edge effects, increasing the effective size of fragments over time. On the other hand, matrices with high-intensity land uses slow the regeneration process along the edges, increasing their retrogression further into the fragment's interior (Gascon et al., 2000).
Our model results also revealed a positive relationship between the age of second-growth vegetation in the matrix and feather growth rate. The importance of matrix vegetation in fragmented landscapes has been long established (Antongiovanni and Metzger, 2005; Ricketts, 2001; Stouffer and Bierregaard, 1995; Stouffer et al., 2006), and this has allowed studies of habitat fragmentation to transition from an island-ocean model to a countryside biogeographic framework (Laurance, 2008; Mendenhall et al., 2014). Although Amazonian second-growth forests may not have conservation value equivalent to undisturbed forests (Barlow et al., 2007; Moura et al., 2013), second-growth forests next to edges act as buffers, serving an important role in lessening some of the detrimental effects of fragmentation (Barlow et al., 2016; Gascon et al., 2000; Lovejoy et al., 1986; Saunders et al., 1991). Vegetation recovery in the matrix can also facilitate dispersal, allowing recolonization of previously isolated fragments (Ferraz et al., 2007; Powell et al., 2013; Stouffer and Bierregaard, 1995; Stouffer et al., 2006). Additionally, second-growth forests can provide habitat and resources for at least a subset of forest species, especially when allowed to regenerate for at least a few decades (Barlow et al., 2007; Chazdon, 2014; Moura et al., 2013; Wolfe et al., 2015).
The importance of second-growth forests and the consequences of land-use history at the BDFFP have been evidenced to influence dynamics inside the fragments in many instances. As early as 9 years after fragment isolation, the importance of second-growth vegetation in the matrix surrounding the fragments was clear; understory bird communities in 10-ha fragments surrounded by Cecropia vegetation resembled pre-isolation communities, and many sensitive species that disappeared after isolation returned to fragments by moving through this type of second-growth (Stouffer and Bierregaard, 1995). As mentioned above, Stouffer et al. (2006) found that second-growth vegetation in the borders and the matrix surrounding the fragments was as important as fragment size for explaining bird abundance inside the fragments. Avian movement across the interface of primary and second-growth forest recovered to pre-isolation levels 13 to 34 years after land abandonment for 9 of 10 guilds studied (Powell et al., 2013). Additionally, when comparing survival and population growth estimates of seven species in 25 year-old second-growth and primary forests, Wolfe (2014) found that although the estimates were higher in primary forest for some species, they all showed stable population growth and survival in both habitats.
Although our study was not aimed at looking at individual species’ responses to landscape change, it is important to highlight the contribution of the two frugivore species to the results we found. These two species are common at the BDFFP, persist in fragments as small as 1-ha, and use second-growth vegetation in the matrix beginning in the early stages of regeneration when most birds avoid it. Despite all these similarities, their responses to fragmentation and landscape change were strikingly different. Mionectes macconnelli fell in line with our predictions with feather growth rate being lower in the 1- and 10-ha fragments, and dropping significantly when the border vegetation was young. Feather growth rate was higher in the 100-ha fragments with and without young borders, and there were no differences between this fragment size class and continuous forest. Also, the effect of second-growth age on the nutritional condition of M. macconnelli was obvious, with feather growth rate being significantly lower when vegetation in the matrix was less than 20 years old. However in the case of D. pipra, we found no differences in feather growth rate across any of the landscape combinations we examined.
Finally, we cannot determine whether the occupation of low quality habitats by birds (i.e. fragments) is the cause or the consequence of the reduced nutritional condition found in those birds. It is possible that birds use low quality habitats because they are unable to obtain and defend territories in optimal habitat patches. However, our long-term analysis allowed us to detect changes in the nutritional condition of birds as the landscape at the study area changed, which leads to suggest that the changes in the landscape are driving the changes in the nutritional condition we observed in birds living in the fragments.
Using a simple, yet informative measure of nutritional condition, we were able to add evidence of the detrimental effects that habitat fragmentation and landscape change have on the processes occurring inside the fragments, while also reinforcing the idea of the promising value of second-growth forests in fragment edges and the matrix, especially when allowed to regenerate for at least a few decades (Chazdon, 2014; Chazdon et al., 2009; Moura et al., 2013; Powell et al., 2013; Stouffer et al., 2006; Wolfe, 2014). However, it is important to remember that the BDFFP is a best-case scenario. The ability of vegetation to regenerate into valuable habitat for wild animals is highly dependent on the intensity and the land-use history of the matrix vegetation, as well as the ratio of second-growth to old-growth forest present in the area (Chazdon et al., 2009). As the ability of edges to regenerate and serve as buffers for forest fragments is highly dependent on activities on the matrix (Gascon et al., 2000). In places where fire, logging, hunting, invasion by exotic species are common, the potential for second-growth vegetation to be valuable for forest species is very low (Chazdon et al., 2009). Impoverishment of soils due to intense agriculture, local extinction of seed dispersers due to hunting and habitat loss, and elimination of the soil seed bank because of fires, halt the process of forest regeneration needed for species persistence in disturbed landscapes (Chazdon, 2014; Chazdon et al., 2009).
Understanding how species respond to changes in the environment is vital for developing effective conservation programs. Although some species might not show decreases in abundance after fragmentation, unfavorable conditions in fragments can affect birds in different ways which could have important implications in their nutritional condition, as evidenced here. Finally, it is important to develop conservation and management programs that integrate forest fragments into the context of the overall landscape for the conservation and future of tropical forest biodiversity.
We thank the many banders, assistants, and mateiros who helped collect the data and feathers for this project, as well as the staff at the Biological Dynamics of Forest Fragments Project for the logistical support. This work was supported by funds from the U.S. National Science Foundation (LTREB numbers 0545491, 1257340), the Departamento Administrativo de Ciencia, Tecnología e Innovación – COLCIENCIAS (Fondo Francisco José de Caldas, Grant Number 529-2011), and the National Institute of Food and Agriculture, U.S. Department of Agriculture, McIntire Stennis projects numbers 94098 and 94327. We conducted this research under applicable Brazilian permits. This is publication 741 of the BDFFP Technical Series and 45 of the Amazonian Ornithology Technical Series of the INPA Collections Program. The manuscript was approved by the Director of the Louisiana State University Agricultural Center as manuscript number 2018-241-32229.