Developing rapid, cost-effective, methods to facilitate forest recovery in degraded tropical habitats is a key restoration goal. Here, we evaluated the efficacy of establishing large (≥2m) vegetative cuttings or stakes of Ficus (Moraceae), keystone species that play essential roles in ecological food webs. We evaluated eight species with broad geographic distribution in the Neotropics in three separate studies in southern Costa Rica: (1) a common garden trial; (2) a multi-site study; and (3) an earlier 2004 study. In the two recent trials, resprouting and survival ranged from 0 to 100%. Six species of hemiepiphytic figs (subgenus Urostigma) resprouted at much higher rates (80–100%) compared to free-standing species in the subgenus Pharmacosycea (0–18%). Contrary to expectations, resprouting did not vary by wood specific gravity. Mean canopy area for established species was 1.1–1.4m2 after 2 yr, diameter growth at this stage was negligible, and one species developed fruit (∼12% of individuals). In the older resurvey, surviving stakes (60%) had mean DBH of 13.7±6.2cm with canopy height of 8.1±2.7m after 13 yr; 50% of individuals were fruiting when surveyed during peak dry season. There was no mortality in the 9 yr lapse since they were last surveyed. Results indicate that this methodology shows promise and could be used to establish enrichment plantings in degraded habitats that augment fruit availability, and thereby facilitate recovery.
A core challenge in contemporary tropical conservation is to facilitate the colonization and establishment of late-successional species in young, regenerating forests. Secondary forests now compose more than half of tropical forests (Chazdon, 2014), and this ratio will continue to increase as old-growth forests are replaced by agriculture (Rudel, 2017) and marginal agricultural lands revert back to forest (Aide et al., 2013). Yet with few exceptions secondary forests do not support complete regional species assemblages (Gibson et al., 2011), and there is a considerable lag-time in recovery (Moreno-Mateos et al., 2017), with late-successional species particularly underrepresented (Martínez-Garza and Howe, 2003). Whereas tropical ecological restoration has often focused on establishing diverse forests on degraded sites (e.g., Lu et al., 2017; Rodrigues et al., 2009), developing mechanisms for secondary forest enrichment may be an important, cost-effective alternative to overcome dispersal and establishment limitations of sensitive species (Bertacchi et al., 2016).
Choosing appropriate species for secondary enrichment is an important consideration, and in the economy of nature, some species play larger roles than others. For example, keystone species are organisms that have a disproportionate ecological impact relative to their abundance or biomass, such that the removal of a keystone species from an otherwise intact ecosystem can precipitate major changes in ecosystem structure and function (Paine, 1974; Power et al., 1996). Reciprocally, establishing keystone species in degraded habitats may be an especially powerful means of catalyzing their recovery (Goosem and Tucker, 2013; Ripple and Beschta, 2007).
In tropical forests, figs (Ficus spp.; Moraceae) are a well-documented example of a keystone group (Terborgh, 1986). Despite their relatively low abundance, fig trees support diverse animal communities through copious fruit production, and often do so at times of the year when few other food resources are available (Diaz-Martin et al., 2014; Harrison, 2005; Shanahan et al., 2001). By attracting a wide array of fruit-eating animals, fig trees also concentrate seed dispersal around them, creating hotspots of plant diversity (Fujita, 2014; Slocum, 2001; Slocum and Horvitz, 2000). With more than 800 species distributed throughout the world, several authors have concluded that figs should be included in efforts to restore degraded tropical and subtropical forests (Cottee-Jones et al., 2016; Dreschel et al., 2017; Kuaraksa and Elliott, 2013; Kuaraksa et al., 2012).
One promising technique for rapidly establishing figs on degraded lands is to propagate them as cuttings. Cuttings have several practical advantages compared to other revegetation practices like direct seeding and planting tree seedlings. Namely, cuttings do not require nursery care, they can be harvested at times when seeds are not available, they carry a lower economic cost than nursery-raised seedlings, and, by virtue of their greater height at planting, they may escape competition with ruderal vegetation more rapidly (Hunter, 1987; Zahawi and Holl, 2014). Moreover, cuttings are scalable; it is possible to plant large individuals that are several meters in length (Zahawi, 2008), and larger cuttings, including figs, tend to establish more consistently than do smaller cuttings (Danthu et al., 2002; Zahawi, 2005).
Successful establishment of large fig cuttings has been related to the time of year in which they are harvested (Alonso et al., 2000; Alonso et al., 2001). Several fig species are known to be easily propagated from cuttings, and planting fig cuttings is regularly practiced by rural people to produce live fence posts and to attract animals (Gautier, 1996; Harvey et al., 2005; Salick et al., 1995; Valerio, 2004). Animal attraction may be accomplished quickly, as fig cuttings taken from mature adults have been observed fruiting in the same year that they were planted (Zahawi, 2008; Zahawi and Holl, 2009). Whereas large cuttings are more cumbersome to transport, the effort may be worthwhile if a few planted individuals have a large effect on ecosystem recovery.
In this study, we address two questions about using fig cuttings for tropical forest restoration. First, what factors predict resprouting and establishment of native fig species in southern Costa Rica? Second, what is the long-term viability of fig trees planted as cuttings? To do so, we draw on three field experiments, two of which were established in 2015–2016 and a third long-term study that was initiated in 2004; we evaluate eight species of Ficus, each of which has broad geographic distribution in the Neotropics (Table 1).
Resprouting, survival, and growth of six Ficus species planted as 4-m tall stakes in a common garden study and censused after 22 mo in southern Costa Rica.
| Species | Geographic rangec | No. planted | No. resprouted | No. surviving | Mean canopy area (m2) (1 SD) |
|---|---|---|---|---|---|
| F. citrifoliaa | US-Arg | 5 | 5 | 1 | 1.4 |
| F. colubrinaea | Mex-Col | 5 | 5 | 3 | 1.5 (0.3) |
| F. costaricanaa,d | Mex-Pan | 5 | 5 | 4 | 1.6 (0.4) |
| F. hartwegiia | Mex-Per | 5 | 5 | 5 | 1.4 (0.4) |
| F. macbrideib | Nic-Per | 4 | 0 | 0 | – |
| F. obtusifoliaa | Mex-Bra | 5 | 4 | 1 | 1.0 |
| Total | 29 | 24 | 14 | 1.4 (0.3) |
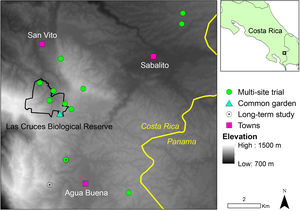
Our study area comprises 13 sites spread across 53km2 of agricultural countryside in Coto Brus County, southern Costa Rica (Fig. 1). Site elevations range from 1000–1200m a.s.l. The predominant native ecosystem is tropical premontane wet forest (Holdridge et al., 1971), but most forest was cleared for coffee cultivation between the 1950s and 1980s with about 28% remaining in the study area at present (Zahawi et al., 2015). Today, the area is a diverse mix of coffee plantations, cattle pastures, crop agriculture, small urban centers, and forest fragments of varying ages. Precipitation varies with microtopography but is ∼3600mmy−1 at the Las Cruces Biological Station (LCBS; 8°47′ N, 82°57′ W), with a distinct dry season from December to March. Mean annual temperature is 21°C (LCBS, 2017).
Stake preparationFor the two planting trials in 2015–2016, we planted 4-m long cuttings (i.e., stakes) of eight native tree species in the genus Ficus. Following Zahawi (2008), stakes were harvested with a machete from multiple adults of each species, and all lateral branches were removed to limit dessication. Stakes were transported to sites in a pick-up truck and were cushioned to avoid damaging cortical tissue. Each stake was girdled 30cm above the base immediately prior to planting in order to stimulate rooting just below the soil surface. Stakes were planted at 50cm depth, and soil was lightly compacted around the base for stability. Mean stake diameter at breast height (DBH; 1.3m) for all species at the time of planting was (8.0±1.7cm; mean±SD), and all stakes were planted within two weeks of cutting. For the long-term study we resurveyed stakes that were planted as 2-m tall cuttings in 2004 (Zahawi and Holl, 2009). Twenty individuals were planted at each of three field sites to a depth of 15–20cm by opening a small hole using a stick and lightly compacting soil around the base of each planted stake. Given the shallower planting depth, stakes were not girdled.
Field trialsCommon garden trialTo evaluate the resprouting capacity of a number of common, native Ficus species, we planted five stakes each of six species in a common garden within a young, secondary forest with a relatively sparse canopy (∼5m height) at the LCBS. Stakes were planted between 25 April and 15 May 2015, and species included F. citrifolia Mill., F. colubrinae Standl., F. costaricana (Liebm.) Miq., F. hartwegii (Miq.) Miq., F. macbridei Standl. (N=4 planted), and F. obtusifolia Kunth. Stakes were planted in a 5×6 grid with 3-m spacing. At planting time, we measured stake height and DBH. We monitored stake survival, establishment, and fruiting after 1, 2, 5, 8, and 22 mo. After 22 mo, we measured stake height, DBH, and canopy area, which we estimated as the area of an ellipse: πAB, where A and B are perpendicular radii.
Multi-site trialTo test stake performance across a heterogeneous landscape, we planted 145 individuals of four Ficus species in 11 young, secondary forests (5–10m canopy height) during three planting periods in 2015–2016. Between 1 April and 30 May 2015, we planted 33 individuals of each of two species, F. americana Aubl. and F. tonduzii Standl. Three individuals were planted at each site, and individuals were separated by ≥30m. Between 12 and 14 August 2015, 33 individuals of F. costaricana were planted, three per site. Finally, between 4 and 17 Aug 2016, we planted an additional 13 individuals of F. costaricana, replacing individuals that had died from the Aug 2015 planting. In this same time period we also planted 33 individuals of F. colubrinae across the eleven sites. We monitored for resprouting, establishment and survival, and fruiting of all planted individuals in Oct 2015, Apr 2016, and Feb–Mar 2017. In Feb–Mar 2017, we also measured canopy area and DBH.
Long-term studyTo evaluate the long-term efficacy of Ficus stakes, in April 2017 we resurveyed survival, height, DBH, and fruiting status of F. americana individuals at ∼13 yr after planting, and at two of the three field sites that were planted originally (the third had since been recleared) (Zahawi and Holl, 2009).
Generalizable predictors of resprouting capacityIn an effort to seek a generalizable predictor of resprouting capacity in Ficus, we tested for relationships with subgenus classification: Urostigma, a predominantly hemiepiphytic group (N=6 spp.) and Pharmacosycea, a free-standing group of trees and shrubs (N=2 spp.) (Berg, 2003). We also evaluated wood specific gravity – a functional trait that integrates many life history attributes (Chave et al., 2006). We used an increment borer to take wood samples from 36 wild-growing Ficus individuals (seven of the eight species evaluated in this study) located within 7km of the Las Cruces Biological Station in Jan 2016 (Appendix S1). Samples were stored at 4°C in watertight containers prior to processing. Samples were then divided into 2-cm segments, weighed wet, dried for 24h at >101°C, and weighed dry (Chave et al., 2006). Specific gravity (G) was calculated by dividing the dry weight of each subsample by its wet volume. Mean specific gravity (Gmean) for each sample was calculated using the formula: Gmean=Gp+2gp/3, where Gp is the gravity at the pith, R is the radius of the tree, and g is the rate of change of specific gravity with respect to the radius (Williamson and Wiemann, 2010).
Data analysisTo determine whether mean specific gravity varied among Ficus species, we used a one-way analysis of variance. We also used a one-way analysis of variance to determine if initial DBH affected survival of planted species at 1 yr. We used a binomial general linear model to test for an effect of species relatedness at the subgenus level on resprouting capacity. Analyses were conducted in R version 3.2 (R Core Team, 2016). Error is reported as 1 standard deviation. Nomenclature follows www.tropicos.org (accessed on 2017-04-20).
ResultsCommon garden trialIn the six Ficus species evaluated, resprouting varied from 0 to 100% (Table 1). Five species in subgenus Urostigma resprouted at rates of 80–100%, whereas one species in subgenus Pharmacosycea did not resprout at all. Among the five resprouting species, survivorship at 22 mo ranged from 20 to 100%, and mean canopy area among the survivors was 1.4±0.3m2.
Multi-site trialAcross two planting seasons at 11 sites, one-year stake survivorship ranged from 97% in F. colubrinae to 74% in F. costaricana, 30% in F. americana, and 12% in F. tonduzii, the only species in subgenus Pharmacosycea used for this trial (Table 2). Most mortality (86–94%) occurred within the first year after planting. Among surviving individuals of all species, canopy area grew to 1.7±1.5m2 after one year and to 4.5±2.7m2 after two years. Diameter and height growth were negligible at this stage. We observed two individuals of F. costaricana (6%) fruiting after one year and four (12%) fruiting after two years. We did not observe other species in fruit.
Survival and growth of four Ficus species planted in a multi-site trial in southern Costa Rica.
| Species | Geographic Rangec | Number planted | Surviving @1 yr | Surviving @2 yr | Mean canopy area (m2) @1yr (1SD) | Mean canopy area (m2) @2yr (1SD) |
|---|---|---|---|---|---|---|
| 2015 planting | ||||||
| F. americanaa | US-Per | 33 | 10 | 7 | – | 1.3 (0.6) |
| F. costaricanaa | Mex-Pan | 33 | 21 | 19 | – | 1.1 (0.8) |
| F. tonduziib | Gua-Bol | 33 | 4 | 2 | – | 1.2 (0.1) |
| 2016 planting | ||||||
| F. colubrinaea | Mex-Col | 33 | 32 | – | 0.4 (0.4) | – |
| F. costaricanaa | Mex-Pan | 13 | 13 | – | 0.5 (0.3) | – |
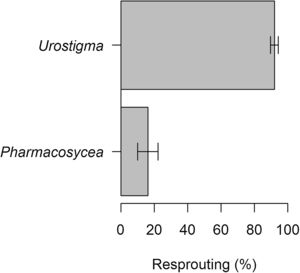
Combining observations from the common garden and multi-site trials, six species in the subgenus Urostigma resprouted at much higher overall rates than the two species in the subgenus Pharmacosycea (Fig. 2). This difference was supported by a binomial general linear model (P<0.0001, difference=76%). For the three species with >5 replicates and at least 20% survival, initial DBH did not affect survival at 1 year (F1,68=1.1, P=0.3), although a broad range in DBH size classes were not utilized in this study. The mean specific gravity of wood did not vary among seven fig species (F6,29=0.6, P=0.7), precluding the possibility of predicting resprouting on the basis of this functional trait (Appendix S1).
Long-term studyF. americana planted as 2-m stakes in two open fields in 2004 suffered no further mortality between three and ∼13 years after planting. Mean DBH of surviving individuals at the time of planting was 3.9±0.9cm, and mean DBH by 2017 was 13.7±6.2cm (range 6.6–29.8cm; N=24). After 13 years, mean tree height was 8.1±2.7m, and 50% of individuals were producing fruit at the height of the 2017 dry season when they were resurveyed.
DiscussionOur results indicate that vegetative establishment of Ficus spp. is a viable planting methodology, at least for some species. Two species in particular, F. colubrinae and F. costaricana, had survival rates in excess of 70%. Although F. americana had low survival in the two more recent field trials (∼20% after 2 yr), it was far more successful in the long-term study with 60% survival and no mortality since they were last surveyed ∼9 yr ago. In turn, F. hartwegii showed considerable promise as an alternate species but replication was low. All species utilized in this study have wide geographic distribution, indicating that this restoration strategy has the potential for broad application within the Neotropics.
Resprouting capacity in the eight fig species evaluated was linked to their evolutionary relatedness and, by association, their lifeform. Species in the subgenus Urostigma had a much greater ability to resprout than members in the subgenus Pharmacosycea. Ficus spp. in the subgenus Urostigma are primarily hemiepiphytic and include species with roots that anastomose (i.e., fuse, as in strangler figs), whereas species in the Pharmacosycea are primarily free-standing trees (Berg, 2003). A similar pattern was noted by Danthu et al. (2002) comparing propagation in the subgenera Sycomorus (a free-standing group) and Urostigma of West African Ficus spp., with resprouting noted exclusively in the latter subgenus. Likewise, all six species evaluated in the study by Kuaraksa and Elliott (2013) using small 10–20cm Ficus spp. cuttings that fared poorly belonged to the subgenera Ficus or Sycomorus that are free-standing (Berg, 2003; Kuaraksa et al., 2012). Accordingly, the ability to establish vegetatively in Ficus could be related to the ability to anastomose or to their hemiepiphytic habit whereby aerial stem tissue readily develops into root tissue upon contact with soil. Regardless of the physiological mechanism involved, circa 280 species of Ficus are in the subgenus Urostigma (a third of all described species) and their distribution is pantropical (Berg, 2003; Shanahan et al., 2001). Thus, the patterns described here may be broadly applicable.
In contrast, wood specific gravity was not a good predictor of resprouting. We expected that lighter-wooded figs would resprout more readily, based on the assumption that lighter wood is typically indicative of a more resilient life history strategy (Curran et al., 2008). Instead, we found little variability in wood specific gravity among the seven species evaluated; wood specific gravity may be a better predictor of resprouting across a more diverse suite of tree species.
In addition to species- and individual-level attributes, the ability to resprout and establish may also vary depending on the characteristics of the translocation site. For example, F. costaricana and F. colubrinae planted in 2015–2016 in young secondary forest habitat established well throughout our study area, which has an elevation range from 1000 to 1200m and 3–4m variability in rainfall. However, we found a strong disparity in survival for F. americana between individuals planted in full sun in 2004 and those planted beneath a secondary forest canopy in 2015–2016. Survival was higher under open sun conditions, suggesting that initial habitat planting conditions may affect establishment success for some species, a result that is supported by noted niche differentiation in the canopy establishment locations of several Urostigma members (Laman, 1996). That said, we cannot directly parse the effect of habitat from the random effects of planting year with our data (Stuble et al., 2017). Lastly, we also noted a marked difference in the size of F. americana individuals (mean height and diameter) planted in 2004 at each of the two resurveyed sites (data not shown). Clearly, developing a better understanding of what parameters affect survival and growth of target species is an important limitation to this planting approach that needs to be further evaluated.
A key reason to evaluate figs is their propensity to produce copious amounts of fruit. In a mature forest, individuals of a population of fig trees often have offset fruiting phenologies, such that there is always a fruiting tree available to frugivores. This is particularly the case with monoecious species which include the entire Urostigma group (Shanahan et al., 2001). Figs in the subgenus Urostigma are also strongly favored by frugivores. In the exhaustive review by Shanahan et al. (2001) the top ten species, in terms of noted number of frugivore species recorded, are all in the subgenus Urostigma. Broad range in fig fruit size is also an important asset given the potential to attract different suites of dispersers (Janzen, 1979; Shanahan et al., 2001). All of these factors are relevant to restoration as attracting large and small frugivores would increase the diversity of seeds dispersed into a site (Moran et al., 2009), whereas continuous fruit production ensures constant visitation and seed dispersal beneath focal fig trees. Moreover, established figs will provide shade, improving microclimatic conditions to facilitate the establishment of dispersed seeds (Slocum, 2001; Fujita, 2014).
Early onset of fruit production is an important feature in species consideration for restoration (Goosem and Tucker, 2013). Indeed, some species of Ficus have been noted to start fruiting within a few years of being planted as seedlings (Kuaraksa et al., 2012). In the multi-site study, several individuals of F. costaricana fruited during the first two years of establishment, indicating that planting large stakes can provide food resources for seed-dispersing animals relatively quickly. This was supported in the long-term study resurvey where 50% of F. americana individuals were producing fruit during the height of the dry season some 13 years after they were planted. Indeed, all of our surveys were conducted during the height of the dry season when fruiting is likely suppressed; fruit production may be higher during less stressful climatic periods of the year and will presumably increase as individuals grow and mature. Other studies evaluating vegetative propagation have found similar results of early fruit onset in other genera (Zahawi, 2005; Zahawi and Holl, 2009) suggesting that this planting technique can lead to faster fruit production than through more traditional planting means. Additionally, given that fruit production in figs is also highly idiosyncratic and varies from one individual to the next (Kattan and Valenzuela, 2013), stakes should be harvested from multiple tree individuals to ensure that fruit production is continuous over time.
Vegetative planting using large cuttings has shown promise in a number of other studies (e.g., Alonso et al., 2001; Danthu et al., 2002; Hunter, 1987), however, it may not work as well in seasonally drier habitats (Douterlungne et al., 2015). Given the difficulty in establishing cuttings in seasonally dry habitats, the timing of planting may be an important factor to consider (Alonso et al., 2001; Danthu et al., 2008, 2002). In addition, the use of growth hormones in such habitats may further facilitate their establishment (Bonfil-Sanders et al., 2007), although field trails for Ficus are lacking. Collectively, our observations suggest that planting figs as large cuttings is relatively simple, and is a more cost-effective restoration strategy compared to traditional seedling planting (Douterlungne et al., 2015; Zahawi and Holl, 2009). It may also be broadly applicable, given the pantropical distribution of Urostigma species and the ability of cuttings to establish in both abandoned pastures and young secondary forests. Further research is needed to develop a robust understanding of the ecophysiological mechanisms that underlie observed differences in stake establishment. It also remains to be seen whether introducing such keystone plant species into regenerating ecosystems will catalyze seed dispersal and produce hotspots of late-successional tree diversity.
We would like to thank Juan Abel Rosales for assistance in field setup and in planting and collecting of stakes. We thank the Organization for Tropical Studies students (OTS 2016-1) for assisting with wood specific gravity sampling and analysis. We would also like to thank the numerous landowners who provided permission to establish field trials on their properties. Finally, we thank Rhett Harrison, one anonymous reviewer, and Jean Paul Metzger for excellent comments that improved an earlier version of this manuscript. Funding for this project was provided by the Center for Conservation and Sustainable Development at Missouri Botanical Garden.