Owing to climate change, species’ geographical distribution may be extended, reduced or displaced in the future. Across species’ ranges, novel climate conditions may also expose species to thermal conditions for which they are not adapted. Migration toward more suitable climates will, however, only be possible if species are able to keep pace with climate change. Here, we analyze different metrics to predict the impacts of climate change on the distribution of Amazon primates. We found that this iconic group will be exposed to novel climate conditions in a large portion of their territories and most species might not be able to track their preferred environmental conditions, even when their range is forecasted to expand. Remaining future populations are expected to become fragmented and to occupy sub-optimal conditions at the periphery of their projected bioclimatic envelopes. Our results suggest that climate change may have unprecedented impacts on Amazon biodiversity, especially for species with low dispersal ability, such as primates. In addition to deforestation, hunting, and disease spread, climate change is likely to add up to conservation-defying feedbacks for Amazon primate populations’ fitness and resilience dynamics.
Climate change will redistribute biodiversity on Earth, with effects from ecosystems health to human well-being (Pecl et al., 2017). Under changing climate conditions, species must tolerate or acclimate to new conditions, suffer population declines up to local extinction, or to move toward preferred environments (Urban, 2015). From a geographical perspective, populations experience different processes, with local extinctions at range boundaries where climates become harsher and colonization of newly suitable environments (La Sorte and Jetz, 2012). Such climate-driven dynamics on range borders ultimately lead to distributional shifts (Thomas, 2010), which are abundant in paleontological records (Davis and Shaw, 2001; Gavin et al., 2014), and have been recently observed following extreme climate events on species with high dispersal abilities (Forero-Medina et al., 2011; Smale and Wernberg, 2013).
As the climate changes, suitable environmental conditions for a given species might move from one place to another, yet not all populations will be able to track their moving climatic niches (Schloss et al., 2012). That is so because climate-driven migrations will only allow species to track their climatic niches if distributional limits move at a minimum velocity that is at least the same speed of climate change (Carroll et al., 2015). To keep pace with climate change, species dispersal ability must therefore exceed the velocity of change in climate (Carroll et al., 2015). In addition, species will require permeable routes across landscapes to move toward novel suitable environments (Lawler et al., 2013). However, deforestation creates landscape mosaics that hamper species movements and prevent climate-driven migrations, especially for canopy-dependent species with low dispersal abilities (Gouveia et al., 2016; Sales et al., 2019). Species that are not able to move across fragmented landscapes might be confined to habitat pockets with changing climate conditions, likely to exceed the extreme, seasonality and amplitude of conditions to which species are adapted (Ribeiro et al., 2016).
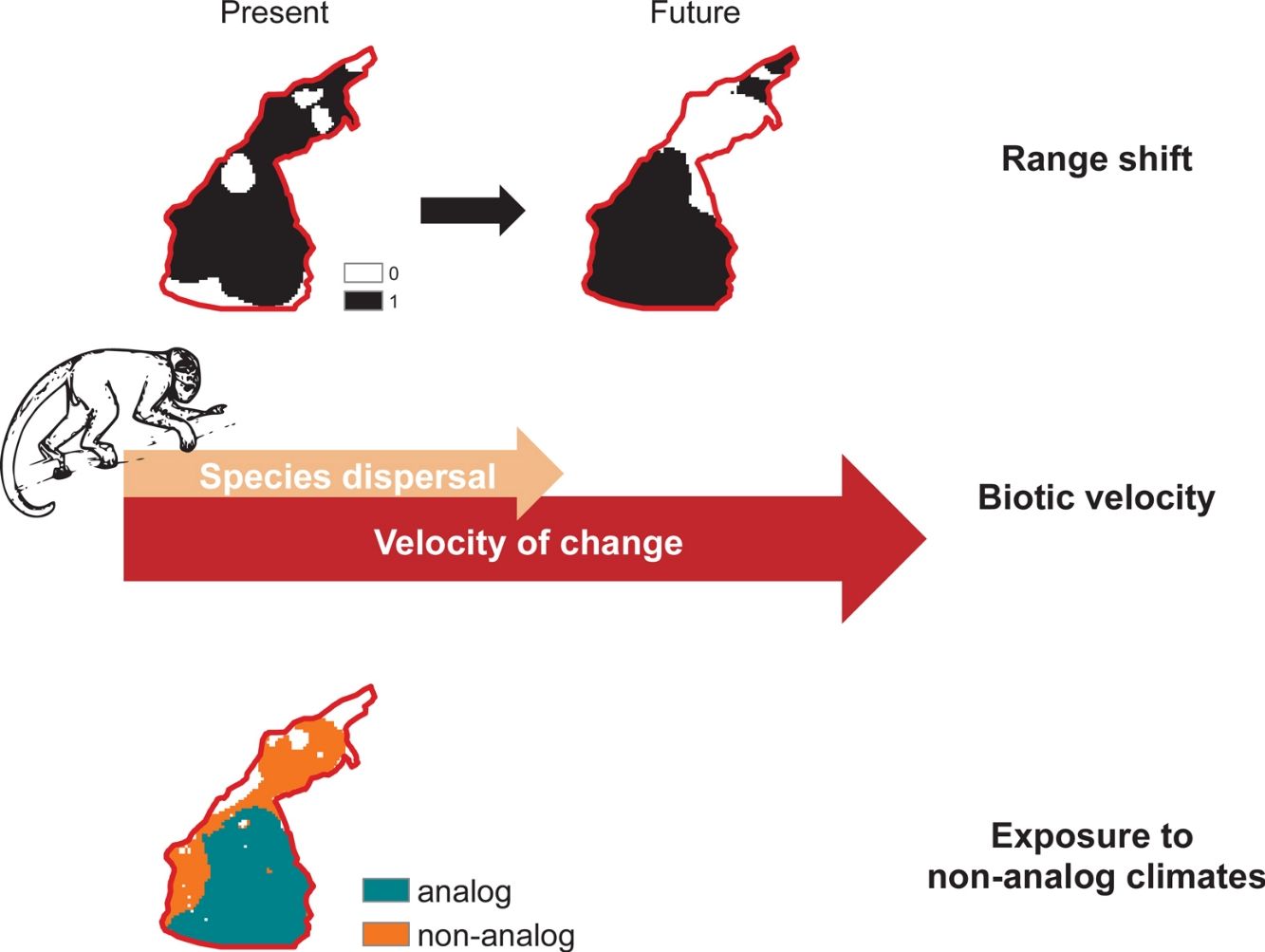
Climate change effects on species distribution, therefore, include: (i) variation in total suitable area, (ii) displacement of optimal environmental conditions and/or (iii) exposure to non-analog climates (Garcia et al., 2014). Here, we assess such multiple dimensions of climate change on the distribution of primates that are endemic to the Amazon basin. Worldwide primates are vulnerable to climate change (Braz et al., 2019; Estrada et al., 2017; Gouveia et al., 2016), but Amazon species might be exposed to novel conditions at a rate greater than the global average (Graham et al., 2016; Ribeiro et al., 2016). In addition to inhabiting regions whose temperatures are close to animals’ upper thermal physiological limits (Khaliq et al., 2014; Sunday et al., 2014), Neotropical Platyrrhini primates rely on forest canopy to feed, reproduce and move across landscapes (Kinzey, 1997; Stone et al., 2009), which agrees on the projected inability of primates to track future climate change (Schloss et al., 2012). Climate change effects have already been observed on primates’ range size (Meyer et al., 2014), population structure (Clee et al., 2015) and dynamics (Wiederholt and Post, 2010), in addition to novel parasitism interactions (Barrett et al., 2013) and multiple feedbacks between climate and deforestation (Struebig et al., 2015).
In this work, we use an innovative approach, combining ecological niche models, deforestation scenarios and dispersal simulations to allow a comprehensive assessment of climate change and deforestation effects on Amazon primates’ distribution. To do so, we analyzed multiple dimensions of climate change, forecasting species-specific and spatial patterns of range shift and exposure to non-analog climates, in addition to a straightforward metric of biotic velocity, searching for metric biases and inconsistencies among them.
MethodsDistribution dataWe defined the species endemic to the Amazon basin as those whose current range boundaries is completely inserted within the basin's territory, plus an additional 200km buffer to account for border uncertainty. We obtained range maps at the International Union for Conservation of Nature database (www.iucnredlist.org, date of access: June 17th, 2019) as polygon shapefiles. Such range maps were used to define species’ environmental requirements, via rasterization – i.e. conversion of a shapefile into a cell-based file – of IUCN range maps into a gridded file of 0.1 degree of lat/long (approximately 10km2 at the Equator line). Random points were sampled within the territory attributed to each species and environmental conditions were characterized (see Climate data section below).
To avoid model overfitting of due to an excessive number of points, we did not use all points to calibrate ecological niche models, following an approach recently described (Sales et al., 2019). Instead, we chose random points within each species’ territory according to its size. Species whose range size was larger than 1000 cells, we randomly chose 12.5% of the total number of cells. For species with range size varying from 501 to 1000 points, only 25% of the number of cells were selected. For species with ranges from 101 to 500 points, we chose 50% of their cells. Finally, for species whose range size was smaller than 100 points had all the cells of their rasterized polygons used in modeling procedures.
The use of maps of extent of occurrence is not considered the first-choice response variable on species distribution modeling (Araújo et al., 2019). The sampling of environmental conditions on locations where species’ presence is not confirmed renders resulting models prone to high commission errors and overly “optimistic” projections (Lobo et al., 2010). Ideally, calibration of bioclimatic envelopes should encompass a well-designed and comprehensive sampling of species occurrences, to obtain a non-autocorrelated representation of species’ realized niche from where species occurs (Araújo and Guisan, 2006). That scenario can rarely, if ever, be met for Amazon species once access constraints, the enormous size of species ranges, and the lack of funding for biodiversity surveys lead to biases in occurrence information (Vale and Jenkins, 2012). Such issues and local extirpations due to defaunation (Dirzo et al., 2014), may result in false relationships between habitat suitability and environmental variables, underestimating biodiversity predictions (Faurby and Araújo, 2018), and overestimating the impact of anthropogenic stressors (Lima-Ribeiro et al., 2017). Furthermore, models calibrated with IUCN range maps are considered useful for providing an initial understanding of species habitat preferences, but these need to be refined with fieldwork (Faleiro et al., 2013; Lemes et al., 2011; Loyola et al., 2012), especially in remote, biodiversity-rich and under-sampled locations, such as the Amazon (Sales et al., 2019, 2017).
Climate dataCurrent climate information was obtained as raster layers, freely available at WorldClim (version 1.4; worldclim.org). Such climate files were produced by interpolation of weather data from ground stations, representative of years 1970–2000 (Hijmans et al., 2005), at 5min resolution (Fick and Hijmans, 2017). Climate information was downloaded in the form of bioclimatic predictors, which derive from raw outputs of surface air temperature and precipitation, but converted into biologically meaningful variables, such as seasonality or climate extremes (Hijmans et al., 2005).
We obtained future climate forecasts referred to year 2050 from the WorldClim database (www.worldclim.org/cmip5_5m, date of access: June 25th 2019) for two extreme greenhouse gases scenarios or representative concentration pathways (RCPs) from IPCC (2014). One scenario represented a stringent Mitigation prospect (RCP 4.5), where greenhouse gas emission rates slow by year 2030, while the other was a Business-as-usual scenario (BAU; RCP 8.5), with no efforts to restrain emissions (IPCC, 2014). Although there are several climate forecasts with global information, all of them result in biases, either in geographical or environmental space, or both (Knutti et al., 2008). In this work, we considered the HadGEM2-ES (HE) model, because its estimates of current temperature and precipitation are considered the least biased for the Amazon (Sierra et al., 2015).
To avoid multicollinearity and overfitting, we reduced the dimensionality of our predictors set using a Principal Component Analysis. By doing so, we extracted the dominant patterns in our group of predictors, summarized into the orthogonal eigenvectors (Reimann et al., 2011) that captured 95% of the information, using the prcomp function of R package stats (R Core Team, 2019). The information related to the future was then projected into this coordinate basis (linear combination), thus respecting the original rotation of the eigenvectors. To do so, applied the function predict onto the prcomp object and the forecasted environmental values from the climate model. Therefore, eigenvector scores, not the original variables, were used here to calibrate our species distribution models.
Species distribution modelingWe modeled the potential distribution of Amazon primates as a function of the environment associated to species’ occurrence. These models were then transferred onto different scenarios of climate change forecasts, to assess potential climate-driven distributional shifts. To do so, we used MaxEnt, a presence-background machine-learning method, known for its high accuracy (Franklin, 2009). Like other machine-learning methods, MaxEnt uses artificial intelligence algorithms to maximize the relationship between occurrences and predictors, while minimizing the number of parameters (Phillips et al., 2006), by comparing environmental conditions from species’ occurrences to the conditions along the study background (Elith et al., 2011). In addition, MaxEnt is robust to the presence of some positional error (Graham et al., 2008) and allows for balancing goodness-of-fit with model complexity, via “tuning” of model settings (Muscarella et al., 2014) by variations on “feature classes” or FCs (Muscarella et al., 2014; Peterson, 2011).
We limited the study area to a species-specific background, as defined by cropping environmental layers by the bounding box from the extent of occurrence (extreme coordinates), plus an additional 10 degrees to each bound. We did so to restrict our study regions to areas that are potentially accessible for species, which is crucial for the reliability of the outcome of species distribution models (Barve et al., 2011). Then, 10,000 background points were randomly sampled from environmental raster files (Barbet-Massin et al., 2012). MaxEnt models were, then, “tuned” by combining feature classes - L, LQ, H, LHQ, LQHP, LQHPT (L=linear, Q=quadratic, H=hinge, P=product, T=threshold) and selected by their values of Akaike Information Criteria (Akaike, 1974), corrected for small sample size (AICc, using the ENMeval R package) (Muscarella et al., 2014). Continuous predictions of climate suitability were, then, converted into binary maps of “presence” and “absence”, using a 10% omission rate threshold. This threshold restricts presumed presences to the 90% more common conditions in the dataset, allowing the models to miss up to 10% of the values.
While recognizing that patterns of deforestation may change, we included deforestation predictions for the Amazon as a potential driver of primate distribution. To do so, we used a previously published model of deforestation in the Amazon (Soares-Filho et al., 2006), based in two scenarios of road paving investment. The first, Business-as-usual, considers historical deforestation rates, yet the second, Governance, establishes an upper limit for deforestation, following the Brazilian environmental law at the time (Soares-Filho et al., 2006). Gridded information on land-use type and proximity to paved roads, in addition to terrain slope and the socio-economic level of micro-watersheds were considered, then, drivers of human occupancy and the best predictors of deforestation in the Amazon (Soares-Filho et al., 2014, 2006).
Here, cells predicted to be deforested by year 2050 were considered permanently unsuitable, as Amazonian primates are canopy-dependent (Stone et al., 2009) and rely on trees to feed, reproduce and to move across human-dominated landscapes (Sales et al., 2019). In addition, deforested areas may disrupt climate-driven migratory routes. Thus, we also considered that deforested cells could prevent migration among suitable cells, using a cellular automata model of dispersal among suitable cells (Engler and Guisan, 2009), implemented in MigClim R package (Engler et al., 2012). As barriers to dispersal, deforested cells reduced the likelihood of colonization of suitable cells among sequential timesteps, unless via stepping-stone “forest routes”.
Biotic velocityTo assess species-specific responses to climate change in terms of range movements, we calculated a distribution-based “biotic velocity”, which corresponds to the time-calibrated distance from a current suitable site to the nearest future site projected to be climatically similar to the species’ suitable conditions (Carroll et al., 2015). In other words, biotic velocity is the minimum speed at which species must migrate to keep track of its preferred climate conditions. However, populations at the center of species range limits are usually buffered against deleterious border effects, so that population viability and abundances usually increase from periphery toward range nucleus (Channell and Lomolino, 2000a). Contagion-like spread of extinction forces on range boundaries probably explain why the ranges of endangered species contract inwards, where core populations persist longer (Channell and Lomolino, 2000b).
We therefore calculated the biotic velocity as the minimum speed at which core populations – those from regions surrounding the centroid of the species current range – should move to remain as future core populations. To do so, we calculated the shortest distance between the centroid of species potential distribution (present to future), according to the Vincenty (WGS84 ellipsoid) method (Vincenty, 1975), within the geosphere R package (Hijmans, 2019) and divided it by the total timeframe of our study (i.e. 50 years). Other metrics of biotic velocity, such as Loarie et al.’s (2009) instantaneous local velocity, or the pace at which each cell needed to move maintain constant temperatures, do not account for species-specific border dynamics driven by moving bioclimate envelopes (Carroll et al., 2015). Because such center-periphery dynamics of local extinction (Channell and Lomolino, 2000a) are particularly relevant in the context of spatially-explicit threats such as deforestation in the Amazon (Soares-Filho et al., 2006), we chose to use this relatively less-used index of biotic velocity.
To evaluate whether species will be able to track their core climatic conditions, biotic velocity was compared to a measure of species’ maximum dispersal ability, modeled as a function of body mass, diet type, and the successive time between generations and obtained from Schloss et al. (2012). In the absence of predictions for all species, we averaged maximum dispersal abilities within taxonomic genera. A species was considered able to track its core climatic conditions when its values of dispersal velocity fell within the confidence interval of its average biotic velocity, taken for each climate scenario. We, therefore, did not calculate the biotic velocity for species whose future potential distribution was considered null (i.e., those with no future analog climates).
Climate change exposureWe considered that a species would potentially be exposed to climate change in cells where future temperature are expected to exceed the maximum temperature at which species is current exposed. We considered “critically exposed” species with more than 80% of their range exposed to temperature changes, as this has proven useful in assessments of climate change effects on biodiversity (Ribeiro et al., 2016). Finally, we mapped areas with highest richness of critically exposed species. We obtained data on temperature (i.e., mean annual temperature) from the WorldClim database (see Climate data section).
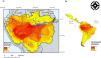
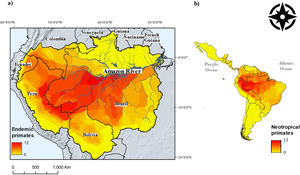
ResultsA total of 143 Neotropical primate species had IUCN georeferenced range maps and of these, 82 species were classified as endemic to the Amazon. Endemic species richness was concentrated south of the Amazon river and follows a west-eastern gradient, from the Andes mountains downstream (Fig. 1). After rasterizing IUCN range maps and selecting random subsets of environmental conditions, per species distribution sample size varied from less than 100 points (seven species) to more than 500 points (51 species), with 23 species exhibiting 101–499 points.
Neotropical primate species richness (right) and richness in the Amazon basin. The red-to-yellow gradient color indicates high-to-low richness of primates that are endemic to the Amazon. (a) A west-eastern gradient from the Andes mountains toward the Atlantic Ocean is observed, where the main tributaries of the Amazon river delimit the distribution of several primate species. (b) The Amazon hosts the higher gridded richness of primates in the Neotropics.
Species response to climate change and deforestation varied among scenarios. Considering the Climate-only scenario our predictions indicate expansion of potential distribution for most primate species (Table S1, Fig. S1), where 59 species expanded ranges up to threefold in a Mitigation greenhouse gas scenario (Range expansionmean=270±30%) and 21 species lost nearly half of their original distribution (Range shrinkmean=−54±3%). On a B.A.U. scenario of climate change, 55 species could still expand their ranges (Range expansionmean=160±15%), but 25 species were predicted to have their potential distribution reduced (Range shrinkmean=−55±12%). Climate change alone could thus lead to more “winners”, i.e. those whose potential distribution could expand, than “losers”.
Including deforestation in the Climate+deforestation scenario, however, led to greater losses for 47 (Mitigation: Range shrinkmean=−78±3%) and 65 species (B.A.U.: Range shrinkmean=−71±17%) and smaller expansions for 33 (Mitigation: Range expansionmean=91±13%) and 15 species (B.A.U.: Range expansionmean=58±11%), under different greenhouse gas emission scenarios. The inclusion of deforestation, therefore, reversed the range shift trend, where synergism among stressors led to a larger number of “losers” than that of “winners” from future environmental change (Figs. S1 and S2). As result of range contraction and expansion, spatial patterns of primate richness were affected in all scenarios. Reductions on primate richness were mostly concentrated at Southwestern regions of the Amazon (Fig. S2), where up to 15 species may be lost in some regions considering a B.A.U. scenario and the combination of climate change and deforestation.
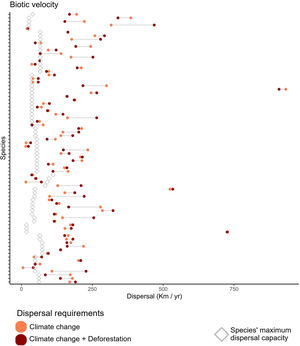
We found that primate species, on average, move at a maximum velocity of 0.74±0.26km/year (Table S2, Fig. 2), but core populations would need to move at least twice as fast in the Climate-only scenario (Mitigationmean: 2.52±2.57km/year; Business-as-usualmean: 2.20±3.10km/year) and Climate change+Deforestation (Mitigationmean: 2.57±2.54km/year; Business-as-usualmean: 2.33±3.09km/year). Range centroids were projected to move in space, while forecasts of potential distribution were scattered by deforestation for 24 species on at least one scenario, so the centroid was positioned outside the species potential distribution (Fig. S3).
Biotic velocity in relation to primate species maximum dispersal capacity. Empty diamonds indicate the maximum dispersal capacity for each species, modeled as function of body size, diet, and generation length (Schloss et al., 2012). Circles indicate the average speed that the centroid of species distribution (here considered to contain the optimal environmental conditions) will move from current time to year 2070. Coral circles refer to a Climate change alone scenario, while dark red circles refer to a Climate change+Deforestation scenario. Primate species might have to migrate at paces almost 10 times higher than expected. Species are shown in alphabetical order from bottom up in y-axis. For species-specific values, please append to Table S2.
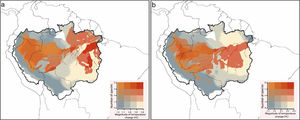
In the future, several Amazonian primates will be exposed to climate conditions that exceed their current thermal amplitudes (Fig. 3). On a Mitigation scenario of greenhouse gas emission, 82% of the studied species (n=67) were predicted to be exposed to temperature conditions that exceed the maximum temperature at which they are currently exposed. Under a B.A.U. scenario, the number of critically exposed species raised to 94% (n=77). Exposure to non-analog thermal conditions was more frequent in Central and Eastern Amazon, especially southwards the Amazon river, where reductions on species richness were also more prominent (Fig. S2).
Species richness of primates that are critically exposed to non-analog temperatures. Critically exposed species are forecasted to experience temperatures that exceed the upper limits observed across current ranges. In the Mitigation scenario (a), most of the studied species (n=67) are expected to be critically exposed to non-analog temperatures, while in the (b) business-as-usual scenario, nearly all (n=77) primate species might be exposed.
Under changing environmental conditions, species must adapt, move or go extinct (Urban, 2015). In this study, we used three distinct metrics of climate change impacts on species distribution (range shifts, biotic velocity, and exposure to non-analog climates) to forecast how climate change might affect Amazonian primates. Despite species-specific divergences, all metrics indicated that Amazonian primates will be imperiled by ongoing climate change, especially in a synergism with deforestation.
We found that most primate species may experience range contractions under future climate change, especially in scenarios including deforestation. Range contractions are expected once climate-driven migrations are hampered by the poor dispersal ability of Amazonian primates in non-forest matrices (Schloss et al., 2012) and deforestation (Soares-Filho et al., 2006). Such range contractions may lead to local extinction (Urban, 2015), by causing physiological stress on populations inhabiting non-optimal climates (Dillon et al., 2010). However, metrics range contractions per se consider only the total area that is climatically suitable for a species, not its spatial configuration. Despite shrinking, we found that the optimal environment for Amazonian primates will move from one place to another. Core populations, considered the most resilient to peripheral disturbance (Channell and Lomolino, 2000a), were in many cases extirpated in our models due to deforestation.
Future remaining populations are expected, therefore, to become fragmented and to occupy sub-optimal conditions at the periphery of their bioclimatic envelope. In such peripheral populations, fitness and resilience to subsequent stressors are usually diminished (Channell and Lomolino, 2000a,b). In addition, the velocity of climate change will likely exceed the maximum dispersal capacity of most species; a pattern consistent for primates worldwide (Schloss et al., 2012). The existence of climate-induced feedbacks on deforestation and fire dynamics in the Amazon (Coe et al., 2013), coupled with increased deforestation rates in recent years (Fearnside, 2015), will further disrupt primate dispersal routes.
Adding the evidence of exposure to non-analog climates does not bring good news for Amazonian primates: most species are expected to experience non-analog conditions in a large fraction of their territory. Exposure to climate conditions to which a primate species is not adapted may cause physiological stress, behavioral change, and fitness reduction (Gillespie and Chapman, 2006; Gould et al., 1999; Milton and Giacalone, 2014). For species with small ranges, logging and subsequent deforestation may further prevent species from moving from their current range to newly suitable habitats (Sales et al., 2019). In such situations, management options could involve ensuring that corridors for dispersal are protected now and into the future or assisted migration; however, the latter option will likely be very expensive and incur in many uncertainties (Strum and Southwick, 1986). Proposed plans for road expansions, such as that in the area of Manu National Park, Peru (Gallice et al., 2017) or the ongoing Manaus-Porto Velho highway paving (Laurance and Balmford, 2013), will therefore threaten primate populations, by disrupting climate-driven faunal migrations and removing canopy cover.
In Brazil, where most of the Amazon deforestation currently occurs (Soares-Filho et al., 2006), the network of protected areas covers >23% of its territory (Veríssimo et al., 2011), although most are outside important migratory routes for primates (Sales et al., 2019). Preserving and expanding this network may thus allow temporary persistence on sub-optimal climates or even adaptation to changing environments (Diniz-Filho et al., 2019). Societal disputes on the fate of Amazon forests, where “ruralists” claim for forest conversion into agro-business landscapes (Ferrante and Fearnside, 2019) and “conservationists” plea for a novel model of economy with the sustainable use of forests and natural resources (Nobre and Nobre, 2019) might be decisive on the future of biodiversity under global changes (Dobrovolski et al., 2018).
Our results strongly suggest a high vulnerability to climate change and deforestation on Amazon primates. We acknowledge, however, a contingency on a series of assumptions of ecological niche modeling, such as equilibrium between occurrences and current climate (Early and Sax, 2014), ecological niche conservatism (Wiens et al., 2010), and absence of evolution (Diniz-Filho et al., 2019) during our study timeframe. In addition, ecological niche models may perform better in predicting total suitable area than the direction of range changes (Fordham et al., 2018). Our metrics of exposure to non-analog climates focus on temperature changes alone, so the inclusion of other environmental stressors could provide different results. Moreover, forecasts of range shifts, biotic velocity and exposure to non-analog climates capture different nuances of the likely effects of climate change on wild species distribution, so that individual species-specific responses may not be homogeneous among indices.
To sum up, we analyzed the three most widely used approaches to understand how climate change will affect the distribution of the potentially threatened group of Amazonian primates. The overall assessment is not good – primates that are endemic to the Amazon basin are expected to experience climate-driven range contractions and may not to be able to disperse rapidly enough to track their preferred environments. Confined to unsuitable sites, several primates will be exposed to novel climate conditions, which may cause physiological stress with deleterious effects on population dynamics.
Conflict of interestThe authors declare no conflicts of interest.
LS is currently funded by a PNPD-CAPES Post-doctoral scholarship. BRR also receives a PhD scholarship from CAPES. RL research is funded by CNPq (grant # 306694/2018-2). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. This paper is a contribution of the INCT in Ecology, Evolution and Biodiversity Conservation founded by MCTIC/CNPq/FAPEG (grant 465610/2014-5).