The protected areas are essential for the conservation of native biota. However, only the protected area establishment does not guarantee the persistence of threatened species. Here, we assessed the efficiency of the Cerrado protected areas in maintaining viable populations of giant anteater and analyzed the impact of roadkills. We used the software VORTEX to model the viability of giant anteater populations in 18 Cerrado protected areas. We evaluated the impact of roadkills through three mortality scenarios (2.5%, 5% and 10% of the initial population). Our results show that in the pessimistic scenario, only three protected areas are able to maintain viable populations of the giant anteater. In the optimistic scenario, 11 protected areas out of the 18 protected areas are capable of maintaining viable giant anteater populations in the next 100 years. Three protected areas are not able to maintain viable populations in any scenario. The roadkills have had a major negative impact on the long-term persistence of giant anteater populations. We suggest that management actions to counteract the negative effects of roadkills are necessary to maintain populations of giant anteater in protected areas affected by this threat.
The maintenance of human well-being and ecosystem services depends heavily on the effective conservation of biological diversity (Rands et al., 2010). However, the anthropogenic pressures on global biodiversity continue to increase which has resulted in an overall decline of the wildlife populations (e.g., Ceballos and Ehrlich, 2002). The greatest anthropogenic threats to the species are habitat degradation, fragmentation and destruction (IUCN, 2014). If the most pessimistic extinction rates (5% per decade) are right, more than half of all species on Earth could be extinct within 150 years (Costello et al., 2013).
Therefore, the global biodiversity will hardly survive without effective conservation measures. One of the most powerful tools for species and ecosystems conservation is the establishment and maintenance of protected areas (PAs) (e.g., Naughton-Treves et al., 2005). Protected areas are representative sites of ecological importance for biodiversity, ecological research, environmental education and local economy (CBD, 2011). However, the protected area establishment per se does not guarantee an appropriate representation or persistence of the threatened biota (e.g., Venter et al., 2014; Eduardo et al., 2012). A global assessment of more than 4000 threatened vertebrate species showed that only 15% of the species have their global geographic range adequately covered by protected areas (Venter et al., 2014).
Well-managed protected areas can achieve benefits beyond biodiversity conservation, such as delivering ecosystem services essential for human well-being (e.g., Chape et al., 2008). However, the protection status does not imply in the absence of human impacts and often protected areas suffer direct or indirect threats of human populations living within or adjacent to these sites (Chape et al., 2008). For example, protected areas can register a high number of roadkill that threaten local fauna (Garriga et al., 2012). The impacts of roadkill are more pronounced in large and slow-moving animals that use the roads regularly (Forman et al., 2003; Coffin, 2007).
The giant anteaters (Myrmecophaga tridactyla Linnaeus, 1758) are commonly dead by roadkills in the Cerrado (e.g., Cunha et al., 2010; Freitas et al., 2014). Only one and a half year of monitoring in a stretch of 216km found 30 giant anteaters killed by vehicles (Cunha et al., 2010). Another monitoring of 10 years on highways in the vicinity of the Parque Nacional da Serra da Canastra, a Cerrado protected area, found that on average 2.5 giant anteaters were lost annually by roadkills (Freitas et al., 2014). The mortality caused by roadkills can threaten profoundly the population persistence of giant anteater (Diniz and Brito, 2013). Therefore, the present study aims to evaluate the effectiveness of the national protected areas in the Cerrado to maintain viable giant anteater populations and to analyze the effects of roadkills on the persistence of these populations.
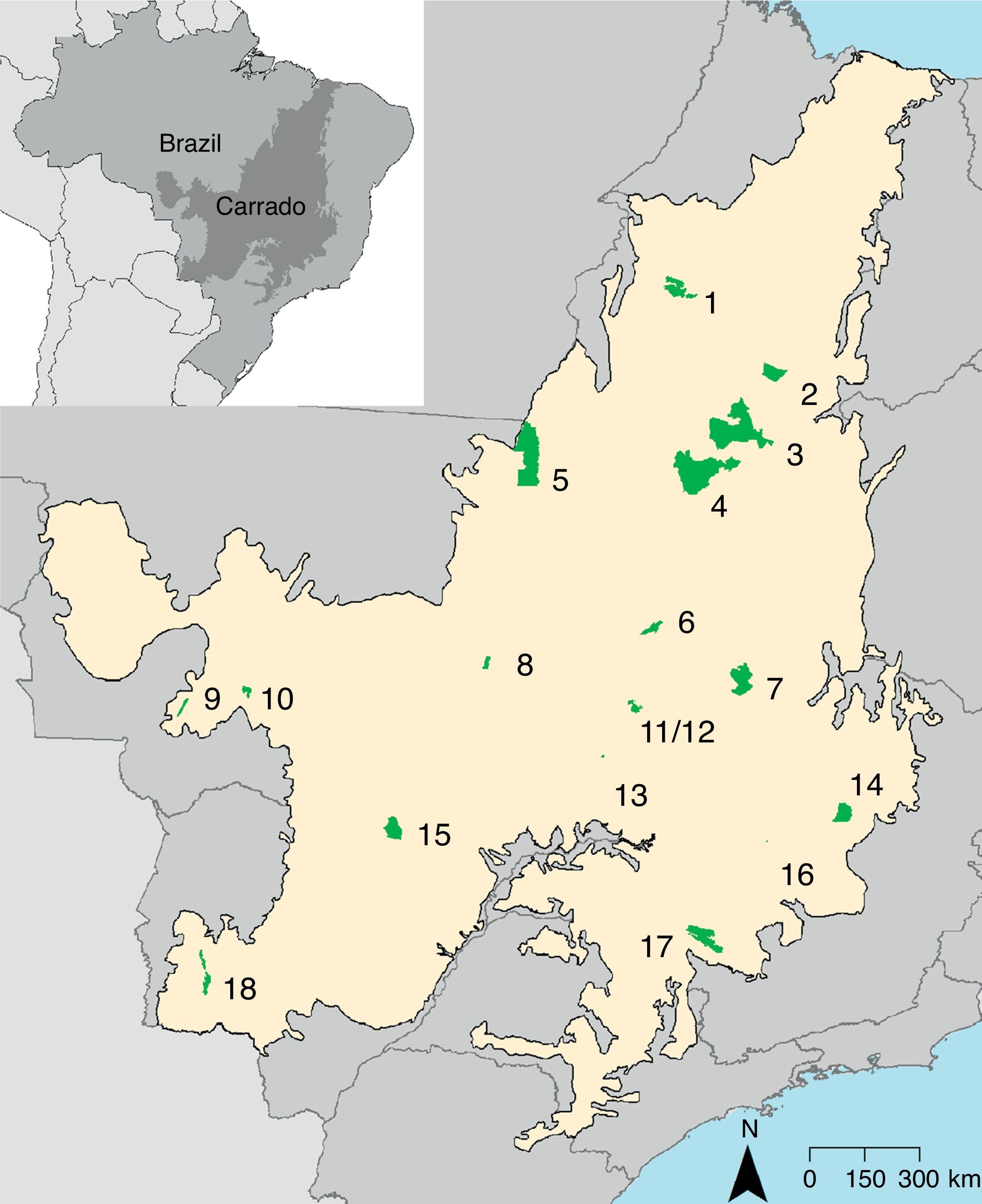
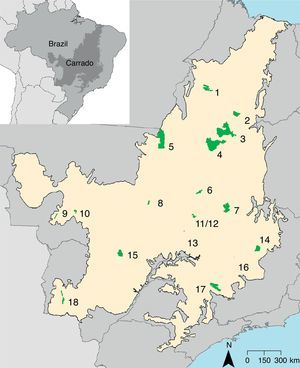
Materials and methodsStudy area and giant anteater natural historyThe expansion of modern agriculture incorporated the Cerrado in the Brazil's agricultural frontier causing in a rapid land-cover change (Brannstrom et al., 2008). Currently, the Cerrado has 285 protected areas (8.3% of the biome) of which 49 are federal protected areas, 27 classified as sustainable use and 22 of strict protection (Françoso et al., 2015). The giant anteater occurs in 18 federal protected areas of the Cerrado, two of sustainable use (Nascimento and Campos, 2011) (Fig. 1). The information about the federal protected areas and the species occurrence records was obtained from Nascimento and Campos (2011).
Location of the 18 Cerrado protected areas with records of giant anteater. Protected areas categories: ES=Ecological Station; NP=National Park; BR=Biological Reserve; NF=National Forest; ER=Extractive Reserve. 1=Chapada das Mesas NP; 2=Uruçuí-Una ES; 3=Nascentes do Rio Parnaíba NP; 4=Serra Geral do Tocantins ES; 5=Araguaia NP; 6=Chapada dos Veadeiros NP; 7=Grande Sertão Veredas NP; 8=Lago do Cedro ER; 9=Serra das Araras ES; 10=Chapada dos Guimarães NP; 11=Contagem BR; 12=Brasília NP; 13=Silvânia NF; 14=Sempre-Vivas NP; 15=Emas NP; 16=Pirapitinga ES; 17=Serra da Canastra NP and 18=Serra da Bodoquena NP.
The giant anteater is found in open grasslands and savanna habitats in the Neotropics and is currently listed as threatened in the IUCN Red List of Threatened Species (IUCN, 2014). The major threats to the giant anteater populations are habitat loss, habitat fragmentation, roadkills, hunting in some regions, fires and feral dogs (IUCN, 2014). Although the giant anteater is a charismatic species, there are still major knowledge gaps about its biology (Diniz and Brito, 2012). A summary of the life history traits of the giant anteater used as input to construct the viability model is given in Table S1. Demographic parameters used were based on Miranda (2004).
The giant anteater occurs in open grasslands and savanna habitats in the Neotropics and is currently listed as threatened in the IUCN Red List of Threatened Species (IUCN, 2014). The major threats to the giant anteater populations are habitat loss, habitat fragmentation, roadkills, hunting in some regions, fires and feral dogs (IUCN, 2014). Although the giant anteater is a charismatic species, there are still major knowledge gaps about its biology (Diniz and Brito, 2012). A summary of the life history traits of the giant anteater used as input to construct the viability model is given in Table S1. Demographic parameters used were based on Miranda (2004).
Population viability analysis modelHere we used the software VORTEX version 9.99 (Lacy et al., 2009). We assumed that each protected area with records of giant anteater represents a population. We also assumed that there was no migration between populations, i.e., between protected areas. The carrying capacities and initial population size of each protected areas were equal, both calculated using population density data (Silveira et al., 1999; Miranda, 2004). We evaluated the protected areas effectiveness through the construction of two scenarios according to different values of population density: pessimistic (0.085ind./km2; Silveira et al., 1999) and optimistic (0.4ind./km2; Miranda, 2004) scenarios.
The fire is considered a natural process in the Cerrado biome. Therefore, we added this factor to estimate the minimum viable populations (MVPs) and in all modeled scenarios. The frequency and severity data were based on a previous PVA study on giant anteater carried out at Emas National Park (Miranda, 2004). For each protected area a total of 1000 interactions were conducted during a 100 year period. We estimated the MVP necessary to achieve demographic stability using a threshold of 5% probability of extinction for the next 100 years. We also estimated a MVP necessary to retaining the genetic variability using a threshold of 90% expected heterozygosity (He) during a 100 year period. Thus, the protected areas were grouped into size categories in accordance with their ability to maintain MVPs in: small (population suffers from the negative effects of genetic erosion and demographic stochasticity), medium (population suffers only from the negative effects of genetic erosion) and large (population is self-sustaining despite the potential effects of demographic and genetic stochasticity).
A sensitivity analysis was performed to identify the model parameters particularly sensitive to initial changes. We consider only the critical giant anteater population sizes (MVPs) to conduct the sensitivity analysis. Sensitivity to mortality, sex ratio and probability of catastrophe (fire) were analyzed with a variation of ±10% of the initial values. The effect of inbreeding was examined by addition of inbreeding depression to the scenarios. A null model (baseline scenario) was built with the basic parameters of the species and compared with those of models of sensitivity analysis. The significance was tested using Student's two-tailed t-tests and data were analyzed using software STATISTICA 7.0.
Effect of roadkillsWe simulated the roadkills for each protected area individually and estimated the mean time to extinction for the populations. We verified the existence of roads through the creation of a 17.27km radius/buffer, home range of the giant anteater (Miranda, 2004), from the boundaries of each protected area. The roadkills were modeled only in protected areas that had roads crossing its extent or roads present at a distance less than or equal to 17.27km from its borders. We excluded from the analysis the protected areas with very small populations (<10 individuals). We used the roadkill rates found in the Cerrado areas (Cunha et al., 2010; Freitas et al., 2014) and constructed three scenarios with different levels of mortality due to roadkills: 2.5%, 5% and 10% of the giant anteater populations per year. We simulate the mortality resulting from roadkill through the annual harvest of individuals (Harvest tool in the Vortex) according to the above mortality rates. We removed the giant anteaters considering the rates of 2.6 males per female and 1.8 adults per young as found by Freitas et al. (2014).
ResultsThe results presented here have as main assumption the total isolation of populations, i.e. there is no migration among populations. Our results show that populations of 150 individuals are necessary to achieve demographic stability and 210 animals for genetic stability in the Cerrado. There was an expressive difference in the amount of protected areas able to maintain viable populations of the giant anteater in the Cerrado according to population density scenarios.
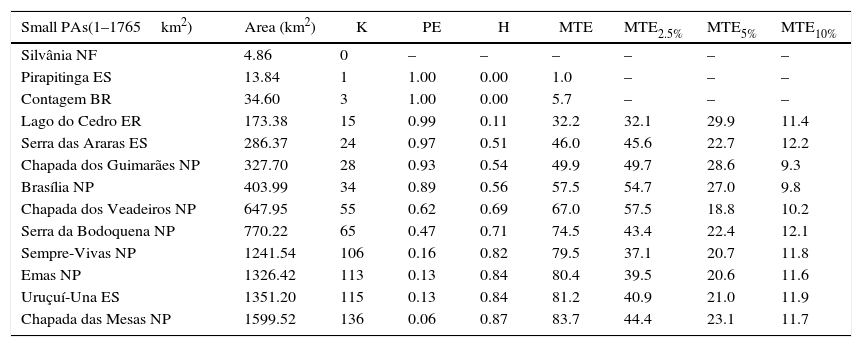
In the pessimistic scenario (population density of 0.085ind./km2) only three (Araguaia National Park, Serra Geral do Tocantins Ecological Station and Nascentes do Rio Parnaíba National Park) out of 18 protected areas are capable of maintaining viable giant anteater populations with demographic and genetic integrity (Table 1). Although the Grande Sertão Veredas and Serra da Canastra National Parks have size to sustain viable populations despite the potential effects of demographic stochasticity (1765–2471km2, 150–210 individuals), both presented high probabilities of falling below the critical size of 150 individuals (91% and 99%, respectively) (Table 1). Three small protected areas (1–1765km2, <150 individuals) (Pirapitinga Ecological Station, Silvânia National Forest and Contagem Biological Reserve) are not able to maintain any populations of giant anteater, with probabilities of extinction of 100% in the next 100 years. The population of the Lago do Cedro Extractive Reserve also presented a high likelihood of extinction (99%), with a mean time to extinction of about 32 years (Table 1).
Results of simulations for giant anteater populations in 18 Cerrado protected areas based on the population density of 0.085 individuals/km2 (Pessimistic scenario). Protected areas categories: ES=Ecological Station; NP=National Park; BR=Biological Reserve; NF=National Forest; ER=Extractive Reserve.
| Small PAs(1–1765km2) | Area (km2) | K | PE | H | MTE | MTE2.5% | MTE5% | MTE10% |
|---|---|---|---|---|---|---|---|---|
| Silvânia NF | 4.86 | 0 | – | – | – | – | – | – |
| Pirapitinga ES | 13.84 | 1 | 1.00 | 0.00 | 1.0 | – | – | – |
| Contagem BR | 34.60 | 3 | 1.00 | 0.00 | 5.7 | – | – | – |
| Lago do Cedro ER | 173.38 | 15 | 0.99 | 0.11 | 32.2 | 32.1 | 29.9 | 11.4 |
| Serra das Araras ES | 286.37 | 24 | 0.97 | 0.51 | 46.0 | 45.6 | 22.7 | 12.2 |
| Chapada dos Guimarães NP | 327.70 | 28 | 0.93 | 0.54 | 49.9 | 49.7 | 28.6 | 9.3 |
| Brasília NP | 403.99 | 34 | 0.89 | 0.56 | 57.5 | 54.7 | 27.0 | 9.8 |
| Chapada dos Veadeiros NP | 647.95 | 55 | 0.62 | 0.69 | 67.0 | 57.5 | 18.8 | 10.2 |
| Serra da Bodoquena NP | 770.22 | 65 | 0.47 | 0.71 | 74.5 | 43.4 | 22.4 | 12.1 |
| Sempre-Vivas NP | 1241.54 | 106 | 0.16 | 0.82 | 79.5 | 37.1 | 20.7 | 11.8 |
| Emas NP | 1326.42 | 113 | 0.13 | 0.84 | 80.4 | 39.5 | 20.6 | 11.6 |
| Uruçuí-Una ES | 1351.20 | 115 | 0.13 | 0.84 | 81.2 | 40.9 | 21.0 | 11.9 |
| Chapada das Mesas NP | 1599.52 | 136 | 0.06 | 0.87 | 83.7 | 44.4 | 23.1 | 11.7 |
| Medium PAs(1765–2471km2) | Area (km2) | K | Q 150 | H | MTE | MTE2.5% | MTE5% | MTE10% |
|---|---|---|---|---|---|---|---|---|
| Serra da Canastra NP | 1978.10 | 168 | 0.99 | 0.87 | 83.9 | 54.6 | 20.7 | 11.2 |
| Grande Sertão Veredas NP | 2308.53 | 196 | 0.91 | 0.89 | 89.0 | 56.3 | 24.1 | 11.7 |
| Large PAs(>2471km2) | Area (km2) | K | Q 210 | H | MTE | MTE2.5% | MTE5% | MTE10% |
|---|---|---|---|---|---|---|---|---|
| Araguaia NP | 5555.18 | 472 | 0.21 | 0.96 | – | 57.1 | 23.9 | 11.5 |
| Serra Geral do Tocantins ES | 7070.79 | 601 | 0.04 | 0.98 | – | 58.5 | 23.8 | 11.4 |
| Nascentes do Rio Parnaíba NP | 7243.25 | 616 | 0.03 | 0.98 | – | 58.3 | 24.2 | 11.3 |
K, carrying capacity of each reserve; H, heterozygosity expected under Hardy–Weinberg equilibrium; PE, probability of population extinction; Q 150, probability of population falling below the quasi-extinction threshold of 150 individuals; Q 210, probability of population falling below the quasi-extinction threshold of 210 individuals; MTE, mean time to extinction of population without roadkills; MTE5%, mean time to extinction of population with 5% of animals killed per year due to roadkills; MTE10%, mean time to extinction of population with 10% of animals killed per year due to roadkills; MTE15%, mean time to extinction of population with 15% of animals killed per year due to roadkills.
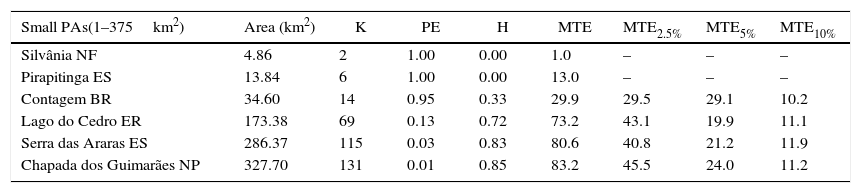
In the optimistic scenario (population density of 0.4ind./km2), we found that 11 out of the 18 protected areas have sufficient area to maintain viable populations of giant anteaters (Table 2). The Brasília National Park should offer protection against the effects of demographic stochasticity but probably its population suffers from the negative effects of genetic erosion. However, this protected area had a high probability (94%) of falling below the population quasi-extinction threshold size (150 individuals) in the next 100 years (Table 2). According to this scenario Silvânia National Forest and Pirapitinga Ecological Station still presented a 100% probability of extinction for the next 100 years.
Results of simulations for giant anteater populations in 18 Cerrado protected areas based on the population density of 0.4ind./km2 (optimistic scenario). Protected areas categories: ES=Ecological Station; NP=National Park; BR=Biological Reserve; NF=National Forest; ER=Extractive Reserve.
| Small PAs(1–375km2) | Area (km2) | K | PE | H | MTE | MTE2.5% | MTE5% | MTE10% |
|---|---|---|---|---|---|---|---|---|
| Silvânia NF | 4.86 | 2 | 1.00 | 0.00 | 1.0 | – | – | – |
| Pirapitinga ES | 13.84 | 6 | 1.00 | 0.00 | 13.0 | – | – | – |
| Contagem BR | 34.60 | 14 | 0.95 | 0.33 | 29.9 | 29.5 | 29.1 | 10.2 |
| Lago do Cedro ER | 173.38 | 69 | 0.13 | 0.72 | 73.2 | 43.1 | 19.9 | 11.1 |
| Serra das Araras ES | 286.37 | 115 | 0.03 | 0.83 | 80.6 | 40.8 | 21.2 | 11.9 |
| Chapada dos Guimarães NP | 327.70 | 131 | 0.01 | 0.85 | 83.2 | 45.5 | 24.0 | 11.2 |
| Medium PAs(375–525km2) | Area (km2) | K | Q 150 | H | MTE | MTE2.5% | MTE5% | MTE10% |
|---|---|---|---|---|---|---|---|---|
| Brasília NP | 403.99 | 162 | 0.94 | 0.86 | 86.6 | 52.2 | 20.1 | 11.6 |
| Large PAs(>525km2) | Area (km2) | K | Q 210 | H | MTE | MTE2.5% | MTE5% | MTE10% |
|---|---|---|---|---|---|---|---|---|
| Chapada dos Veadeiros NP | 647.95 | 259 | 0.79 | 0.93 | 78.5 | 47.5 | 23.4 | 11.5 |
| Serra da Bodoquena NP | 770.22 | 308 | 0.57 | 0.95 | 91.0 | 38.5 | 27.1 | 11.3 |
| Sempre-Vivas NP | 1241.54 | 497 | 0.06 | 0.97 | – | 57.6 | 24.6 | 11.6 |
| Emas NP | 1326.42 | 531 | 0.04 | 0.97 | – | 55.1 | 23.8 | 11.6 |
| Uruçuí-Una ES | 1351.20 | 540 | 0.03 | 0.97 | – | 52.7 | 24.0 | 11.4 |
| Chapada das Mesas NP | 1599.52 | 640 | 0.01 | 0.97 | – | 53.7 | 25.2 | 11.7 |
| Serra da Canastra NP | 1978.10 | 791 | 0.00 | 0.97 | – | 54.2 | 24.9 | 11.4 |
| Grande Sertão Veredas NP | 2308.53 | 923 | 0.00 | 0.98 | – | 53.1 | 25.2 | 11.3 |
| Araguaia NP | 5555.18 | 2222 | 0.00 | 0.99 | – | 55.3 | 24.9 | 11.5 |
| Serra Geral do Tocantins ES | 7070.79 | 2828 | 0.00 | 0.99 | – | 52.3 | 24.0 | 11.5 |
| Nascentes do Rio Parnaíba NP | 7243.25 | 2897 | 0.00 | 0.99 | – | 52.9 | 25.3 | 11.4 |
K, carrying capacity of each reserve; H, heterozygosity expected under Hardy–Weinberg equilibrium; PE, probability of population extinction; Q 150, probability of population falling below the quasi-extinction threshold of 150 individuals; Q 210, probability of population falling below the quasi-extinction threshold of 210 individuals; MTE, mean time to extinction of population without roadkills; MTE5%, mean time to extinction of population with 5% of animals killed per year due to roadkills; MTE10%, mean time to extinction of population with 10% of animals killed per year due to roadkills; MTE15%, mean time to extinction of population with 15% of animals killed per year due to roadkills.
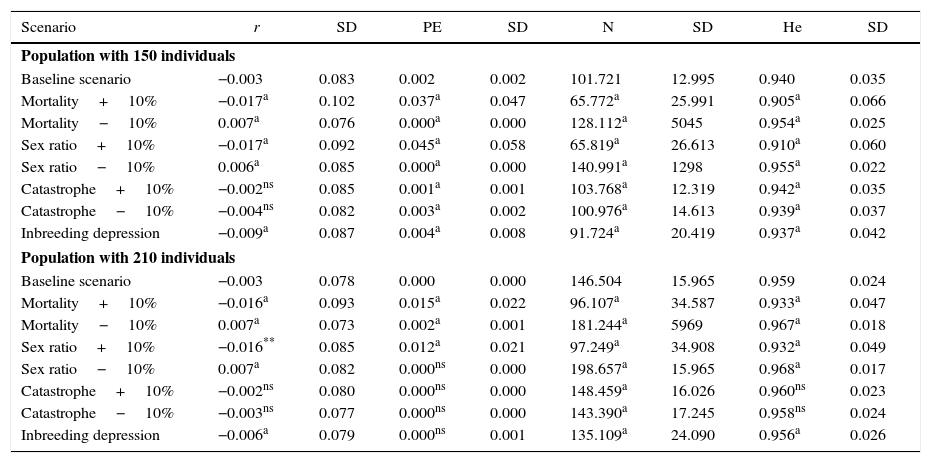
The sensitivity analysis showed that the population of 150 giant anteaters presented a high sensitivity to all factors. The changes in the modeled population output values influenced significantly the population mean growth rate, probability of extinction, mean final population size and expected heterozygosity (Table 3). In this population only the probability of catastrophes had no significant influence on the population mean growth rate. For the population threshold size of 210, the probability of catastrophes, inbreeding depression and reductions in sex ratio did not affect the probability of extinction. The changes in mortality rate and a 10% deviation in the sex ratio for males affected all parameters in both populations (Table 3).
Sensitivity analysis for two critical population sizes (150 individuals and 210 individuals) of the giant anteater. The scenarios were created from ±10% change in mortality, sex ratio and probability of catastrophe of the initial parameters (baseline scenario). The effect of inbreeding was examined by introducing inbreeding depression. The variables used to compare the models were: population mean growth rate (r), probability of extinction (PE), mean final population size (N) and expected heterozygosity (He).
| Scenario | r | SD | PE | SD | N | SD | He | SD |
|---|---|---|---|---|---|---|---|---|
| Population with 150 individuals | ||||||||
| Baseline scenario | −0.003 | 0.083 | 0.002 | 0.002 | 101.721 | 12.995 | 0.940 | 0.035 |
| Mortality+10% | −0.017a | 0.102 | 0.037a | 0.047 | 65.772a | 25.991 | 0.905a | 0.066 |
| Mortality−10% | 0.007a | 0.076 | 0.000a | 0.000 | 128.112a | 5045 | 0.954a | 0.025 |
| Sex ratio+10% | −0.017a | 0.092 | 0.045a | 0.058 | 65.819a | 26.613 | 0.910a | 0.060 |
| Sex ratio−10% | 0.006a | 0.085 | 0.000a | 0.000 | 140.991a | 1298 | 0.955a | 0.022 |
| Catastrophe+10% | −0.002ns | 0.085 | 0.001a | 0.001 | 103.768a | 12.319 | 0.942a | 0.035 |
| Catastrophe−10% | −0.004ns | 0.082 | 0.003a | 0.002 | 100.976a | 14.613 | 0.939a | 0.037 |
| Inbreeding depression | −0.009a | 0.087 | 0.004a | 0.008 | 91.724a | 20.419 | 0.937a | 0.042 |
| Population with 210 individuals | ||||||||
| Baseline scenario | −0.003 | 0.078 | 0.000 | 0.000 | 146.504 | 15.965 | 0.959 | 0.024 |
| Mortality+10% | −0.016a | 0.093 | 0.015a | 0.022 | 96.107a | 34.587 | 0.933a | 0.047 |
| Mortality−10% | 0.007a | 0.073 | 0.002a | 0.001 | 181.244a | 5969 | 0.967a | 0.018 |
| Sex ratio+10% | −0.016** | 0.085 | 0.012a | 0.021 | 97.249a | 34.908 | 0.932a | 0.049 |
| Sex ratio−10% | 0.007a | 0.082 | 0.000ns | 0.000 | 198.657a | 15.965 | 0.968a | 0.017 |
| Catastrophe+10% | −0.002ns | 0.080 | 0.000ns | 0.000 | 148.459a | 16.026 | 0.960ns | 0.023 |
| Catastrophe−10% | −0.003ns | 0.077 | 0.000ns | 0.000 | 143.390a | 17.245 | 0.958ns | 0.024 |
| Inbreeding depression | −0.006a | 0.079 | 0.000ns | 0.001 | 135.109a | 24.090 | 0.956a | 0.026 |
The three roadkill rates (2.5%, 5% and 10%) were evaluated in 15 protected areas and 16 protected areas in the optimistic and pessimistic scenarios, respectively. Our results suggest that roadkill mortality is a serious threat to species persistence (Tables 1 and 2). The removal of individuals resulted in a rapid extirpation of the populations in all protected areas. In both pessimistic and optimistic scenarios, the mean time to extinction was not exceeding 60, 30 and 15 years for roadkill scenarios of 2.5%, 5% and 10% of the population, respectively (Tables 1 and 2).
DiscussionThe protected areas of Brazil might not be effective for the conservation of the some large mammals such as the lowland tapir (Tapirus terrestris) (Eduardo et al., 2012), and might be only reasonably effective for the conservation of the some small mammals (e.g., Brito and Grelle, 2004). The results presented in our study might raise concern because based on the pessimistic scenario only three protected areas have sufficient size to maintain giant anteater populations in the long-term. The Pirapitinga Ecological Station, Silvânia National Forest and Contagem Biological Reserve should have special attention from managers because they have no areas able to maintain any giant anteater population. The persistence of the giant anteater and other large and medium-sized species in these protected areas depends primarily on the habitat availability. Some potential solutions to increase the efficiency of these sites are the increase in size and/or in connectivity between protected areas.
The Lago do Cedro Ecological Reserve and Silvânia National Forest are sustainable use protected areas. These reserves allow the exploitation of natural resources in an environmentally responsible manner, at least in theory. However, a current study demonstrated that many natural resources exploited (in an alleged sustainable manner) in some sustainable use protected areas in Brazil do not obey this criterion (Fernandez et al., 2012). Thus, these protected areas do not have the minimum spatial requirements to ensure the persistence of the giant anteater and other species in the long-term, and the quality of habitat within these areas may not be suitable to the objectives of wildlife conservation.
A genetic study conducted in Emas National Park found that the population of giant anteater of this area has low levels of genetic diversity and high levels of inbreeding (Collevatti et al., 2007). We do not consider the inbreeding depression in our model. However, the effects of inbreeding depression in addition to the demographic and environmental instability could make small populations of giant anteaters even more vulnerable to extinction. This certainly might raise concern particularly in the context of the pessimistic scenario. The environmental heterogeneity and some threats such as hunting pressure and interference from domestic animals were not considered in our analysis. However, giant anteaters are hunted (e.g., Koster, 2008) and are attacked by dogs (Lacerda et al., 2009) in certain areas of its distribution. Both the critical population sizes (150 and 210 individuals) were sensitive to changes in mortality rate. The MVPs sizes identified here do not consider the threats mentioned above and therefore in such cases they cannot be sufficient to guarantee the persistence of species in the next 100 years.
Roadkills are one of the main sources of mortality for terrestrial vertebrates, surpassing even the effects of hunting in some places (Forman et al., 2003). The reduction in population size caused by direct mortality of animals is the primary and most obvious consequence of roadkill. However, the secondary effects range from behavioral changes, population fragmentation, genetic changes to increased risk of local extinction (Forman et al., 2003; Coffin, 2007). The giant anteater is a large mammal of slow movements and a limited vision, characteristics that make it more susceptible to be killed by vehicles. A study in the Brasília National Park showed that the roadkill is the greatest threat to the persistence of giant anteaters, having a more serious negative effect than fire and inbreeding (Diniz and Brito, 2013).
The implementation of mitigation measures against roadkills (e.g., installation of speed bumps and traps, revision of the speed limits, more effective traffic warning signals and construction of wildlife overpasses) was suggested as an important conservation action for a giant anteater population in the Cerrado (Diniz and Brito, 2013). In order to reduce the impacts of roadkills, we strongly recommend that these measures should be adopted in all Cerrado protected areas under the influence of roads. These measures should be complemented by the implementation of monitoring programs (to monitor both wildlife populations and roadkills) in order to estimate qualitative and quantitative impacts of roadkills and to evaluate the effectiveness of the measures implemented.
Conflicts of interestThe authors declare no conflicts of interest.
Milena F. Diniz thanks CAPES for the PIBIC scholarship. Daniel Brito's work is funded by CNPq (305446/2012-6).