Although the Brazilian Atlantic Forest and other global biodiversity hotspots are rapidly urbanizing, there is little information on the potential of metropolitan regions to safeguard biodiversity within their reserves. We sampled bird assemblages in nine protected areas (14.8–1058ha) of the metropolitan region of João Pessoa, Northeast Brazil, to test the hypothesis that larger urban reserves are more effective than smaller ones in protecting the regional species richness, as more habitat and environmental heterogeneity are available for birds. We recorded 126 species belonging to 41 families, including seven endemics and two introduced species. Thraupidae and Tyrannidae were the most representative families with 16 species each. Larger areas clearly protected greater proportion of the regional species richness, but the smaller areas accounted for at least 35.7% of the regional richness. Differences in size and isolation significantly influenced species similarity, but hunting, catching for illegal trade, presence of exotic predators and noise pollution were likely to affect composition as well. The remarkable bird diversity suggests good potential for legal-based, well-planned birdwatching activities in the studied region. The results highlight that urban reserves of any size are valuable for bird conservation in metropolitan regions, though larger ones should be prioritized.
Although the Brazilian Atlantic Forest is considered one of the world's largest repositories of biodiversity (Myers et al., 2000), only 11.4–16% of the original forest cover were left (Ribeiro et al., 2009). More than 80% of the almost 250,000 forest fragments are smaller than 50ha, about half of the remaining vegetation is less than 100m from the nearest edge, and the average distance between the fragments is 1440m (Ribeiro et al., 2009). During this long process of habitat loss and fragmentation, many metropolitan regions have raised and dominated agricultural landscapes that first replaced the forest (Joly et al., 2014; Stevens, 2014). As in other regions of the world, cities and dense settlements show a clear trend for growth in the Brazilian Atlantic Forest (Seto et al., 2013; United Nations, 2014), which has been somehow counterbalanced by the creation of urban protected areas. However, to date there is little empirical evidence showing the ability of such protected areas to safeguard biodiversity and ecosystem services that positively affect biodiversity.
With nearly 2000 species of birds, including 277 endemics and 1692 resident species, Brazil has one of the most diverse avifauna in the world (Piacentini et al., 2015). Studies have shown that forest destruction and fragmentation have resulted in species loss at local and regional scales (Anjos et al., 2015). The microclimatic changes, especially the increase in light and decrease in air humidity arising from landscape modification, eliminate most sensitive species (Morante-Filho et al., 2017). The reduction of food and sites for nesting also lead to the decline of many species, especially those that require large areas (Marini and Garcia, 2005). This decline, in turn, results in negative effects on the plants that birds pollinate and disperse, insects and other animals they consume as well as predators and parasitoid they are attacked by (Ford et al., 2001). Birds are also the basis for birdwatching tourism, considered one of the most biodiversity-friendly activities with potential to conciliate economic development with human well-being (Sekercioglu, 2002; Millennium Ecosystem Assessment, 2005). Nonetheless, the provision of this ecosystem service in urban reserves of the Brazilian Atlantic Forest has been poorly explored.
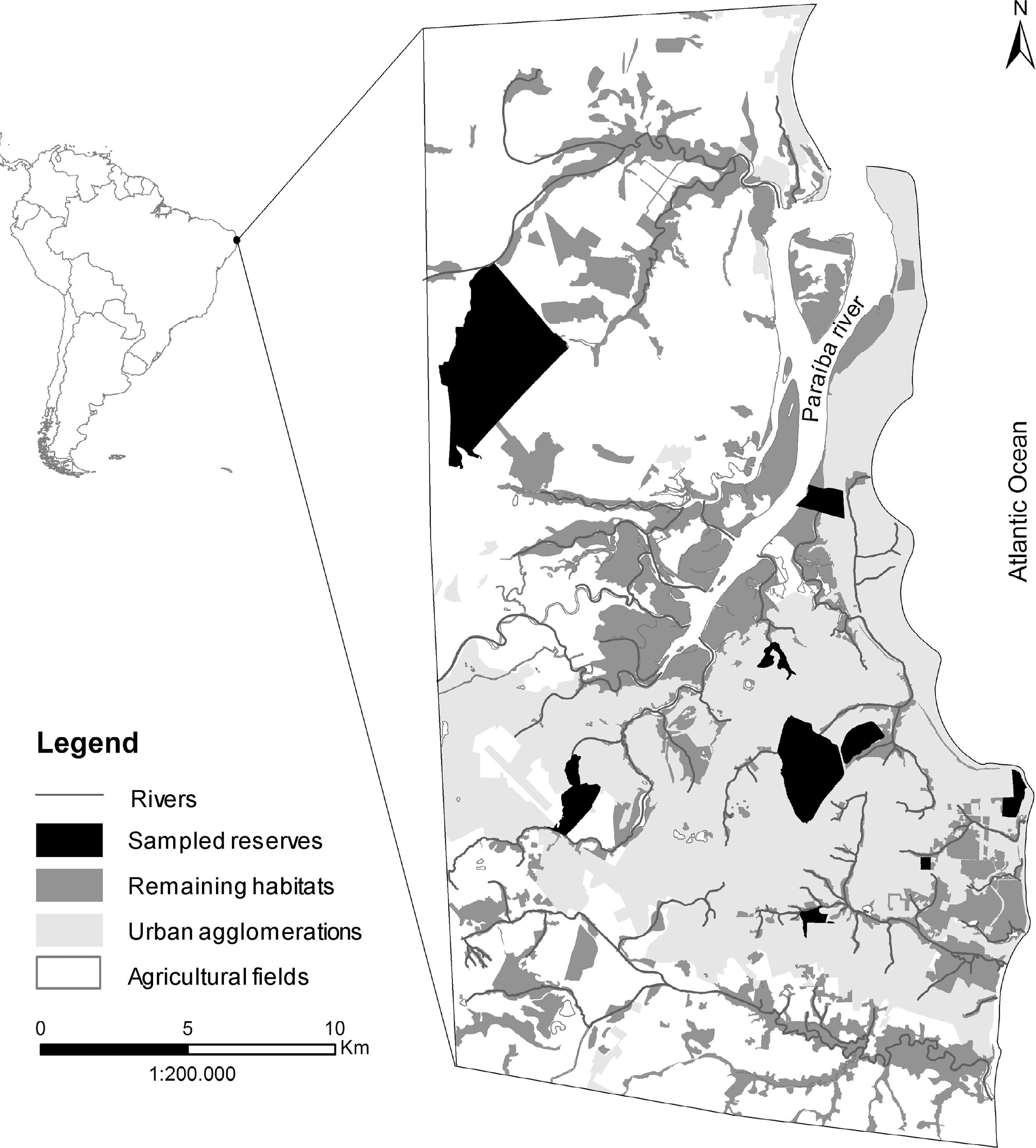
In the Northeast of Brazil it is located the Pernambuco Center of Endemism of the Brazilian Atlantic Forest, a ∼56,000-km2 area that includes forests north of the São Francisco River in the states of Alagoas, Pernambuco, Paraíba and Rio Grande do Norte (Porto et al., 2005). This center homes at least 434 bird species, including 35 endemics and 40 officially recognized as threatened by the Brazilian Ministry of Environment (MMA) (Roda et al., 2011; Lobo-Araújo et al., 2013), as well as four medium-sized cities that encompass metropolitan regions of 1–5 million inhabitants (United Nations, 2014). One of them is the metropolitan region of João Pessoa, state of Paraíba, where this study was conducted. This region has faced intense urbanization since 1970 and a peak of protected area creation in the 2000s, following the promulgation of the federal law 9985/2000 that established the National System of Protected Areas. Currently, 18 protected areas varying from 14.8ha to 1058ha safeguard a variety of ecosystems under distinct administrative levels (i.e., municipal, state, federal areas) (Stevens, 2014). These areas are expected to home a large portion of the 395 species, 28 orders and 67 families of birds reported for the whole state (Marinho, 2014), but no estimate has been done so far, precluding the adoption of legal-based, environmental-friendly management of the birds.
In this study we record the avifauna of nine protected areas in the metropolitan region of João Pessoa to test the hypothesis that larger urban reserves are more effective than smaller ones in protecting the regional richness, as more habitat and environmental heterogeneity are available for birds (Sekercioglu et al., 2002). We quantify the relative contribution of each protected area for regional bird species richness and assess how differences in protected area size and isolation affect species composition. We also provide a brief recommendation for the development of legal-based, well-planned birdwatching activities in the region.
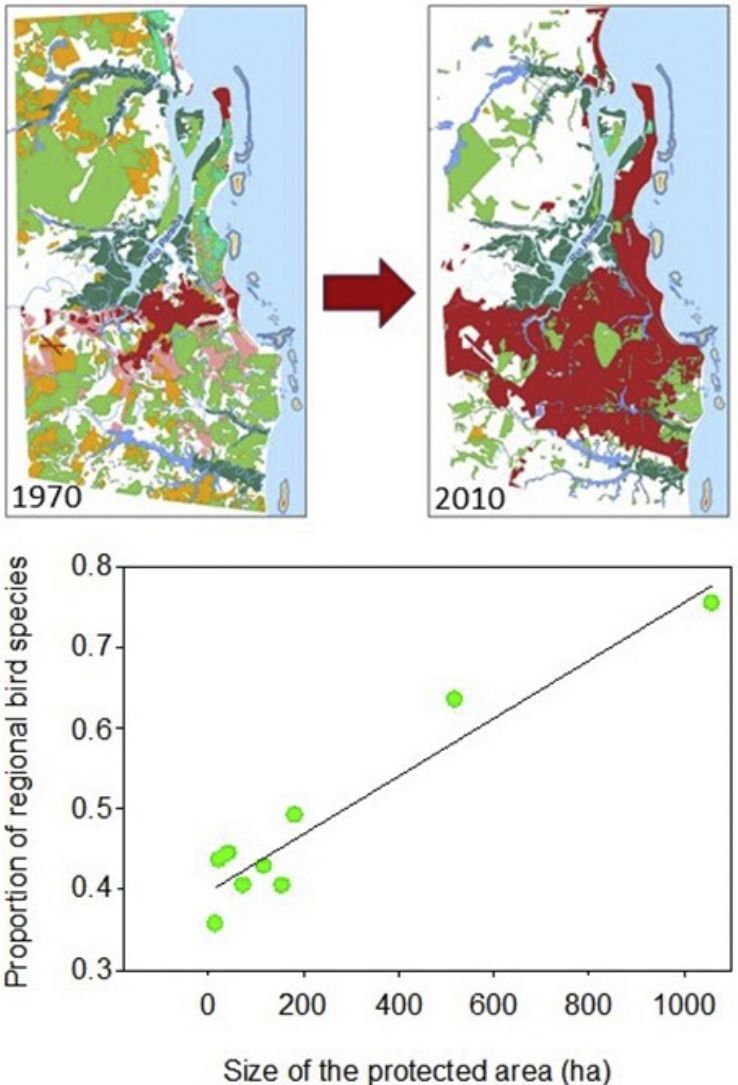
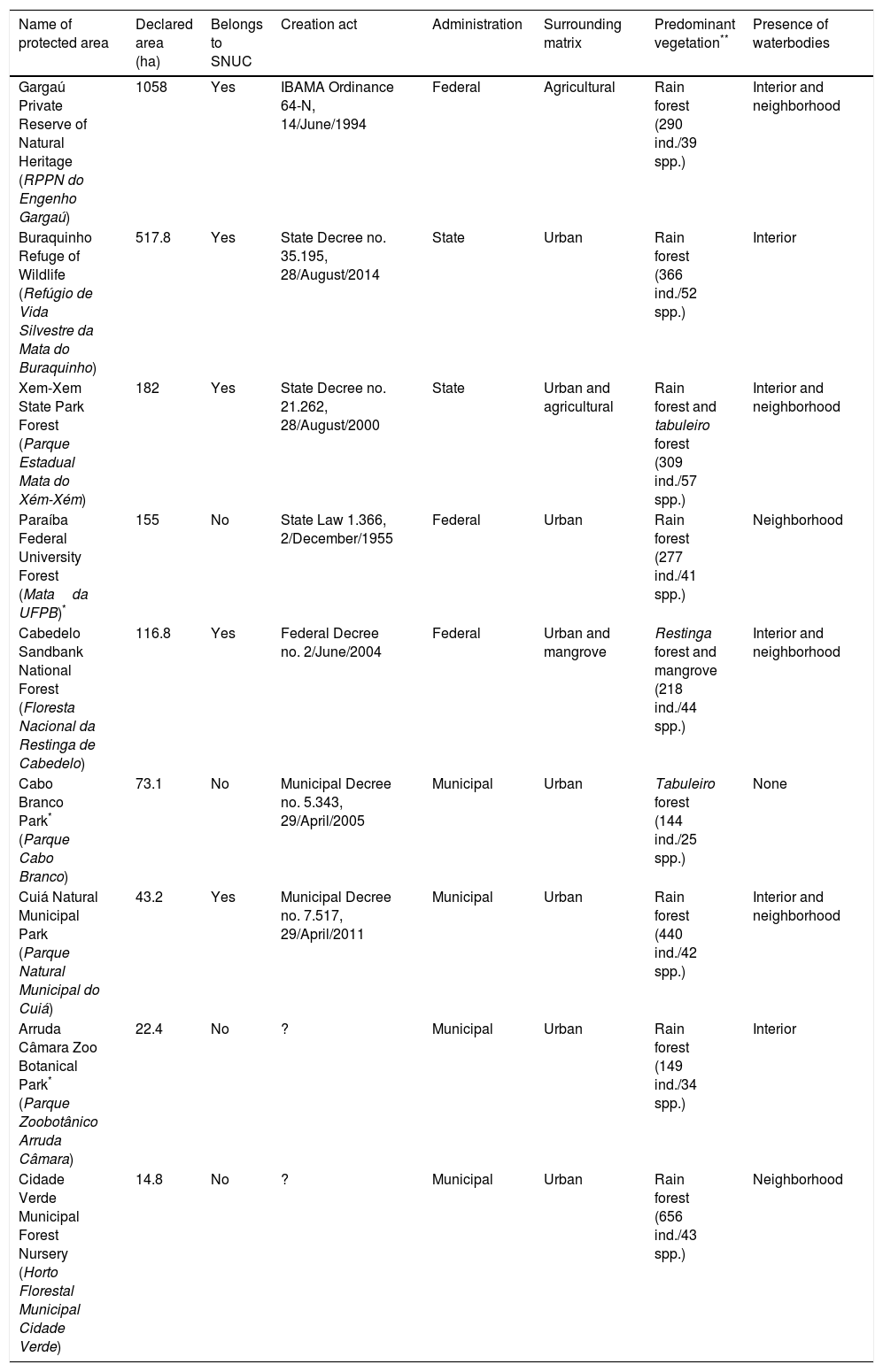
Materials and methodsStudy areaOur study area has 63,000ha and encompasses the most densely populated portion of the metropolitan region of João Pessoa, including the municipalities of Cabedelo, Santa Rita, Bayeux, Lucena and João Pessoa (34° 58′87″W 7° 15′46″S and 34° 47′19″W 6° 54′43″S) (Fig. 1). A recent study indicates that 57% of the original vegetation cover were lost between 1974 and 2010, and the most affected areas were the rain forests, sandbank forests (restingas) and tabuleiro forests (Stevens, 2014). Currently, the 14,847ha of native vegetation left are scattered over 236 forest remnants, most smaller than 50ha and surrounded by urban agglomerations or agricultural fields (mainly sugarcane plantations) under peri-urban conditions. About 20% of the remaining vegetation is protected by law within 18 protected areas, either within the federal law 9985/2000 or other legal instruments at the state and municipal spheres. Nine protected areas showed minimum level of security to permit access and fieldwork inside. They vary in size, internal level of fragmentation, surrounding matrix, predominant vegetation, presence of waterbodies, and administration regime (Table 1). Altogether, they conserve a mosaic of ecosystems composed of distinct types of forests, from rain to drier forests (restinga and tabuleiro forests), salt marshes, mangrove swamps and estuaries. In this study we consider urbanization as a multidimensional process that manifests itself through rapidly changing human population and changing land cover (sensuSeto et al., 2013) and, consequently, assume that all remaining vegetation inside the study area are facing urbanization.
The nine protected areas studied in the metropolitan region of João Pessoa, Northeast Brazil, sorted by their size (from the largest to the smallest area).
| Name of protected area | Declared area (ha) | Belongs to SNUC | Creation act | Administration | Surrounding matrix | Predominant vegetation** | Presence of waterbodies |
|---|---|---|---|---|---|---|---|
| Gargaú Private Reserve of Natural Heritage (RPPN do Engenho Gargaú) | 1058 | Yes | IBAMA Ordinance 64-N, 14/June/1994 | Federal | Agricultural | Rain forest (290 ind./39 spp.) | Interior and neighborhood |
| Buraquinho Refuge of Wildlife (Refúgio de Vida Silvestre da Mata do Buraquinho) | 517.8 | Yes | State Decree no. 35.195, 28/August/2014 | State | Urban | Rain forest (366 ind./52 spp.) | Interior |
| Xem-Xem State Park Forest (Parque Estadual Mata do Xém-Xém) | 182 | Yes | State Decree no. 21.262, 28/August/2000 | State | Urban and agricultural | Rain forest and tabuleiro forest (309 ind./57 spp.) | Interior and neighborhood |
| Paraíba Federal University Forest (Matada UFPB)* | 155 | No | State Law 1.366, 2/December/1955 | Federal | Urban | Rain forest (277 ind./41 spp.) | Neighborhood |
| Cabedelo Sandbank National Forest (Floresta Nacional da Restinga de Cabedelo) | 116.8 | Yes | Federal Decree no. 2/June/2004 | Federal | Urban and mangrove | Restinga forest and mangrove (218 ind./44 spp.) | Interior and neighborhood |
| Cabo Branco Park* (Parque Cabo Branco) | 73.1 | No | Municipal Decree no. 5.343, 29/April/2005 | Municipal | Urban | Tabuleiro forest (144 ind./25 spp.) | None |
| Cuiá Natural Municipal Park (Parque Natural Municipal do Cuiá) | 43.2 | Yes | Municipal Decree no. 7.517, 29/April/2011 | Municipal | Urban | Rain forest (440 ind./42 spp.) | Interior and neighborhood |
| Arruda Câmara Zoo Botanical Park* (Parque Zoobotânico Arruda Câmara) | 22.4 | No | ? | Municipal | Urban | Rain forest (149 ind./34 spp.) | Interior |
| Cidade Verde Municipal Forest Nursery (Horto Florestal Municipal Cidade Verde) | 14.8 | No | ? | Municipal | Urban | Rain forest (656 ind./43 spp.) | Neighborhood |
Between June 2014 and May 2015, we used 50 Mackinnon lists of 10 species to sample birds in each of the nine protected areas, starting early in the morning (around 6 am) to maximize species detection (Bibby et al., 1998). In the Mackinnon method (Ribon, 2010), each species is recorded only once in each list until the maximum number of species in the list is reached (10 in our study). After that, a new list is started. We produced 10 lists per day per area during five consecutive days, totaling 450 lists. An advantage of this method is that the list may be generated throughout a day without application time, or on subsequent days. Furthermore, it allows the calculation of species frequency by list and area, uncovering common and rare species (Manhães and Loures-Ribeiro, 2011). Nonetheless, we were conservative and excluded any doubtful record from our lists.
We recorded the birds visually with binoculars Nikon 7×50, aurally through species vocalization and confirmed species identification with specialized literature and sound recordings of private collection (Sick, 1997; Erize et al., 2006; Sigrist, 2009). We alternated consecutive field surveys between smaller and larger protected areas to control for possible seasonal effects on bird detection, activity and occurrence along the gradient of protected area size. Furthermore, we restricted the surveys to the forest formations of each protected area (i.e., rain forest, tabuleiro forest, restinga forest; Table 1) to minimize compositional differences attributed to habitat type. The taxonomic classification of the species followed the Brazilian Ornithological Records Committee (Piacentini et al., 2015). We searched for the conservation status of identified species in the red lists of IUCN and the Brazilian government. Introduced species were also identified.
Data analysesTo quantify the relative contribution of each protected area for the regional bird species richness, we divided the species richness of each area by the regional species richness. Our expectation was that smaller areas showed smaller proportion of the regional richness than larger areas. A simple linear regression was performed in JPM 8 (SAS Institute Inc., Cary, NC, USA) to test this relationship. We used the Jackknife method of first order to estimate the regional species richness following the equation: Sjack1=Sobs+Q1 (m−1/m), where Sjack1 is the estimated species richness; Sobs is the observed number of species in all lists; Q1 is the number of species recorded in a single list; and m is the number of lists. We obtained the estimates and species accumulation curves with the software Estimates 9.1 with 1000 randomizations (Colwell, 1997).
To assess how differences in protected area size and isolation affected species composition, we constructed a dissimilarity matrix of taxonomic composition based on the Jaccard distance (1−J) and used Mantel tests to correlated it with matrices of geographic distance and difference in size. We expected that less isolated and similar-sized protected areas showed an avifauna more similar to each other than more isolated, different-sized areas. We produced matrices and ran Mantel tests in Primer 6 (Clarke and Gorley, 2006). To identify the most common and rarest species in the region, we calculated the frequency of occurrence of each species by dividing their number of occurrence by the total number of occurrence of all species in the lists.
ResultsContribution of protected areas to the regional species richnessWe recorded a total of 126 species (7 endemics), 17 orders and 41 bird families (Table S1). The richness estimated with Jackknife method of first order was approximately 136 species (Fig. S1), indicating the presence of 10 species more in our study region. Thraupidae and Tyrannidae, with 16 species each, were the most representative families, followed by Trochilidae (8 spp.), Thamnophilidae (6 spp.), Rhynchocyclidae (5 spp.), Picidae (5 spp.) and Columbidae (5 spp.). Eighteen of the 41 families were represented by only one species (Fig. S2).
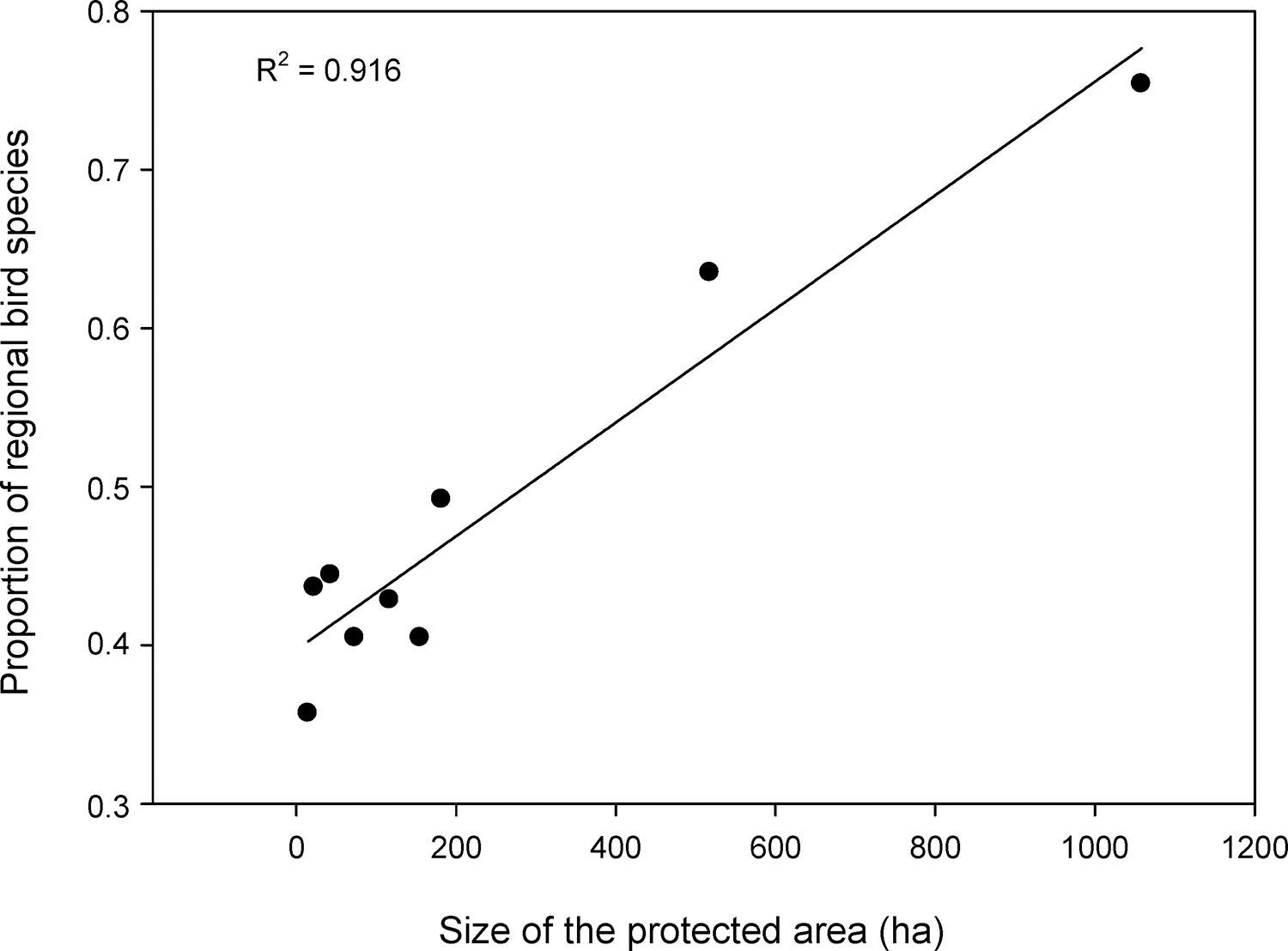
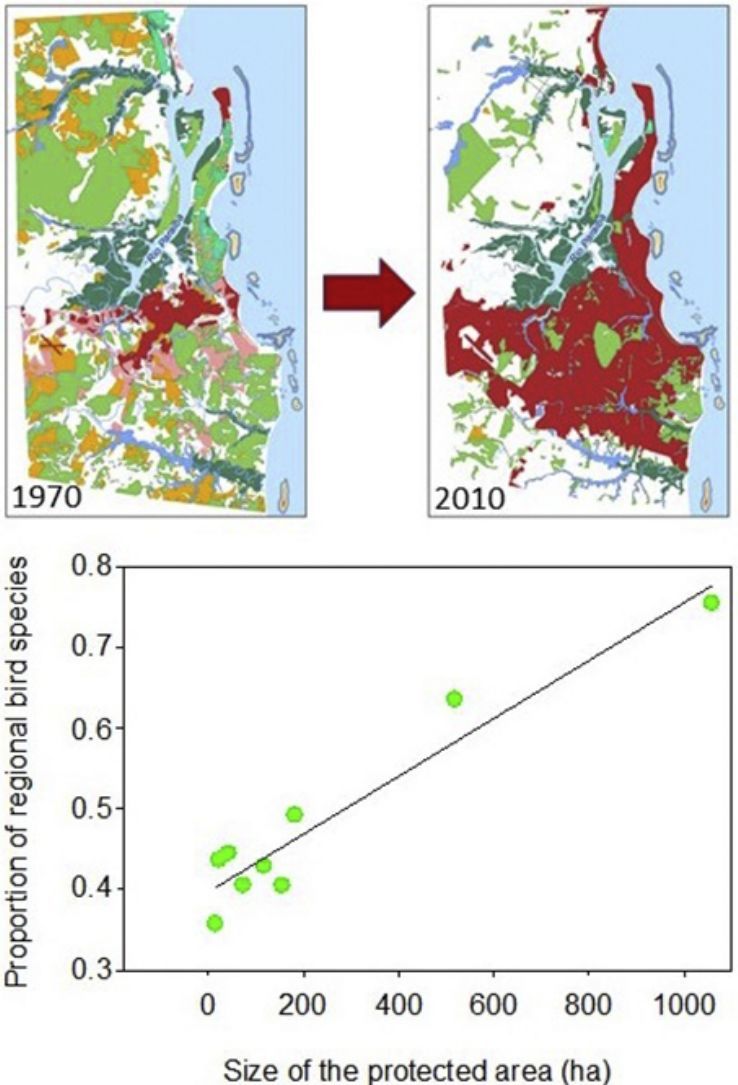
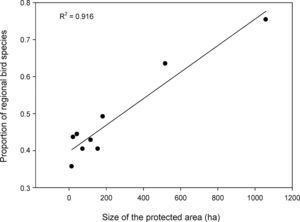
As expected, the richest area was the largest reserve (Gargaú Private Reserve of Natural Heritage, 1058ha), with 95 species, accounting for 75.4% of the regional species richness. The Buraquinho Refuge of Wildlife (518ha) and the Xem-Xem State Park Forest (182ha), second and third largest areas, followed with 80 and 62 species, respectively (63.5% and 49.2% of the regional richness). Five areas ranging from 22ha to 155ha protected between 40.5% and 43.7% of the regional richness: Cuiá Natural Municipal Park (56 species), Arruda Câmara Zoo Botanical Park (55 species), Cabedelo Sandbank National Forest (54 species), Cabo Branco Park (51 species) and Paraíba Federal University Forest (51 species). The smallest area, the Cidade Verde Municipal Forest Nursery (14.8ha), protected as much as 35.7% of the regional richness (45 species). When we modeled the proportion of regional richness in each protected area as a function of its size, a clear positive relationship arose (R2=0.916; F1,7=77.2; P<0.0001; Fig. 2), showing the remarkable contribution of larger areas for bird conservation.
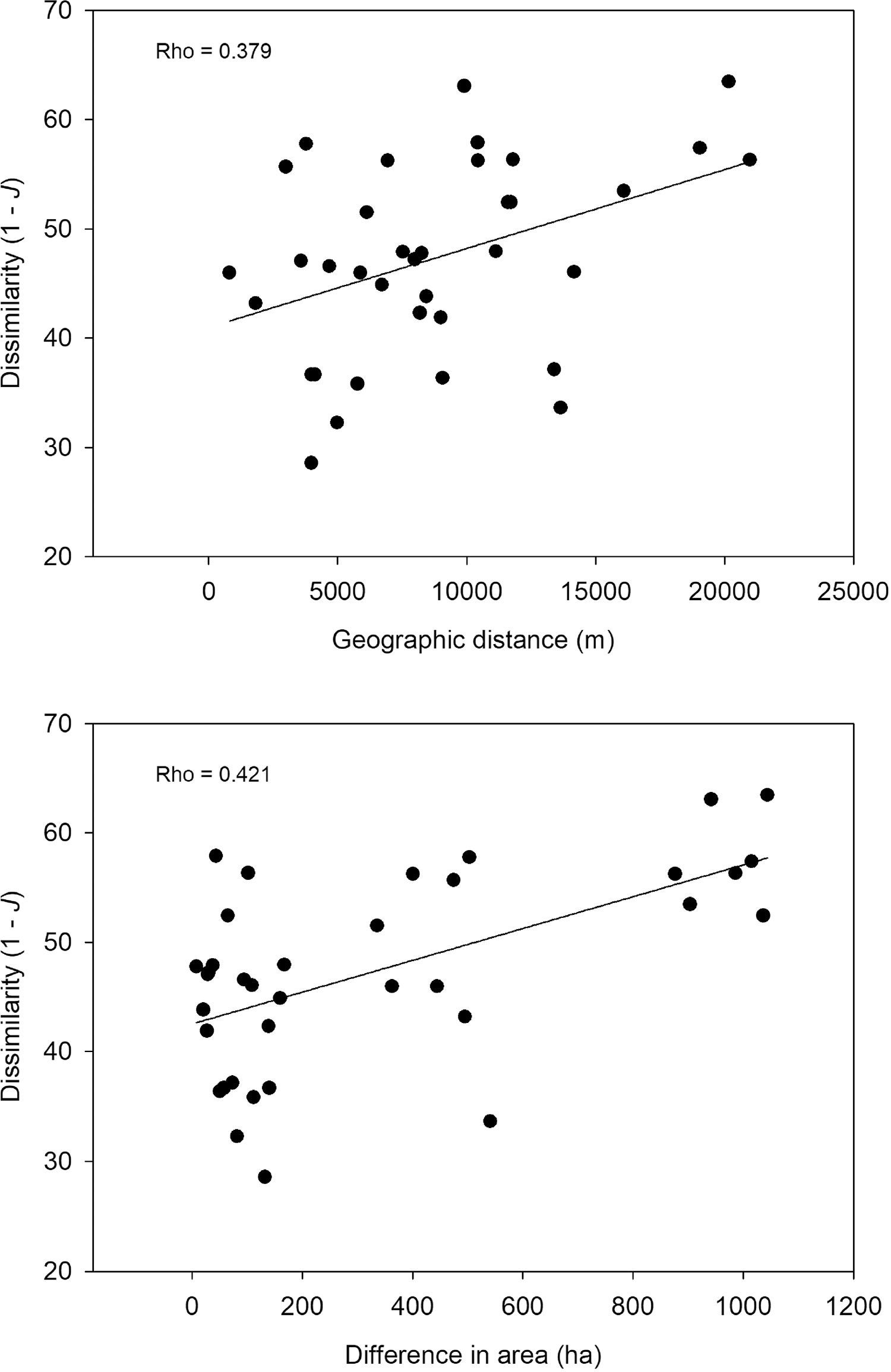
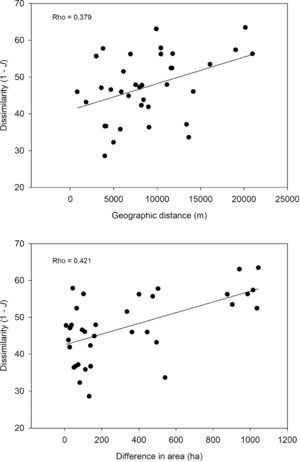
Species similarity across the protected areasPairwise similarity averaged 52.6% across the protected areas, varying from 36.5% (Gargaú Private Reserve of Natural Heritage vs. Cidade Verde Municipal Forest Nursery) to 71.4% (Arruda Câmara Zoo Botanical Park vs. Paraíba Federal University Forest). There was a trend for distance-decay in species composition (Rho=0.379; P=0.055) supported by lower similarity between more distant areas (Fig. 3). Differences in size also influenced species composition (Rho=0.421; P=0.04), with similar-sized areas being more taxonomically similar than different-sized areas (Fig. 3). Nonetheless, in both cases the strength of the relationship (Rho values) was low, suggesting that the size and isolation of the protected areas are not the main drivers of species composition.
Common species and conservation statusThe most frequent species was Pitangus sulphuratus (Great kiskadee; Tyrannidae), taking place in all areas and in more than half of the 450 lists. Other common species included Coereba flaveola (Thraupidae), Vireo olivaceus (Vireonidae), Fluvicola nengeta (Tyrannidae), and Columbine talpacoti (Columbidae), occurring in 30%-37% of the lists (Table S1). In addition to P. sulphuratus, other 18 species occurred in all protected areas, but at lower frequencies. Ten species were recorded only once in the entire study.
Only one species, Picumnus fulvescens (Picidae), is recognized by the IUCN as near threatened (NT). However, six species appear in the Brazilian Red List of Endangered Species in worrying categories: Penelope superciliaris (Cracidae; CR – Critically endangered), Momotus momota (Momotidae; EN – Endangered), Conopophaga melanops (Conopophagidae; VU – Vulnerable), Xenops minutus (Xenopidae; VU), Platyrinchus mystaceus (Platyrinchidae; VU) and Xiphorhynchus atlanticus (Dendrocolaptidae; VU). Only two introduced species were detected: Estrilda astrild (Estrildidae) and Passer domesticus (Passeridae), but P. domesticus occurred in 25.8% of the lists.
DiscussionOur findings support the hypothesis that larger urban reserves are more effective than smaller ones in protecting the regional richness, emphasizing the critical role of large reserves (>1000ha) for bird conservation in the studied metropolitan region. However, even the smallest and more isolated area significantly contribute to the regional avifauna, safeguarding more than one third of the species richness found in the region (see also Silva et al., 2014). This clearly demonstrates that small urban reserves are not negligible for bird conservation. The results also highlight the great conservation value of the metropolitan region of João Pessoa for bird conservation at broader spatial scales, protecting at least 32% of the 395 species reported for the state of Paraíba (Marinho, 2014) and 29% of the 434 species reported for the Pernambuco Center of Endemism (Roda et al., 2011; Lobo-Araújo et al., 2013). This regional richness is actually underestimated according to the species accumulation curve, possibly because migratory, wandering and regionally rare species require greater sampling period to be detected.
The greater richness observed in larger areas is not surprising. Compared with smaller areas, larger areas usually offer a wider variety of food resources, micro-habitats and suitable conditions for reproduction, dispersion and survival (Sekercioglu et al., 2002). Species with different physiological and ecological requirements are sorted across the micro-habitats available, increasing the turnover within the area and resulting in the co-occurrence of a large number of species. In our study, all but one reserve had waterbodies inside and/or nearby, two were composed of more than one vegetation type and three were internally fragmented and urbanized. These features aggregate environmental heterogeneity to all, but certainly more to the largest areas (i.e. the Gargaú Private Reserve of Natural Heritage, 1058ha, and the Buraquinho Refuge of Wildlife, 518ha), allowing them to contribute more than the others to the regional richness.
Although 23 bird species have left the Brazilian list of endangered species (MMA Ordinance 444/2014), 234 species/subspecies are still threatened. In our surveys we recorded six endangered species, demonstrating that the urbanizing region is still able to harbor threatened birds. However, if future urbanization in the region follows the trend of the 1970–2010 period (Stevens, 2014), a massive erosion in bird diversity is expected (Sol et al., 2017). During the fieldwork, it was also evident the unauthorized use of the protected areas for illegal hunting, firewood extraction, livestock, fishing, garbage deposition, and other destructive uses, especially in those under state and municipal administration (see also Silva-Junior and Santos, 2017). These chronic management failures affect the biota as whole and may also jeopardize the long-term bird conservation.
The presence of the introduced species Estrilda astrild and Passer domesticus is also worrisome, as biological invasion is a powerful driver of biodiversity loss at multiple spatial scales (e.g. Jones et al., 2008). Estrilda astrild entered accidentally in the Brazilian territory, but P. domesticus was introduced intentionally to control agricultural pests (Sick, 1997). The effects of the introduction of exotic species are usually irreparable, especially if the exotics is a superior competitor able to rapidly reproduce, establish and spread over the new areas (Shaw et al., 2008; MacGregor-Fors et al., 2010). In this scenario, native species are not able to fight back and the exotics becomes an invasive species. In West Mexico, for example, areas invaded by P. domesticus have heavily dominated avian communities with low species richness, while non-invaded areas exhibit highly-even and species-rich bird communities (MacGregor-Fors et al., 2010). These might be the case for E. astrild and P. domesticus in our study region, but more studies are needed to quantify their invasiveness, if any.
On the other hand, the high representation of the native Thraupidae and Tyrannidae was expected (Hilty, 2011), given that both are commonly found in urban parks, squares, gardens and other altered habitats. Tyrannidae is considered the largest family of the Western Hemisphere, with 413 species representing approximately 18% of the passerines known for South America (Sick, 1997; Fitzpatrick, 2004). Thraupidae is less specious (240 spp.), but is also very common along the Brazilian Atlantic Forest. In our study region, one in four species belongs to these two families. These birds usually feed fruits, seeds and insects (Lopes et al., 2005). The frugivorous species usually consume small seeds and fruits (<1.5cm length), favoring the dispersal and proliferation of plant species that bear such attributes. These plant attributes are generally correlated with soft wood, fast growth, copious production of small seeds, and low capability to store carbon, being typical in pioneer tree species that do not characterize old-growth conserved forests (Santos et al., 2012). The dominance of Thraupidae and Tyrannidae might therefore help to maintain the flora at early to mid successional stages and boost the regional floristic homogenization driven by the proliferation of native tree species (Lôbo et al., 2011).
Regardless this dominance of Thraupidae and Tyrannidae, the species similarity varied considerably across the protected areas, following our expectation that less isolated, similar-sized areas showed an avifauna more similar to each other than more isolated, different-sized areas. This suggests certain level of dispersal limitation to more distant sites and major environmental differences between small and large areas. Both possibilities have been reported elsewhere (Sekercioglu et al., 2002; Uezu et al., 2005; White et al., 2005). However, the weakness of the relationships, though statistically significant, indicates that species composition are also influenced by factors other than size and isolation. Based on field observations, we hypothesize that varying levels of hunting, catching for illegal trade, pressure by exotic predators (e.g. cats and dogs) and noise pollution also play important role in taxonomic composition.
Implications for management and conservationIn addition to more investment in basic infrastructure and full-time staff (Silva-Junior and Santos, 2017), we strongly recommend the responsible agencies to follow the federal law 9.985/2000 and create the management council and the management plan of the four areas officially recognized as ‘conservation units’. Lawfully, these actions should have been taken in the five years after creation, but to date only the Cabedelo Sandbank National Forest is in conformity with legislation. This legal noncompliance threatens the protected areas with downgrading, downsizing and degazettement (Bernard et al., 2014) and make public use of their ecosystem services almost unfeasible. Three municipal protected areas, which are not yet recognized as conservation units, face even more legal insecurity as their creation act may be revoked more easily. In this sense, we also recommend the official recognition of these areas as conservation units under federal law 9.985/2000.
Despite these challenges, the remarkable bird diversity of small and large urban reserves suggest that birdwatching for contemplative and educational purposes may help to change the course of conservation in the region, provided that it is well planned and based on the current legislation. Birdwatching is the act of observing and identifying birds in their native habitats; it is becoming the most rapidly growing and most environmentally conscious segment of ecotourism and provides economic hope for many threatened natural areas around the world (Sekercioglu, 2002). Positive impacts of birdwatching include link between avian diversity and local income, financial incentive to conserve wildlife, less impact and more income than typical tourism, increased local control due to unique bird species, visitation of areas outside traditional tourist itineraries, protection of unprotected areas with desired species, valuation of local natural history knowledge, education and employment of local guides, generation of funds for bird conservation and contribution to ornithological knowledge (Sekercioglu, 2002). Negative impacts involve disturbing birds by playing tapes and by approaching, increased nest predation and nest abandonment, increased disturbance of rare and/or threatened birds, visitor-related pollution and habitat destruction, cash leaks from local communities, resentment by excluded locals and cultural degradation associated with tourism (Sekercioglu, 2002).
Following Sekercioglu's recommendations for optimal birdwatching, we suggest that birdwatching initiatives in ours and other urbanizing regions of the Brazilian Atlantic Forest adhere to and insist on ethical birding conduct, avoid nests and young birds as much as possible, show particular care with threatened and rare species, minimize tape use and try to minimize being seen, do not approach further once a bird notices the watcher, stick to established roads/trails/walkways, use scopes for observation and photography, educate locals about birds and their financial benefits, support local and low-impact establishments and contribute to non-governmental organizations active in bird conservation. Providing a comprehensive birdwatching planning extrapolates the limits of this study, but its principles are presented here. We hope conservationists and decision makers find this information useful and give more attention to the urban reserves under their responsibility.
This work was supported by the National Council for Scientific and Technological Development (CNPq) (grant numbers 476135/2013-3 and 310340/2016-0). We thank the Higher Education Personnel Improvement Coordination (CAPES) for the scholarship to the TER and the UFPB Graduate Program in Biodiversity for institutional support. We also thank the Chico Mendes Institute for Biodiversity Conservation (ICMBio) and the Paraíba Environment Administration (SUDEMA) for authorization to carry out this work within the protected areas. We are grateful to Pamela Stevens for producing the figure of the study area, Orione Alvares da Silva for discussing ideas and supporting fieldwork, and two anonymous reviewers for their contributions and suggestions on earlier versions of this manuscript.