Macroecological and biogeographical studies have assumed that range map data should be used only at coarser grains due to false presences (errors of commission) at small grains. This has been explored using mostly species richness, underrepresenting other potentially informative biodiversity metrics. Here, we evaluated these issues by quantifying the extent to which taxonomic and phylogenetic alpha and beta diversity patterns calculated using anuran range maps at three cell sizes (1×1km, 5×5km, and 10×10km) differ from the patterns calculated based on checklists in 14 protected areas along the southern range of the Brazilian Atlantic Forest. We found that range maps and checklists generated reasonably similar spatial richness patterns in all cell sizes (r≥0.80 in all cases) and slightly weaker, but still correlated alpha phylogenetic diversity patterns (0.78≤r≤0.81). We also found that taxonomic (r≤0.76) and phylogenetic (r≤0.68) beta diversities had lower correlations than alpha spatial patterns. Therefore, range maps have value in documenting alpha biodiversity patterns, as well as beta diversity at more marginal levels, for tropical species at scales relevant to local conservation efforts.
The increased availability of spatial data on species distributions, coupled with the development of Geographic Information System (GIS) software, has allowed new approaches to investigate biodiversity patterns (Kozak et al., 2008). Studies of species occurrences usually rely on one of two general data types with different spatial characteristics and intrinsic errors (Hulbert and White, 2005; Hortal, 2008; Cantú-Salazar and Gaston, 2013): (i) smaller-scale field plots (i.e., limited areas sampled with standardized survey techniques) or local checklists (i.e., long-term surveys from sites of known limits and varying areas) describing species that occur at specific locations; or (ii) larger-scale atlas data (i.e., obtained from natural history collections, literature, and citizen science programs) or range map data (i.e., expert drawn polygons depicting species ranges). However, the type of data is well known to influence pattern detection (Hulbert and White, 2005; McPherson and Jetz, 2007). Therefore, looking for congruence among biodiversity patterns obtained from different data sources has important implications for basic spatial ecology and spatial conservation planning. For example, independent of the error (i.e., false positives or negatives), the congruence among spatial distribution of facets of biodiversity (i.e., species richness, endemism, complementarity and/or phylogenetic diversity) obtained from different sources should help offset the limitations of each data set, supporting the delimitation and management of priority areas for conservation. This is particularly true in the Neotropics with large Linnean (i.e. species still unknown) and Wallacean (i.e. incomplete knowledge of geographical species distributions) shortfalls (Hortal et al., 2015).
Previous studies have found that range maps are reasonable tools to describe biodiversity patterns at coarse grains (Hulbert and White, 2005; Hulbert and Jetz, 2007; Hawkins et al., 2008), but the minimum grain at which they should be used is still unclear. Most studies exploring biodiversity patterns have compared only range maps and atlas data, ignoring other sources (i.e., local checklists, but see Cantú-Salazar and Gaston, 2013). Atlas data generally have broad spatial coverage but relatively coarse resolutions. Conversely, local checklists have high spatial resolution, but low spatial coverage (McPherson and Jetz, 2007). Previous studies (Hulbert and Jetz, 2007; McPherson and Jetz, 2007; Hawkins et al., 2008) concluded that range maps and atlas data generated similar species richness patterns at a minimum grain size of 220km, 100km and 50km, respectively. Furthermore, when the quality of survey data increases, the grain at which range maps and survey data converge decreases (Hawkins et al., 2008). Consequently, if range maps are reliable only at broader spatial scales (Hulbert and Jetz, 2007), we would expect that range maps to be poor predictors of patterns at fine grains, compared to those generated with local checklists.
A related issue is that previous studies have addressed only a single aspect of biodiversity: species richness or taxonomic diversity (TD). However, understanding the spatial distribution patterns of alternative facets of diversity can guide future management and conservation actions. For example, phylogenetic diversity (PD) quantifies a substantially different component of biodiversity than species richness (Devictor et al., 2010), since two communities with equal richness may contain different phylogenetic histories. Based on this approach, highly phylogenetically clustered communities would contain less evolutionary information than those with a distantly related set of species from species-poor clades or phylogenetically overdispersed communities. Additionally, there could be incongruence between taxonomic and phylogenetic alpha diversity when measured using different types of data. Indeed, previous studies have pointed out the spatial incongruence between different measures of biodiversity for several groups (e.g., Forest et al., 2007; Devictor et al., 2010). Another important issue is that using compositional similarity (i.e., beta diversity, complementarity) could be a better alternative than alpha diversity alone (i.e., species richness and/or phylogenetic diversity), which can indicate a poor representation of species in protected areas (Williams et al., 1996). For example, Williams et al. (1996) found that by selecting 5% of the areas with the highest bird species richness, only 89% of the species in Britain would be represented. Alternatively, all species would be represented by using complementarity as a criterion taking the same number of areas. Nonetheless, the spatial grain at which measures of alpha and beta TD and PD converge when calculated using different data sources remains unexplored.
Amphibians are the most threatened vertebrate group globally, with about one-third of species being currently threatened with extinction and half of them in decline (Catenazzi, 2015). The main threats include fungal diseases, habitat destruction and alteration, and climate change (Catenazzi, 2015). Also, many evolutionarily distinct species are prone to extinction, making amphibians a group of high conservation concern (Wakea and Vredenburg, 2008). Paradoxically, amphibians also have one of the highest species description rates among vertebrates (Catenazzi, 2015), indicative of our incomplete state of knowledge about them, particularly in megadiverse regions. A global analysis of PD (Fritz and Rahbek, 2012) found that the southern range of the Atlantic Forest has high lineage richness of anurans, coincident with high species richness. As successful conservation plans should aim to preserve both species richness and the evolutionary potential of assemblages (e.g., Forest et al., 2007), understanding both the spatial distribution of multiple facets of biodiversity and their relationships with scale is key to guide future systematic conservation planning for this threatened vertebrate group. This is because, varying spatial grain and extent of investigation we alter the processes and mechanisms influencing the biodiversity patterns.
Here, we examine the extent to which alpha and beta biodiversity patterns calculated from extent-of-occurrence maps of anurans are congruent with local checklists in protected areas along the southern range of the Atlantic Forest. This is a species-rich region with many reliable site surveys. We used checklists of local anuran communities that have been intensively sampled over recent decades. We also used range maps overlaid onto grids with three cell sizes (1×1km, 5×5km, and 10×10km), all of which smaller than used previously to estimate species co-incidence. Therefore, we tested the congruence of alpha and beta phylogenetic diversity between range maps and local checklists. Our goals are to further explore the minimum grains at which range maps may be useful for assessing diversity gradients and to expand the scope of the use of range maps in estimating multiple patterns relevant to conservation planning at small scales. Nonetheless, it is beyond the scope of this study to define which is the best data source or the best biodiversity metric to be used in conservation strategies. Rather, we focus on the congruence of distribution patterns of alternative facets of biodiversity metrics considering range maps and checklists data sets. Because both data sources have advantages and disadvantages, congruence patterns between data would assist conservation strategies mainly in megadiverse areas suffering of Linnean and Wallacean shortfalls (Hortal et al., 2015).
Material and methodsLocal checklistsWe gathered data on anuran records from the literature (see Table S1 in Appendix) for 14 areas. We limited this study to forest communities within protected areas because checklists of amphibians in these areas are more complete than the rest of the Atlantic Forest (da Silva et al., 2014). We selected only sites whose checklists met the following criteria: (i) they used at least two of four survey methodologies (audio, active search, casual observations, and pitfall traps, and (ii) samples were taken in all seasons for at least one year. Finally, anuran species with incomplete or uncertain identification were excluded.
Range mapsWe overlaid extent-of-occurrence maps of amphibians (version 2015.2, IUCN, 2015) onto a grid to generate a presence–absence matrix for the locations of the checklists using three cell sizes (1×1km, 5×5km, and 10×10km). These cell sizes were selected because they represent extents normally sampled in area inventories. Species do not occur everywhere within their geographical range due to gaps and/or disjunctions in species distributions caused by unfavorable areas, biotic interactions, and source-sink population dynamics (Hulbert and White, 2005). Also, Ficetola et al. (2014) recently found that the accuracy of range maps differs among continents, with higher variation in megadiverse tropical regions, such as Tropical Asia and South America. Despite these uncertainties, range maps are widely used to investigate biodiversity patterns of South American anurans at a range of spatial scales (e.g., Villalobos et al., 2013; Loyola et al., 2014; Vasconcelos et al., 2014).
Data analysisTo evaluate the discrepancies between species composition obtained from checklists and range maps, we calculated for each grid grain the proportion of species listed in checklists that: (i) are shared with range maps, (ii) are not predicted to occur from range maps, i.e., range map omission error, and (iii) are predicted to occur from range maps but are not present in checklists, i.e., commission error (Cantú-Salazar and Gaston, 2013).
We built a regional phylogeny by pruning the time-calibrated tree of Pyron and Wiens (2013), which contains 2871 species (40% of known extant species) from 432 genera (85% of the 500 currently recognized genera). Species not represented in the phylogeny were added as intrageneric polytomies, whereas species belonging to genera not present in the tree were inserted based on phylogenetic relationships from other sources. We acknowledge that polytomies underestimate branch length differences among species. However, phylogenetic metrics are generally more sensitive to a loss of resolution near the root than the tips of the phylogeny (Swenson, 2009).
To calculate phylogenetic diversity we used Faith's metric (PD), which is the sum of branch lengths connecting all species in an assemblage (Faith, 1992). To remove the influence of species richness, we calculated the standardized effect size (SES) based on a null model (Swenson, 2014). First, we simulated PD values by shuffling the tips of the phylogeny 999 times (i.e., null communities). Then, the PD observed was subtracted from the average of simulated PD and divided by standard deviation of simulated PD.
We estimated taxonomic beta diversity (TBD) using the Sørensen index, which uses the fraction of taxa shared by two samples (Soininen et al., 2007). This index was chosen as it is relatively independent of species richness and accurate even with small samples (Soininen et al., 2007). We also estimated phylogenetic beta diversity (PBD) using the Phylosor index, which use the fraction of branch lengths shared between two samples (Bryant et al., 2008). For both TBD and PBD, we used as the beta diversity estimate the averaged similarities for each site (checklists) and grid cells (range maps).
To test the spatial congruence of alpha and beta diversities between range maps and local checklists we conducted significance tests of Pearson correlation coefficients after accounting for spatial autocorrelation (Dutilleul, 1993). Dutilleul's method corrects the number of degrees of freedom associated with the distribution of estimated variance but not affect the correlation coefficient. Because this method is very conservative (it assumes all spatial structure is artifactual), and we are interested in how well checklists and range maps represent the spatial distribution of diversity rather than claiming yes or no (see Hawkins, 2012 for discussions of this issue) we do not base interpretation of the results on the significance tests. Even so, because significance testing in non-experimental geographical data still occurs in the literature, we report the results of the significance tests of the correlations for those who consider them informative.
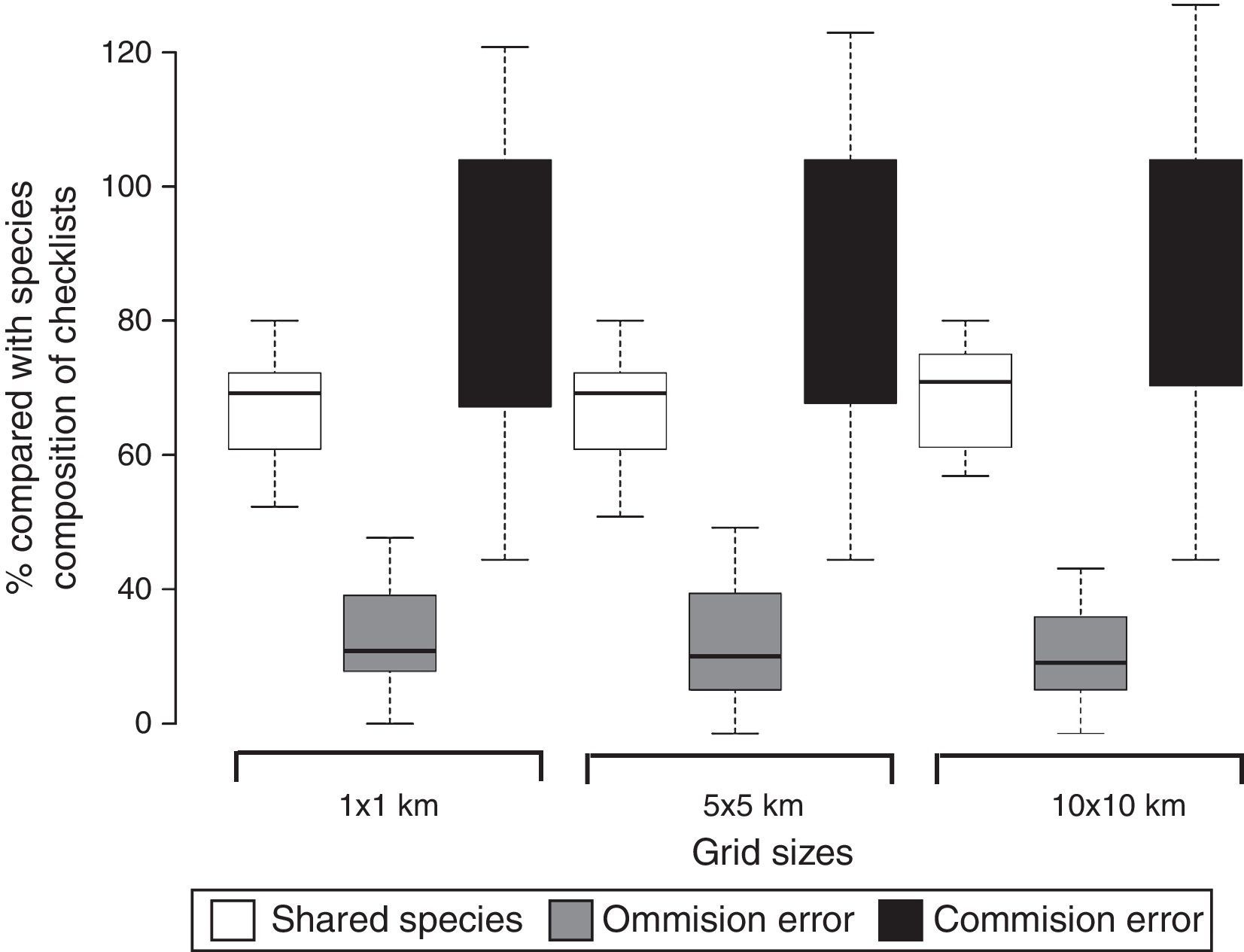
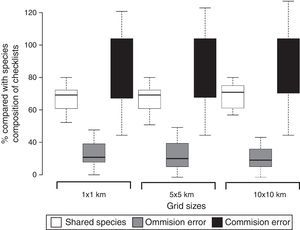
ResultsOverall, we recorded 262 anuran species, of which 208 were recorded in the checklists (47 species exclusive to this source) and 215 species in the range maps (54 exclusive to this source). On average, 67.6% of anuran species present in checklists were detected in range maps, although range maps overestimated species richness when compared to checklists (Fig. 1 and Fig. S2 in Appendix). The proportion of shared species (on average 67.6%), and errors of commission (on average 84.7%) and omission (on average 31.8%) from range maps compared to checklists were similar at the three grid sizes (1×1km, 5×5km, and 10×10km; Fig. 1).
Boxplot showing the proportion of shared species and range maps commission (false presence) and omission (false absence) errors of 14 area inventories (checklists) in the southern range of Brazilian Atlantic Forest and the three grid cell size (1×1km, 5×5km, and 10×10km) used to extract species composition from range maps available at IUCN (2015). The horizontal line and box show the median and 50% quartiles, respectively, and the error bars display the range of the data.
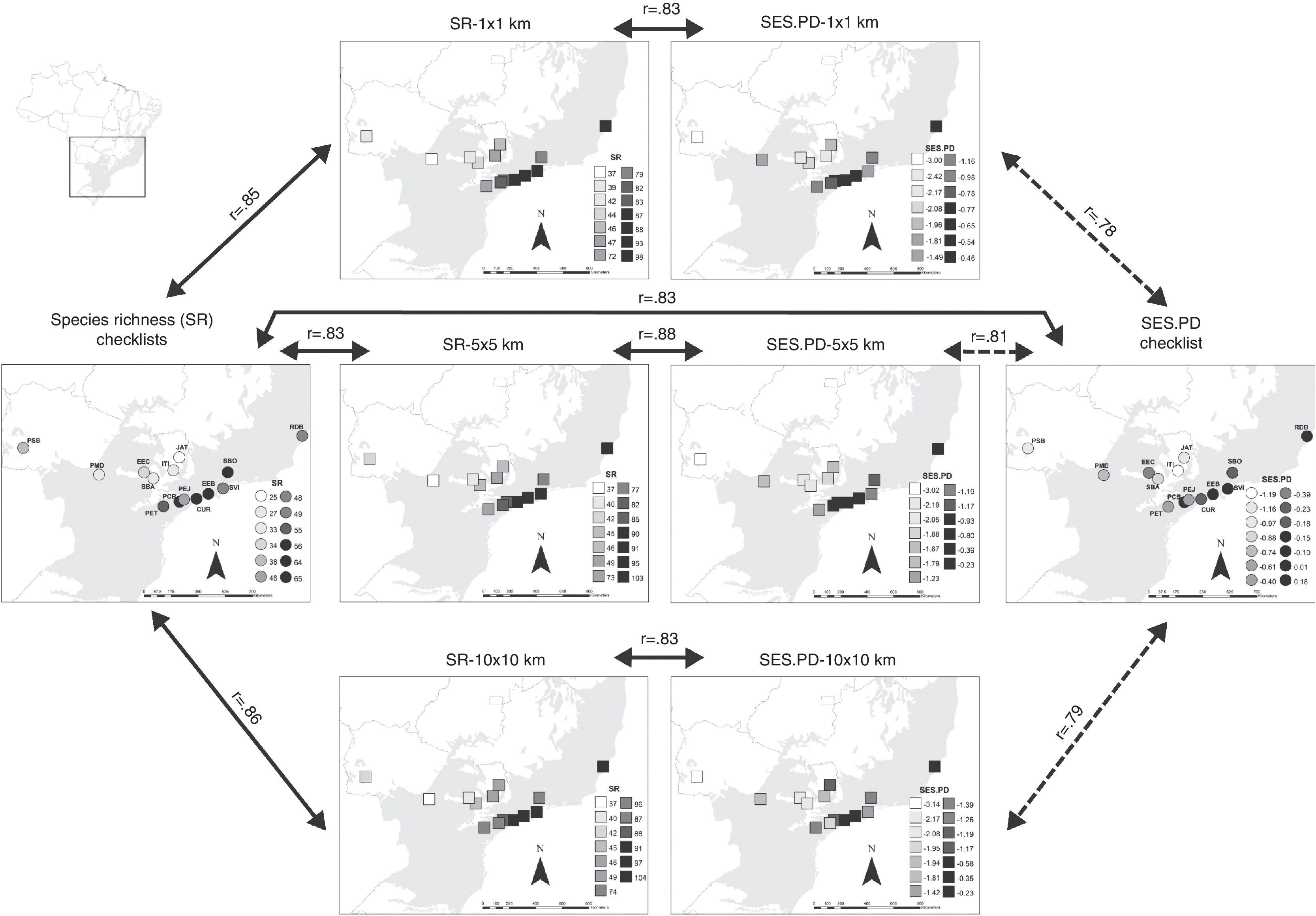
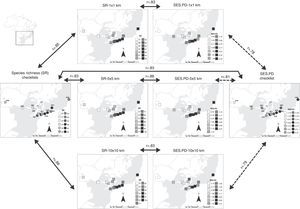
We found that species richness obtained from checklists was positively correlated with species richness at the three grains at fairly high levels (Fig. 2), 1×1km (r=0.85), 5×5km (r=0.83), and 10×10km (r=0.86). These results indicate that sites in eastern Atlantic Forest have higher species richness than those in the west, a general pattern captured by both data sources (Fig. 2). Associations of PD obtained from range maps and checklists were also positive, albeit slightly lower than for species richness (Fig. 2), 1×1km (r=0.78), 5×5km (r=0.81), and 10×10km (r=0.79). We also observed a strong positive correlation between species richness and PD of local checklists (r=0.83) and species richness and PD for each of the three grid cell sizes (Fig. 2), 1×1km (r=0.83), 5×5km (r=0.88), and 10×10km (r=0.83).
Species richness (SR) and standardized effect size of Faith's phylogenetic diversity (SES.PD) of the 14 areas in the southern range of Brazilian Atlantic Forest and the three grid cell size (1×1km, 5×5km, and 10×10km) used to extract species composition from range maps. Dark shading indicates the Atlantic Forest biome. Continuous arrows indicate ‘significant’ (P<0.05) Pearson correlations after taking into account spatial autocorrelation in the data, while dashed arrows indicate no statistical significance. For illustrative purposes we increased cell sizes of 1×1km, 5×5km, and 10×10km to enhance visibility.
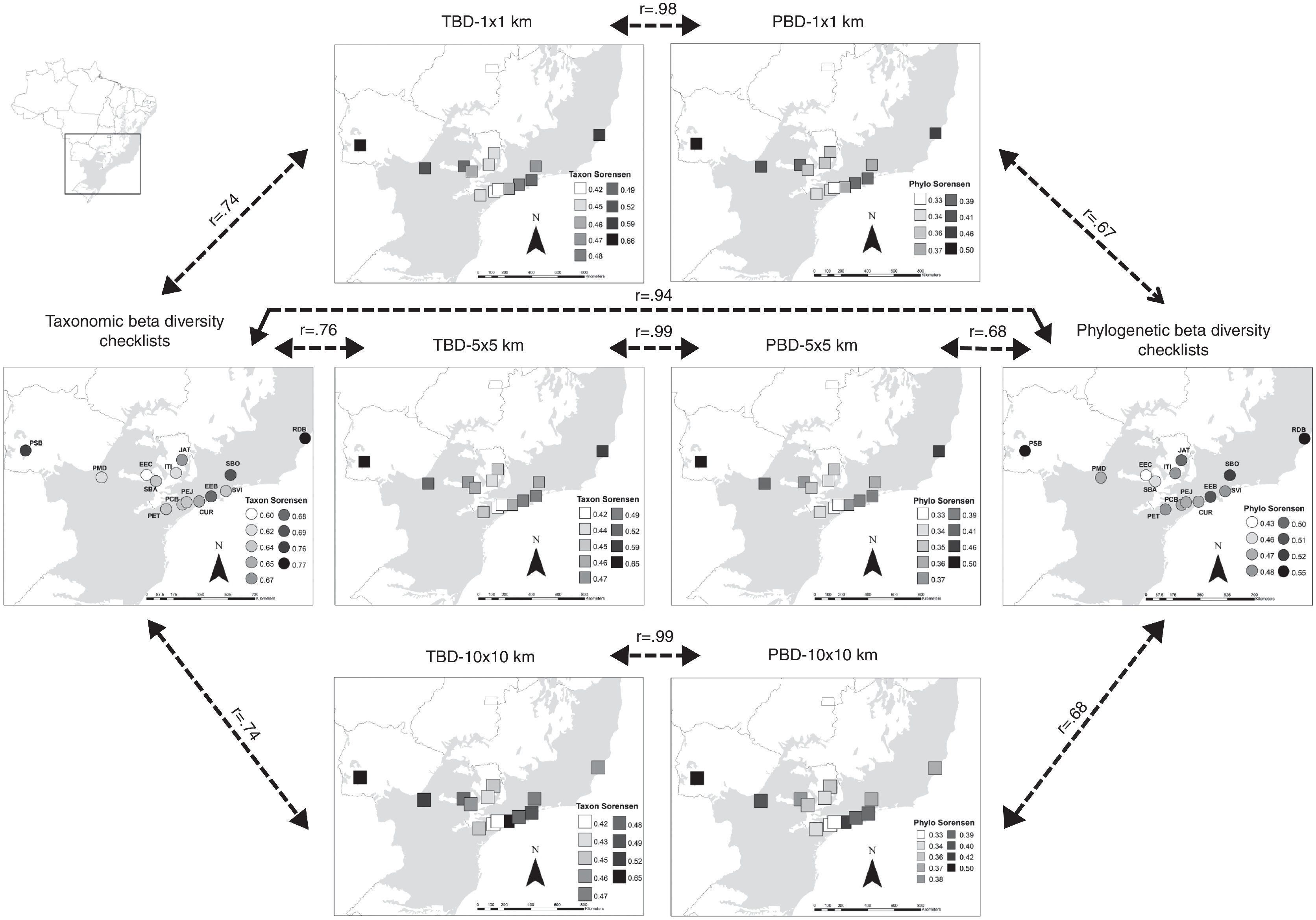
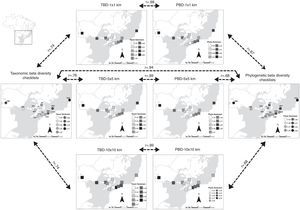
Although TBD and PBD of checklists and range maps indicated the same sites (PSB and RDB) and their correspondent grid cells as the highest values of dissimilarity, we found a marginally non-significant congruence between range maps and checklists (Fig. 3), 1×1km (r=0.74), 5×5km (r=0.76), and 10×10km (r=0.74). Furthermore, TBD and PBD calculated from checklists were higher than range maps (Figure S3 in Appendix). Thus, congruence between beta diversity indexes was not as strong as alpha diversity (Fig. 3). However, we observed a strong positive correlation between TBD and PBD calculated from local checklists (r=0.94) and TBD and PBD for each of the three grid cell sizes (Fig. 3), 1×1km (r=0.98), 5×5km (r=0.99), and 10×10km (r=0.99).
Taxonomic (TBD) and phylogenetic (PBD) beta diversities of the 14 areas in the southern range of Brazilian Atlantic Forest and the three grid cell size (1×1km, 5×5km, and 10×10km) used to extract species composition from range maps. Dark shading indicates the Atlantic Forest biome. Continuous arrows indicate ‘significant’ (P<0.05) Pearson correlations after taking into account spatial autocorrelation in the data, while dashed arrows indicate no statistical significance. For illustrative purposes we increased cell sizes of 1×1km, 5×5km, and 10×10km to enhance visibility.
In contrast to studies that found that range maps and checklists generate similar species richness patterns only at coarse grain sizes (Hulbert and Jetz, 2007; McPherson and Jetz, 2007; Hawkins et al., 2008), we found that they generated similar alpha patterns at a grain size as fine as 1×1km, but less so for the beta diversity indexes. The low detectability of some species is also an issue in checklists (Gooch et al., 2006) that it is not usually considered in surveys, especially in coastal regions of the Brazilian Atlantic Forest that harbor high species richness with restricted ranges (Villalobos et al., 2013).
We found that range map richness overestimates were higher for species-rich than species-poor checklists. Cantú-Salazar and Gaston (2013) found that species richness estimates obtained from checklists and range maps in the western hemisphere showed a significant positive relationship for amphibians, mammals, and birds. They also found that correlations were stronger for amphibians than for birds and mammals, whose range distributions and point occurrence are best known. Therefore, although we found that range map data can provide reasonable spatial patterns of species richness relative to ground-truthed data at finer resolutions, they are likely going to overestimate absolute levels of richness, unless they underestimate the true ranges of many species.
Beta diversity is being increasingly used as an alternative biodiversity metric to guide spatial conservation planning (Wiersma and Urban, 2005; Nobrega and De Marco, 2011; Socolar et al., 2016). We found that TBD and PBD of range maps and checklists were not significantly correlated at small grain sizes. This suggests that the choice of data source alters the spatial pattern for our study area. Thus, selecting protected areas based solely on beta diversity indexes using either of the two data sources will not ensure the conservation of the same assemblages at fine scales. We also observed that range maps consistently show high commission and omission errors at the grid cells when compared to checklists. Consequently, these two data sources provide different answers about which areas should be protected and which processes are associated with the spatial distribution of TBD and PBD but not alpha diversity. Because checklists are associated with local processes while range maps are associated with regional processes (Hortal, 2008), we have to take into account qualities and limitations of such datasets at different extents and resolutions when designing conservation plans based on beta diversity indexes.
Investigating spatial patterns of biodiversity obtained from multiple data types can improve our knowledge of ecological processes driving them. For example, checklists capture better the effects of local scale factors such as density-dependent interactions, habitat selection, and community assembly processes, while range maps reflect the effects of environmental filters and dispersal barriers more prominent at broad scales (Hortal, 2008). But generating local checklists over large areas is time consuming and expensive, whereas range maps are available for a large number of taxonomic groups. Our results provide evidence that range maps can help in selecting protected areas considering alpha diversity even at fine scales but perhaps not taxonomic and phylogenetic beta diversities. Therefore, the results of previous studies using range maps to calculate beta diversity metrics and evaluate the representativeness of protected areas under future climate scenarios or guide reserve selection should be taken with caution, at least for our study extent. It is not surprising that range maps do not generate identical patterns of species richness compared to local surveys, but it is unexpected that they can capture the general spatial patterns in a threatened biodiversity hotspot in sites down to 1km2.
Conflicts of interestThe authors declare no conflicts of interest.
F.R. da Silva thanks Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Proc. 2013/50714-0) for support.