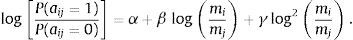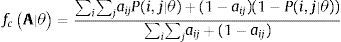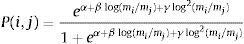Rewilding encompasses management actions such as reintroductions and translocations with the purpose of restoring ecological processes and ecosystem functions that were lost when species were locally extirpated. The success of a species introduction is conditioned by multiple factors, in particular, ecological interactions. To predict the fate of the introduced population and the community-level outcomes of the introduction, species interaction patterns need to be considered. Here I propose that ecological network models can help in rewilding projects in at least three ways. First, combining ecological information and probabilistic models it is possible to infer the most likely ways whereby the introduced species will integrate the community and which will be its role in the topology of the food web. Second, by determining the species more likely to interact directly or indirectly with the introduced species, it is possible to identify those species that may affect the success of the introduction and those that are more likely to be affected. Third, by constructing potential interaction networks representing the rewilding scenario, one can infer the possible ways by which the overall structure of the network will change and thus devise more efficient plans to monitor the community. Network models can be an important asset in rewilding, helping in feasibility and risk assessment as well as in monitoring the consequences after species release.
Over the past decades, conservation biology underwent a shift from a field whose main mission was to evaluate extinction risk and halt diversity loss, with particular focus on threatened species (Meine, 2010), to a more process-centered view, whose focus is the conservation of functional ecosystems (Tylianakis et al., 2010). The functioning of ecological systems depends on the integrity of the ecological networks formed by the multiple interactions that species establish with each other (McCann, 2007; Molnar et al., 2004). The main strategy for the conservation of ecosystems is arguably the establishment and management of large interconnected reserves (Lovejoy, 2006). Larger areas can harbor a greater diversity of habitats, organisms and thus of ecological processes (Peres, 2005). However, maintaining large areas does not guarantee ecological processes will be preserved. In fact, most regions of the planet are already largely defaunated (Dirzo et al., 2014), and without large stocks of organisms in neighboring areas, reserves may be nothing more than large patches of empty forests (Redford, 1992) amidst the urban and agricultural landscape matrix.
The extirpation of large vertebrates began when humans expanded their distribution in the Pleistocene and continued in historical times, having profound consequences for ecological systems (Malhi et al., 2016). Large bodied vertebrates are often key players in ecosystems, participating in several processes such as nutrient cycling (Doughty et al., 2013), long-distance seed dispersal (Pires et al., 2017) and exerting top-down control on species on lower trophic levels (Ripple et al., 2015; Terborgh, 2001). Moreover even smaller-sized vertebrates, which might be able to compensate to some extent the absence of large-bodied species, are now declining in most areas (Donatti et al., 2009). This scenario calls for more active restoration approaches in order to reestablish animal populations in the wild (rewilding) and their ecological interactions (rewiring), thus reinstating ecological processes and ecosystem functions (Seddon et al., 2014).
Soulé and Noss (1998) proposed the use of the Pleistocene as a baseline for ecosystem restoration in North America and introduced the rewilding concept. Rewilding was originally defined as “the restoration and protection of big wilderness and wide-ranging large animals – particularly carnivores” (Soulé and Noss, 1998). The use of the Pleistocene fauna as a baseline implies in the introduction of taxon substitutes, such as large felids, feral horses, cattle and elephants, that would be able perform the roles that vacated since the native megafauna, like saber-toothed cats, lions, camels, sloths and mastodons died out (Donlan, 2005; Galetti, 2004). More recently the definition has been loosened and rewilding now encompasses both the reintroduction of locally extinct species (Galetti et al., 2017; Svenning et al., 2016) and conservation translocations using surrogate species whose ecological roles would be equivalent to the species that have been lost (Seddon et al., 2014). Different from traditional reintroduction, which focuses on recovering declining populations, the ultimate aim of rewilding is to restore ecosystem processes that were lost due to local extirpation, generating a self-regulated community, without the need of continued management (Sandom et al., 2013; Svenning et al., 2016).
To reestablish ecological processes, a rewilding project envisages rewiring an emptied food web with the desired links. Rewiring is the reconfiguration of the interaction patterns of network elements (Watts and Strogatz, 1998). I refer to network rewiring as the establishment of novel ecological interactions, which will generally involve the introduced species, but also reconfigurations of the interaction network that occur as an indirect effect of a species introduction.
If the goal in rewilding is to restore certain ecological processes, the viability of the introduced population over time has to be secured. However, a number of examples from accidental introductions and biological invasions show that the introduction of a species into a local food web may trigger cascading effects via direct and indirect pathways that can result in diversity decline and changes in ecosystem properties (Lodge, 1993). Thus, a rewilding program should also be able to ensure that the negative impact on other populations will be minimal. Considering the amount of resources a rewilding initiative demands and the potential mishaps, it is compulsory to use techniques that allow foreseeing the suite of possible outcomes after an introduction.
The “Guidelines for Reintroductions and Other Ecological Translocations” (IUCN/SSC, 2013) from the IUCN Reintroduction Specialist Group (RSG) highlight the need of assessing the match between the abiotic and biotic needs of the candidate species and features of the target area. Basic information about the abiotic conditions determining species occurrence are available for many species, especially the ones that are often considered as potential rewilding candidates. Distribution modeling techniques allow predicting, sometimes with a very high level of confidence, where are the suitable regions for a species (Elith and Leathwick, 2009). Yet, without careful consideration of how biotic interactions affect the dynamics of the managed population and how the introduced species will affect other organisms, a reintroduction program is sentenced to failure. In short, the main challenge of any reintroduction is how to guarantee that the introduced species will subsist in a biotic context where it is able to sustain its population and will not harm the others.
Here I first review a set of cases where success and failure of species introduction was related to the effects of biotic interactions. Next, I argue that approaches derived from network science can be an asset for planning, assessing the viability, and for monitoring the success of rewilding.
Biotic interactions and rewilding successAs soon as individuals of the candidate species are released they will create novel interactions with several other species, which will influence the likelihood that the population establishes and will have consequences to the local community. Looking at the outcomes of past reintroductions and translocations is the key to understand how biotic interactions affected their success or failure. Most of the early attempts of reintroduction were ill-planned, with no post-introduction monitoring (Seddon et al., 2007). Available data regarding introductions in the 70s and the 80s show many programs were unsuccessful in reestablishing populations, although the causes of failure were mostly unaddressed (Griffith et al., 1989; Seddon et al., 2007). Examining more than 500 cases of reintroduction to understand what aspects were being reported, Seddon et al. (2007), found that only 7% of the studies addressed the ecological effects of the introduction, i.e., the interactions of the released species with the environment and other organisms.
The RSG (Reintroduction Specialist Group) reports include detailed analyses on the consequences of introductions, but tend to report mainly the successful attempts. Actually, this is a trend in the restoration literature (Fischer and Lindenmayer, 2000; Moehrenschlager et al., 2013), which may be harmful to our understanding on the reasons underlying failure or negative impacts of introductions and translocations. Yet, most of the reports on introductions and translocations covered by the RSG list interactions with predators, pathogens, competitors and resource availability (prey or plants) as potential causes determining the success of introduction attempts.
Predation, either by native or invasive predators, is often identified as a major determinant of post-release mortality, limiting the success of released individuals to form a viable population (Innes et al., 1999; Moseby et al., 2011; Seddon et al., 2007). High mortality due to predation after release has been associated mainly with the naivety of captive-bred individuals (Aaltonen et al., 2009; Biggins et al., 2011). Exposure to predators during captivity has been shown to reduce predation-related mortality for different species (e.g., Heezik et al., 1999). A careful assessment of the predator–prey interactions the rewilding candidate establishes in its location of origin may allow determining the potential predators in the target area and how the introduced species will integrate the local interaction network. Such knowledge may help devising strategies that minimize loss due to predation, including rearing schemes that foster anti-predator behavior.
Large-bodied rewilding candidates are presumably less likely to be victims of predation, especially in already defaunated areas. Still, other natural enemies such as pathogens may induce high mortality in released individuals. The stress produced during capture and transportation may affect the immune system of introduced individuals making them more susceptible to pathogen infection, a phenomenon described for different species such as beavers (Castor fiber; Nolet et al., 1997) and the Eurasian lynx (Lynx lynx; Schmidt-Posthaus et al., 2002). Knowledge of the pathogens carried by resident organisms and their potential to infect the introduced species is essential to the development of countermeasures that reduce mortality risk.
Another important source of mortality related to the biotic component is starvation. Examples include introductions of the river otter (Lontra canadensis; Day et al., 2013), the Arabian oryx (Oryx leucoryx; Mésochina et al., 2003), and the Canadian lynx (Lynx canadensis; Devineau et al., 2010). Failure of several reintroduction attempts of African predators has also been associated with reduced prey availability in the target area or inefficient hunting skills of captive-bred individuals (Hayward et al., 2007). In a compilation of carnivore introduction success, Jule et al. (2008) found that starvation was the second cause of mortality after human related causes such as shooting and vehicle collision. Competition with resident species for resources may also hinder the establishment of the introduced population (Hayward et al., 2007; Jule et al., 2008).
Starvation may only become a prevalent mortality cause when populations grow unchecked due to low top-down control. In the Oostvaardersplassen, a fenced nature reserve in the Netherlands where cattle, red deer and horses were introduced, populations undergo die-offs as the availability and quality of the forage drops during the winter (Vera, 2009). In the absence of predators, introduced species may also cause undesirable impacts on the vegetation, affecting several other species, such as happened with reintroduced elk (Cervus canadensis) in Kentucky (Cox, 2011), introduced red deer (Cervus elaphus) in Scotland and mustang horses in North America (Sandom et al., 2013). Yet, the effects of introduced herbivores on vegetation are likely complex, including desirable and undesirable outcomes (Johnson and Cushman, 2007). Being able to predict in which species the rewilding candidate will feed on, which are the potential competitors in the target area, and whether it will be able to shift to alternate resources in case the main resource declines is important to foretell not only the success of the introduction, but also the consequences it will have for other members of the community.
A number of examples illustrate the potential of introductions to produce adverse effects (Moehrenschlager et al., 2013; Ricciardi and Simberloff, 2009). The introduction of foxes (Vulpes vulpes) in Australia in the nineteenth century for recreational hunting still threatens several native species (Saunders et al., 2010). Beavers (Castor canadensis) introduced in the 1940s in Southern South America for fur trading have devastated the endemic Nothofagus forests and constructed dams produce a number of effects on riparian communities, water chemistry, and nutrient cycling (Vázquez, 2002). Introduction of the Nile perch (Lates niloticus) in Lake Victoria resulted in population decline and extinctions of several2 native species of fishes (Harrison and Stiassny, 1999). In all these cases the introduced species impacted resident populations via negative direct and indirect effects, causing diversity loss and changes in ecosystem functions.
Despite the conceivable risks, well-planned and well-monitored rewilding has the potential to produce the desired effects. The replacement of extinct endemic giant tortoises in Mauritius by an ecologically similar tortoise (Aldabrachelys gigantea) has restored ecological functions such as seed dispersal and control of invasive weeds (Griffiths et al., 2010). The release of wolves in Yellowstone became the paramount example of the overarching effects that rewilding can produce. The introduction of wolves is thought to have regulated the population of elks, numerically or by inducing behavioral changes, allowing woody plants such as aspen (Populus tremuloides) and willows (Salix spp.) to recover, but also benefitted beavers and songbirds that depended on the trees and bears who benefit from carcasses (Ripple and Beschta, 2012).
Collectively the examples discussed above highlight a salient feature of rewilding, the introduction of a novel species in a community will create a suite of novel direct and indirect interactions, which determine the fate of the introduced population and the consequences of the introduction for the community. The interactions established by an introduced species are part of a larger network of interactions. The network approach offers the tools to characterize interaction patterns and thus infer how species affect each other in the community. Using a network approach in the rewilding context might allow determining which other species may represent potential threats or assets to the establishment of the introduced population, affecting the outcome of a rewilding project.
A network approach for rewildingIf the motivation for rewilding is to restore ecological processes, the proximate goal is to restore past interactions or establish novel ones that will help reinstating community function (Seddon et al., 2014). The multiple biotic interactions species establish in ecological communities make it hard to predict how an introduction will play out. Ideally, a rewilding program should be able to foresee: (i) which interactions will be established once a species is introduced; (ii) how the introduced population will react, taking into account the positive and negative interactions; (iii) the impacts of the introduction on the multiple species in the community and how these impacts will feedback to the introduced population. I argue that network modeling may not only help devising such predictions but also monitoring the outcomes of an introduction.
The network approach has a long history in ecology, from the diagrammatic representation of feeding interactions between species in communities or energy flow in ecosystems, to more sophisticated quantitative analysis of network structure in search for general patterns and underlying processes (Dunne, 2006). The overall architecture of the network is determined by the ecology of its constituent species. The number and strength of interactions of a given species in the network are determined by its degree of ecological specialization (Blüthgen et al., 2008). Thus, the level of ecological specialization of the species in the studied community determines network properties such as the distribution of the number of links, the distribution of interaction strengths and the overall density of links in the network. Complex network patterns such as compartmentation, the presence of groups of species densely connected to each other, can also be mapped into ecological mechanisms such as habitat preferences or morphofunctional constraints (Dunne, 2006; Baskerville et al., 2011). Therefore, examining the structure of ecological networks offers insights on the mechanisms underlying community assembly and functioning.
The main information required to study interaction networks is basically the information on who interacts with whom in a given locality. Ideally we wish to know what are the demographic consequences of each interaction, but this information is often unavailable (Vázquez et al., 2005). The inherent complexity of real ecological networks makes the outcome of interactions in terms of their demographic effects hard to predict (Vucetich et al., 2011). Even the dynamics of apparently simple systems where a predator and prey seem to control each other's densities, are actually more complicated and involve several trophic levels (Krebs et al., 2001). In this sense predicting the demographic responses of all community elements to such a drastic intervention as a species introduction is unfeasible. However, networks can help us predict how the introduced species will integrate in the community and point in the direction of what are the most likely ways a community is expected to change.
The network approach offers the toolset to quantitatively assess the overall architecture formed by species interactions and the role of each species in the network. A rewilding candidate will usually be chosen based on the expectation that it will be able to restore key processes lost after species extirpation. In this sense the introduced species is expected to display the characteristics of keystone species, those whose influence on ecological processes are disproportionate in relation to abundance (Power et al., 1996). Although identifying keystone species is fraught with challenges and requires experiments or manipulation in the field (Power et al., 1996), the network approach offers ways to detect potential keystone species based on the species connection patterns and its degree of topological centrality (Jordán et al., 2006). If the introduced species take on a central position in the network, interacting directly and indirectly with many other species, it is very likely to become a key species in the community (Soulé et al., 2003).
One of the main shortcomings of the network approach is that obtaining detailed information on interactions to build high resolution ecological networks is demanding (McCann, 2007). Moreover, ecological systems are dynamic adaptive systems where species compositions and interaction patterns can change over short and long timescales. For instance, Olesen et al. (2008) have shown that interaction turnover in plant–pollinator networks may be high, changing from day to day. Therefore, the best way to think on an ecological network is not as a snapshot, which is often how networks are represented and studied. Instead, predictions should incorporate the uncertainty on interaction patterns, which is inherent to any real ecological network.
In the same ways that considering stochastic processes helped to devise more representative models for population viability analyses (Morris and Doak, 2002), by dealing with ecological interactions under a probabilistic framework it is possible to encompass the uncertainty in the outcomes of rewiring after a species is introduced. The toolset to analyze the structure of probabilistic interaction networks is now available (Poisot et al., 2016), and probabilistic models that predict species interaction patterns based on species traits are becoming more popular in the literature of ecological networks (e.g., Pires and Guimarães, 2013; Rohr et al., 2010; Williams and Purves, 2011).
Probabilistic network models are statistical or rule-based models in which the probability of pairwise species interactions is predicted from a set of parameters, which can often be mapped into one or a small set of species traits (Williams and Purves, 2011). There is a growing body of studies showing a large part of the interaction patterns in real ecological networks can be predicted based on basic biological information, such as habitat use (Baskerville et al., 2011), body mass relationships (Brose et al., 2006; Gravel et al., 2013) and abundance (Krishna et al., 2008).
Interactions between any pair of species can only occur if species co-occur in space and time. For instance, diverging habitat preferences may result in low encounter rates diminishing the likelihood of interactions (Baskerville et al., 2011). Species may also miss each other due to phenological mismatches, as happens in many seasonal plant–pollinator systems where the activity patterns of pollinators are decoupled from the flowering periods of certain plants (Olesen et al., 2011). If individuals from different species do come into contact, some level of trait matching may be required for interactions to take place. For instance, body size ratio has been found to be a good predictor of predator–prey interactions (Brose et al., 2006). Fruit size and gape-width are often found as relevant traits conditioning seed-dispersal interactions between plants and frugivores (Dehling et al., 2016). Likewise, the match between the depth of the corolla and the length of the pollinator proboscis or bird beak constrains interactions in plant–pollinator networks (Stang et al., 2009; Vizentin-Bugoni et al., 2014). In fact, the overall structure of interaction networks seems to be predicted by a small number of species traits (Eklöf et al., 2013; Pires et al., 2011). Finally, abundance determines the likelihood that individuals from different species will run into each other. Thus, high interaction frequency may simply reflect high local abundance, whereas two rare species are unlikely to interact (Vázquez et al., 2007).
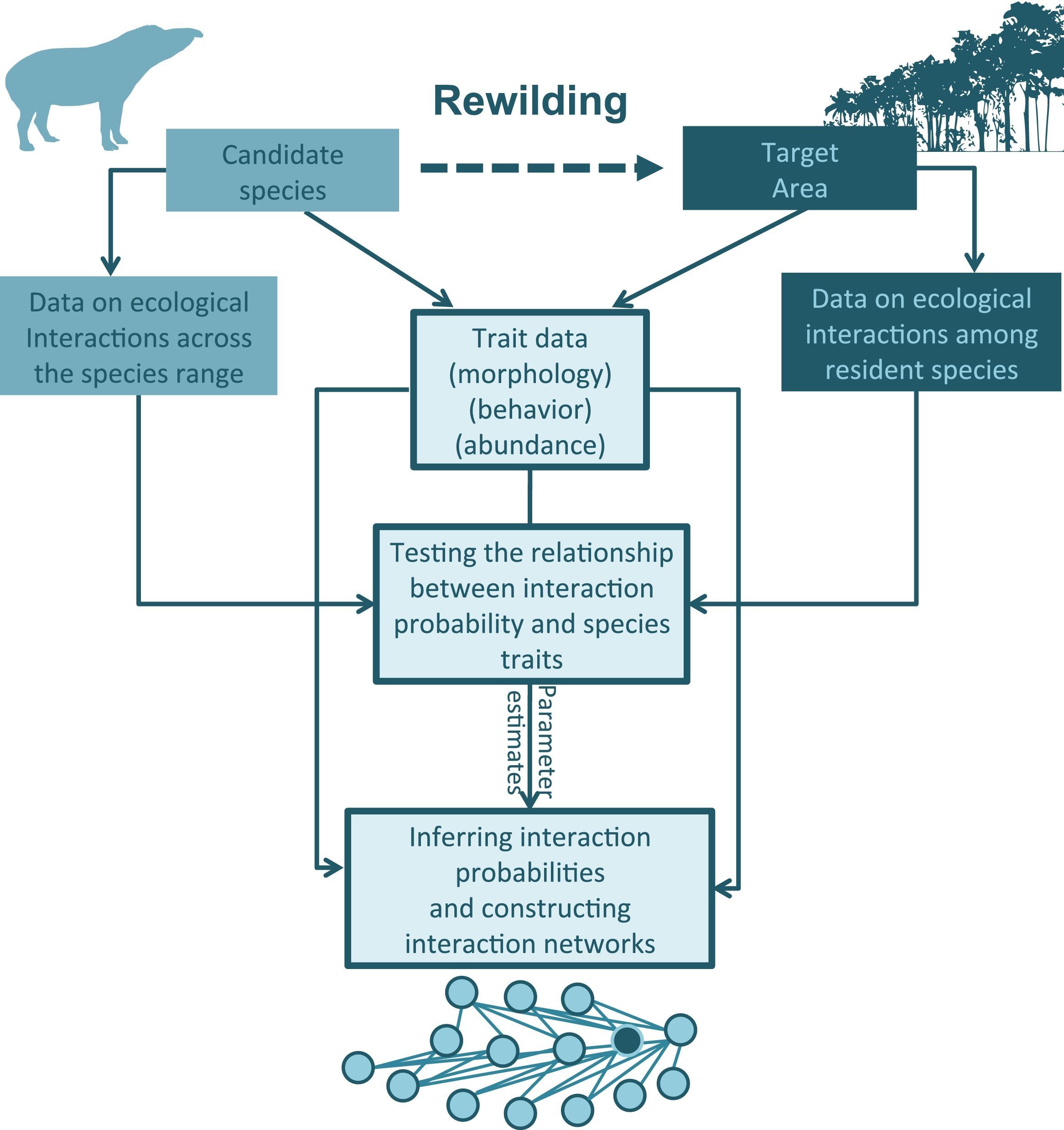
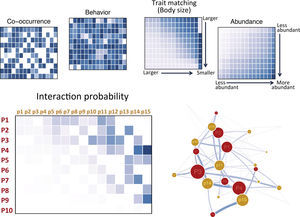
The first step to build predictive network models is to compile information on the interactions between the species in the target area, and interaction patterns of the candidate species with other species across its distribution (Fig. 1). Although any species is involved in multiple types of interactions, both as consumer (predator, herbivore, seed disperser, pollinator) and as resource (prey, host), studied networks usually focus on a certain interaction type, which presumably is the most relevant for the phenomena of interest. Accordingly, different types of interactions can be modeled separately. To model trophic interactions such as predator–prey or herbivore–plant interactions, for instance, the required information can be drawn from dietary assessments, which are available for a large number of species, especially those that are often considered for rewilding. Next, one should gather information on the traits likely to affect interaction patterns such as morphology, habitat preferences, activity patterns and behavior (Fig. 1). Of course the lack of information for certain traits will be the main limitation at this point. However, one or a few trait dimensions may be enough to build networks models that are able to predict a reasonable share of species interaction patterns (see Box 1). Moreover, if a species is being introduced in a given locality it is expected that basic information on morphology and behavior, for instance, is known.
An outline for building ecological networks to inform rewilding programs. Data on the traits and interaction patterns of the candidate species can be used to test the relationship between specific traits and the probability of interactions with other species. Similar tests can be performed using data on the traits and interactions among the species occurring in the target area. The estimated parameters and trait data can then be used to compute interaction probabilities and build ecological networks simulating the rewilding scenario. The position of the introduced species in the network (as highlighted by the node with a different color in the model network) can then be inferred and network structure can be examined.
Constructing probabilistic predator–prey networks using body mass data
In previous studies we used the network modeling approach to reconstruct predator–prey interactions between large mammals that occurred in the past (Yeakel et al., 2014; Pires et al., 2015). First, we compiled information on the interaction patterns of mammalian predators and their prey in Africa to investigate how body size affected the probability that we observed an interaction between two species in the dataset. To determine the relationship between body mass and predator–prey interactions, we used a statistical model (Rohr et al., 2010) where the logit of the probability of an interaction (aij=1 in matrix A) is a function of the ratio between the body mass of predator (mi) and prey species (mj):
Using model fitting techniques we estimated the parameters of the model (α, β, γ) that yielded the best fit to the observed data. The expected fraction of interactions (and non-interactions) correctly predicted by the model can be computed by summing the estimated probability assigned to each pairwise interaction (and absences of interactions) and dividing it by the total number of interactions.
This metric allows characterizing the performance of the model in a straightforward way, and it showed that the model was able to correctly predict between 70% and 83% of the observed interactions in different datasets (Pires et al., 2015).
For a given a set of parameters the probability of interactions can be computed as:
We then used the model and estimated parameters to infer the probability of interactions and reconstruct potential interaction networks between species known to occur in past assemblages in Africa (Yeakel et al., 2014) and in the Americas during the Pleistocene (Pires et al., 2015). The underlying assumption here is that the rules governing the relationship between body mass and predator–prey interaction patterns would be similar in past and present assemblages.
In these examples we only used qualitative information on interactions and used only body–mass relationship to predict predator–prey interactions. If more information were available for the assemblages we wanted to reconstruct, such as abundance estimates, it would have been possible to weight pairwise interaction probabilities and obtain more realistic estimates. The purpose of this type of model is not to obtain highly accurate inferences on the probability that two species will interact in a given place, but to generate an approximation that shows which interactions are more likely or less likely to happen in a given assemblage.
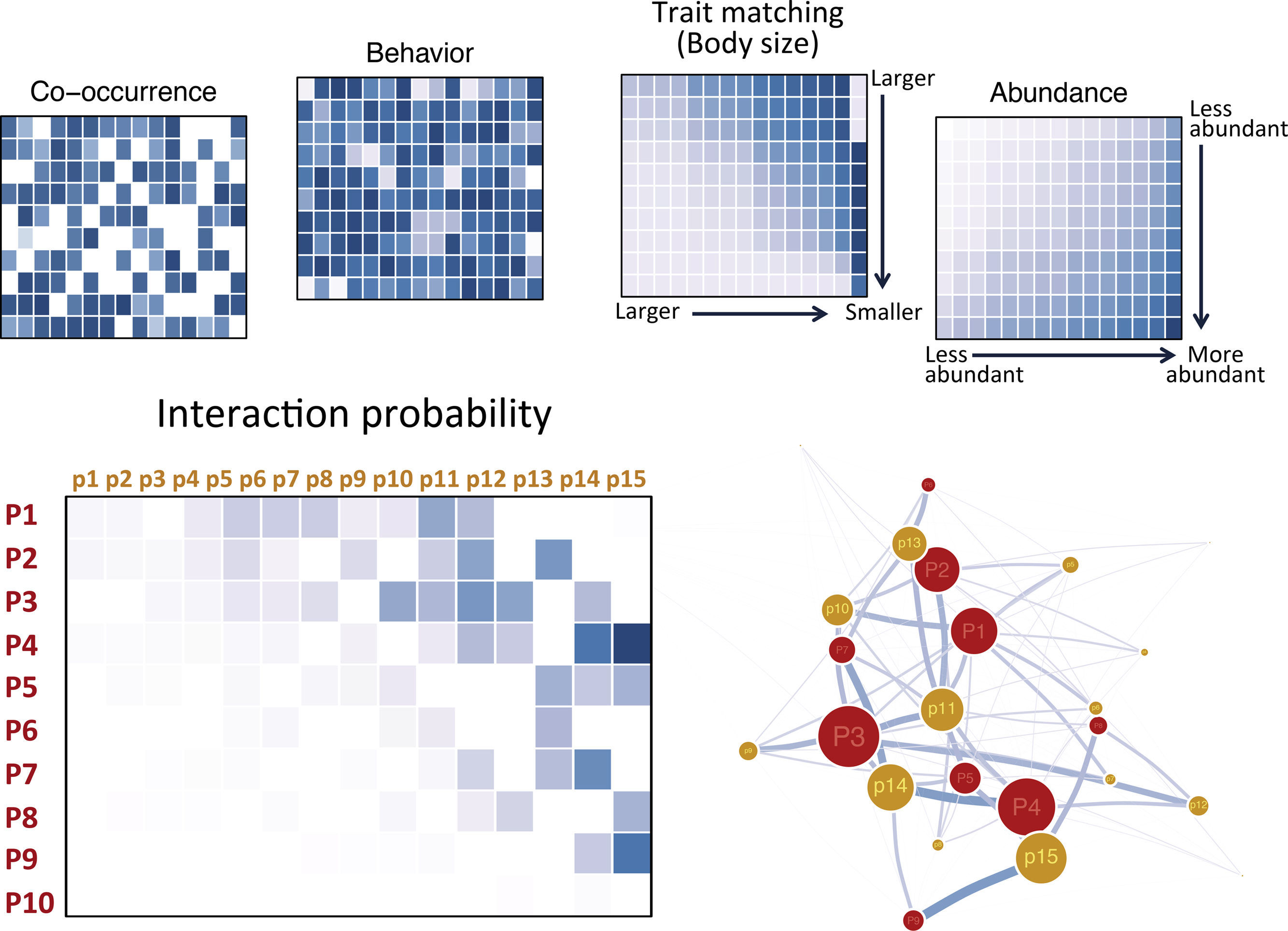
Morales-Castilla et al. (2015) suggest a hierarchical framework for combining these different layers of biological information in a single model and build theoretical ecological networks that encompass the effects of multiple factors (Fig. 2). This approach starts by identifying species unlikely to interact based on basic biological information such as trophic level or guilds. This coarse categorization allows identifying forbidden links (e.g., herbivores are not allowed to feed on carnivores). The next step is to refine the inference on the interactions by establishing the relationship between interaction probability and species traits. Using information on interaction patterns for the species occurring in the target area and assuming interaction sampling was thorough, it is possible to use statistical approaches such as generalized models to test for the effectiveness of certain traits in predicting interactions and estimate the parameters governing the relationship (Eklöf et al., 2013; Rohr et al., 2010; Sebastián-González et al., 2017). Once the important traits are identified, the estimated parameters of the fitted models can be used to compute interaction probabilities (Figs. 1 and 2; Morales-Castilla et al., 2015). Combining information on the traits of the species in the target area and the estimated parameters, interaction probabilities can be computed providing a picture of which are the species likely to interact with the introduced species (Figs. 1 and 2; Box 1).
Building a probabilistic network. Each hypothetical matrix contains information on the probability of pairwise interactions between predator (rows, labeled P#) and prey (columns, labeled p#) species. Darker colors depict higher probabilities. Species are ordered according to decreasing body size from top to bottom and left to right. Interaction probabilities can be estimated according to different variables: co-occurrence, behavioral or natural history information known to affect interactions, trait matching (body–mass ratio is depicted in the example), and relative abundances. Overall interaction probabilities can be obtained from the element-wise product of the probabilities computed according to each variable and a probabilistic network or and ensemble of theoretical networks based on probabilities may be built and analyzed.
The interaction probabilities estimated from different traits can be combined in multidimensional models resulting in joint probabilities, which reflect the combined effects of different independent traits on the likelihood that an interaction occurs (Fig. 2; Eklöf et al., 2013; Williams and Purves, 2011). Further layers depicting habitat use or abundance can be used to weight the estimated probabilities (Fig. 2). Actually, the use of a hierarchical framework allows considering any modifier known to affect interactions, including behavior, natural history information or abiotic characteristics of the environment (Fig. 2). Layers representing mechanisms known to affect the efficiency of interactions, the actual effects of interactions on individual fitness or population dynamics, may also be considered, delivering more functionally meaningful networks (Vidal et al., 2013).
The relationship between interaction probability and species traits can also be analyzed under a Bayesian approach, which uses information on the observed interactions and empirical trait distribution to obtain posterior probability distributions for the parameters (Bartomeus et al., 2016). This approach also allows weighting the trait distribution by species abundances, thus taking into account the neutral component of the interaction. Multiple traits can be considered at the same time by computing the joint probabilities under the assumption that the considered traits are independent (Bartomeus et al., 2016). Even in the absence of information on specific traits governing interactions it might be possible to obtain information on interaction probabilities using information on the patterns of interactions of other species, as done using the framework of latent traits (Rohr et al., 2016) or by using proxies that encompass a combination of traits such as phylogenetic data (Mouquet et al., 2012).
The final outcome of the outlined modeling approaches is an array of pairwise interaction probabilities. The pairwise interaction probabilities can be represented as a matrix or as a weighted network where the weights represent the inferred probabilities (Fig. 2). This network has the desirable property of encompassing the uncertainty on interaction patterns. The interaction probabilities obtained using any of the approaches listed above, allow constructing an ensemble of potential networks, each representing a hypothesis about how species interact with each other in the community. The properties of the whole network and of each species can be analyzed using metrics designed specifically for probabilistic networks (Poisot et al., 2016) or by applying the conventional metrics to the networks constructed from the interaction probabilities. From these interaction networks other networks depicting indirect interactions, such as competition, can also be inferred.
Having inferred networks for the rewilding scenario, it is possible to identify those species that are more likely to be affected by the introduction, e.g., by quantifying the distances separating the species in the food web. Furthermore, using basic descriptors of network structure it is possible to infer what are the most likely responses of the community as a whole to rewilding. Properties such as connectance, the proportion of interactions that actually occur relative to the theoretical maximum, and compartimentalization, are related to the vulnerability of a network to change (Dunne et al., 2002; Stouffer and Bascompte, 2011). Thus, given the straight relationship between network structure and community dynamics, analyzing the structure of the local interaction networks before and after management actions such as rewilding should be a standard procedure.
ChallengesThe main challenge to the implementation of network methods in rewilding projects is data availability. Obtaining information on ecological interactions in the wild is expensive and time consuming (Tylianakis et al., 2010). In the best scenario, interactions among species in the target area will be well known and a model will only be required to fit the candidate species in the network. Nevertheless, in the proposed framework a first approximation on the interaction networks can be obtained even in the absence of data on the local interactions patterns. Using the data already available in the literature, one may be able to infer the relationship between interaction patterns and traits in a different locality and generate potential interaction networks for the species in the area of interest under the assumption that the relationship between traits and interaction occurrence is consistent.
Interaction patterns can vary over time and across space. One way of dealing with this variation is to perform separate analyses on the relationship between interaction probability and species traits for all locations from where good quality information on interaction patterns is available. By conducting separate analyses for different locations one can evaluate the consistency of the relationship between interaction patterns and traits and estimate a range for the parameter values. Different networks could then be generated using a range of parameters to account for such context dependence.
Another potential source of variation in the interaction patterns is intrapopulation variation. Interactions take place at the individual level (Poisot et al., 2015) and the traits of individuals affect how exactly they will be behave in a novel ecological context. Using population averages to predict interactions is reasonable if intrapopulation variation is small or if the sample of individuals to be introduced is not a biased one. Otherwise variation among individuals should also be taken into account. Some of the trait-informed network models discussed above can also incorporate trait information at the individual level when this information is available (Bartomeus et al., 2016).
Any species participate in different types of interactions, playing multiple ecological roles as consumers, resources, hosts, competitors and mutualists. Networks are often constructed based on a single type of interaction, but the ideal scenario would be to generate predictions on multiple interaction types, so as to anticipate the consequences of an introduction for different groups of organisms and different ecological processes. Obtaining information on all the possible interactions of species in a community is unrealistic. Yet, some information on the main interactions is better than none. The studied networks will always include only a subset of the innumerable interactions that species participate. The main assumption shared by any community ecology study is that the subset of studied interactions is representative of the interactions governing the dynamics of the studied system. Network ecologists have recently begun to merge different types of interactions in a single network and study its properties (Pilosof et al., 2017). As novel methods are devised for the analysis of these multilayered networks a more complete assessment of the impacts of the introduction of a new species in the community will be possible.
Concluding remarksThe assessment of the results of species introductions and translocations often focus on population aspects of the target species or on a single or a few pairwise interactions (Svenning et al., 2016). However, to fully understand the impact of such interventions, variables related to the whole community and ecological processes should be monitored over time. In this sense using a network approach to monitor the aftermath of the release may help unraveling broader consequences and the need for further management. The main risk is that the new community with the introduced species have unanticipated emergent properties of its own (Seddon et al., 2014). Although network approaches cannot replace the need of detailed information on demographic trends of species to assess how community functioning was impacted, the use of networks may allow detecting which are the species that should be monitored closely. Moreover the network approach is not intended to replace any other tool already established in conservation practice, but to be used as an ancillary approach that combined with others, may assist conservation planning and monitoring.
Monitoring a rewilding program using networks may also help in adaptive management actions, where management options are reassessed based on results from previous implementation steps to guarantee efficient establishment of the population or reduce impacts (Moehrenschlager et al., 2013). After release the several possible hypothesis on interaction patterns generated by network models can be refined according to observation and the interaction networks can be constantly reevaluated. Depending on the observed changes, the need for interventions, such as resource provisioning or predator control (in the case of exotics) (e.g., Innes et al., 1999), may be anticipated.
Network science can also benefit from the use of network models in rewilding. Rewilding episodes may allow testing theoretical predictions on the relationship between network structure and community dynamics (e.g., Ripple and Beschta, 2012) and guide theoreticians on how to make models more accurate. With each species introduction instance where the changes in networks structure are not evaluated we miss a unique opportunity of learning more about how ecological communities respond to change.
The success of any introduction depend on several factors, such as the number of released individuals (Fischer and Lindenmayer, 2000), variation in habitat quality (Wolf et al., 1998), and human interference (Jule et al., 2008). Yet, a key component in a rewilding program is how biotic interactions affect and are affected by a species introduction. Because biotic interactions can affect the success of introduction in multiple ways, using probabilistic approaches and network models in the different stages of program implementation can offer important insights on community-level responses to rewilding while incorporating uncertainties, thus helping evaluating the balance between the benefits and risks.
When rewilding involves species that are not originally from the target region the risks are potentially greater and less predictable and meticulous assessment of the these risks are necessary (IUCN/SSC, 2013). Although introductions of species outside its range would better be avoided (Ricciardi and Simberloff, 2009) the severity of the ongoing biodiversity crisis asks for proactive measures to maintain functional ecosystems (Svenning et al., 2016). As highlighted by Jepson (2016) rewilding is already happening and we need to employ the right tools to minimize negative impacts and strengthen the desired effects. Being able to foresee how interactions will rewire the food web is critical to predict the success of an introduction and its potential outcomes.
FundingMMP was supported by São Paulo Research Foundation (FAPESP: grant #2013/22016-6) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
I thank Paulo R. Guimarães and Tiago B. Quental for comments and suggestions.