Several studies have found a positive relationship between habitat complexity and species richness. Here, we sampled Arctiinae moths in a savanna-forest gradient to test if (1) structurally complex habitats harbour more species and individuals than simple habitats, (2) composition of Arctiinae moths in forests is dissimilar from the other vegetation types, and (3) due to its strong association with the vegetation the tribe Arctiini will present more consistent results to the first two hypotheses when compared to the tribe Lithosiini. Species richness was higher in more complex vegetation types. Forest and savanna had distinct Arctiinae species composition. These findings were more consistent to Arctiini than to Lithosiini because Arctiini feeds on a great range of plant species whereas Lithosiini has a specialized diet on lichens, algae and bryophytes.
Arctiinae moths are a subfamily of Lepidoptera used as bioindicators as they respond rapidly to environmental changes, are abundant and easy to sample (Kitching et al., 2000). These moths are divided into four tribes, and only Arctiini and Lithosiini occur in the Neotropics (Zahiri et al., 2012). The larvae of many Lithosiini feed on mosses, lichens and algae (Wagner, 2009). The Arctiini feed during the larval stage on a variety of host plants, including herbaceous and woody plants (Wagner, 2009). Morphological and life-history differences among species in the diverse tribes and subtribes of the Arctiinae reflect their choices for distinct host plants (Hilt and Fiedler, 2006). Thus, the variation in plant species structure, diversity and composition is linked to the richness and composition of Arctiinae assemblages among different sites (Hilt and Fiedler, 2006).
Here, we use this group of moths as a model to test three hypotheses. First, Arctiinae species richness and individual density are greater in more structurally complex vegetation sites. As showed by several classic and recent researches, when the structural complexity of the habitat increases, the number of animal species also increases (MacArthur and MacArthur, 1961; Karr and Roth, 1971; Root, 1973; Tews et al., 2004). According to the habitat heterogeneity hypothesis, structurally complex habitats provide a greater variety of microhabitats and microclimatic conditions and more refuges against natural enemies and against unfavourable weather conditions than less-complex habitats (Bazzaz, 1975; Tews et al., 2004), allowing more species to co-exist in a given area. The second hypothesis is related to the differentiation of species composition among different vegetation sites. We expect that Arctiinae species composition in forests is dissimilar to the fauna of savannas because these vegetation types have distinct plant species and microclimates (Oliveira-Filho and Ratter, 2002). The third hypothesis predicts that the Arctiini and Lithosiini tribes will not show the same responses to the two previous questions, since Arctiini are considered true herbivores (feeding mainly on herbaceous, shrubs and trees) and Lithosiini generally feed on lichen, algae and bryophytes (Wagner, 2009). Therefore, we expect that Arctiini should have a stronger response to the variation of the vegetation structure and composition than the Lithosiini since in our study we used the density of woody plants and herbaceous cover as surrogate of habitat complexity and Lithosiini larvae did not feed these plants.
Material and methodsWe conducted the study in Emas National Park, situated in the Brazilian Central Plateau (17°49′–18°28′S, 52°39′–53°10′W). We selected 40 plots of 100m2 each, distributed among four Cerrado biome vegetation types: six plots in grassland, 14 plots in savanna, ten plots in woodland savanna and ten plots in semideciduous forest. This represents a typical savanna-forest gradient where tree density and height increases towards the forests while herbaceous cover and density decreases (Oliveira-Filho and Ratter, 2002).
We collected moths at each plot from dusk to dawn, using a Luiz de Queiroz light trap with a 15-W black light fluorescent light bulb. In each light trap, we attached a plastic bag containing a glass bottle with ammonium hydroxide. The light traps were suspended 1.5m above the ground in the centre of each plot. Sampling was performed in the dry (June–July 2010) and rainy (December 2010–February 2011) seasons. We collected moths during each season in all plots on two nights, totalling four sampling nights (two in the dry and two in the rainy season) in each of the 40 plots. Plots sampled in the same night were the most distant possible to avoid pseudoreplication. Each plot was sampled once at the new moon and once during the waning moon. The plots are at least 100m apart to avoid the sample of individuals from others plots. Arctiinae individuals were sorted into morphospecies and identified them to the lowest taxonomic level possible by comparison with the types at V.O. Becker Collection (Camacan, Brazil) and with the published literature. All individuals were deposited in the Coleção Zoológica da Universidade Federal de Goiás (Goiânia, Brazil).
We used shrub and tree density and herbaceous cover as surrogates of the structural complexity of each plot. In the grassland, savanna and woodland savanna plots, we included all woody plants with a diameter at soil level greater than 3cm, whereas in the semideciduous forest plots, we included all woody plants with a diameter at breast height greater than 3cm. To measure the herbaceous cover we used a 1m2 quadrat subdivided into 100 squares of 10cm×10cm. We conducted two measurements per plot in each season and calculated the mean number of squares filled with herbaceous vegetation.
Quantitative analysisTo demonstrate the structural differences among the four vegetation types we performed two one-way ANOVA. One to compare shrub and tree density and the other to compare the herbaceous cover among the four vegetation types. We used a first-order jack-knife to estimate the total moth richness in the study area and in each vegetation type. We calculated individual-based rarefaction curves for the entire subfamily and for the Arctiini and Lithosiini tribes to compare the richness difference among the four vegetation types.
To test whether moth density was higher in plots with more structurally complex vegetation we performed a Generalised Linear Mixed-effects Model (GLMM). We assumed that there was variation in the environmental conditions that influence the capture of moths (such as moon phase, temperature, humidity, rainfall and wind). Because of the proximity among the collection points (maximum distance <12km) we inferred that all these conditions are homogeneous among the collection points in one night. Thus, we used the collection date as a random factor and used the Poisson distribution, which is recommended for count data or density (Bolker et al., 2009). The GLMM model was composed by: (1) shrub and tree density and herbaceous cover in each plot (continuous, independent variables); (2) moth density (dependent variable); and (3) the sampling date of each plot (random effect). The GLMM was conducted for all species, and separately for the Lithosiini (mostly lichenivorous), and the Arctiini (mostly herbivorous) tribes. The best model for each insect group was selected using Akaike Information Criterion.
We used a non-metric multidimensional scaling (NMDS) based on Bray–Curtis distances to assess differences in moth composition among the plots. We applied a square root transformation to compensate for deviations caused by the low frequency of high individual densities. We assessed the differences in species composition among vegetation types using an Analysis of Similarities (ANOSIM) with 1000 random permutations. Because we performed multiple comparisons, a Bonferroni correction was used to reduce Type I errors. The NMDS were conducted for all species, and separately for the Lithosiini (mostly lichenivorous), and the Arctiini (mostly herbivorous).
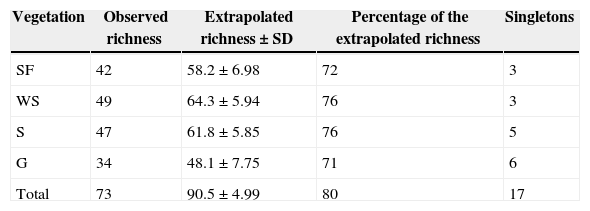
ResultsWe collected 1509 Arctiinae individuals representing 73 species. The estimated richness for the region was 90.5 species (Table 1). Eighteen species were collected in only one plot (Table S1) and 17 species were singletons (Table 1 and Table S1). We found a higher density of shrubs and trees in the woodland savanna plots, followed by semideciduous forest, savanna and grassland plots (Fig. S1a). Grassland and savanna plots had great herbaceous cover (Fig. S1b).
Arctiinae species (Lepidoptera: Erebidae) from an area of the Brazilian Cerrado (Emas National Park). Observed and extrapolated (first-order jack-knife) richness, percentage of the extrapolated richness that was sampled, and the number of singletons per vegetation type and in all plots (Total). SF, semideciduous forest (n=10 plots); WS, woodland savanna (n=10); S, savanna (n=14); G, grassland (n=6).
| Vegetation | Observed richness | Extrapolated richness±SD | Percentage of the extrapolated richness | Singletons |
|---|---|---|---|---|
| SF | 42 | 58.2±6.98 | 72 | 3 |
| WS | 49 | 64.3±5.94 | 76 | 3 |
| S | 47 | 61.8±5.85 | 76 | 5 |
| G | 34 | 48.1±7.75 | 71 | 6 |
| Total | 73 | 90.5±4.99 | 80 | 17 |
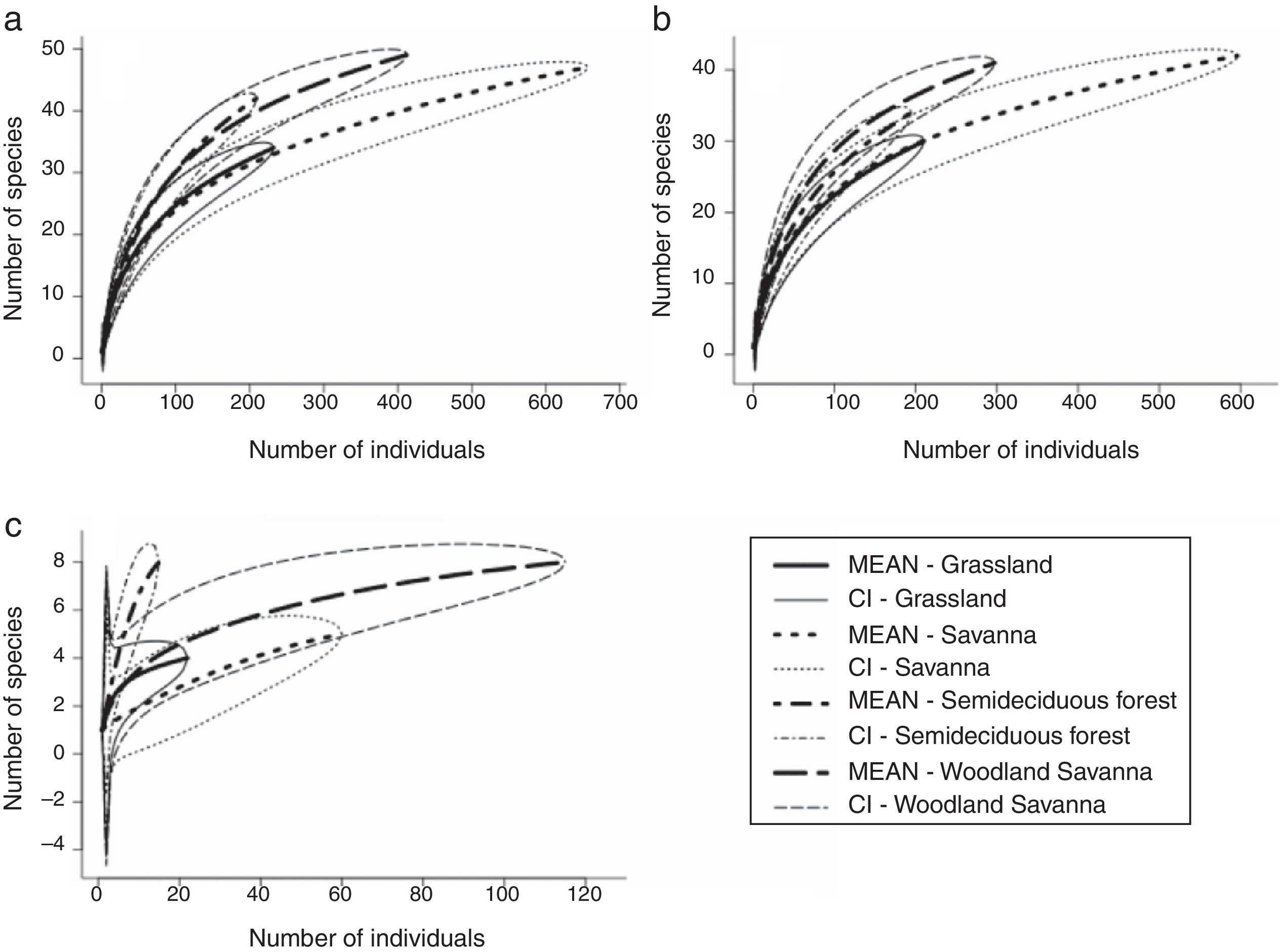
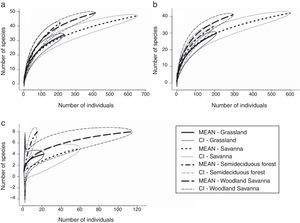
The highest moth species richness was observed in the woodland savanna, followed by savanna, semideciduous forest and grassland plots (Table 1). The same pattern was observed for the extrapolated richness (Table 1). However, when we standardised the sampling effort (by the number of individuals) woodland savanna and semideciduous forest plots were richer in Arctiinae species than savanna and grassland plots (Fig. 1a). The number of Arctiini species was higher in the woodland savanna and semideciduous forest than in the grassland (Fig. 1b), but there were more Lithosiini species in the semideciduous forest than in the woodland savanna, savanna and grassland (Fig. 1c).
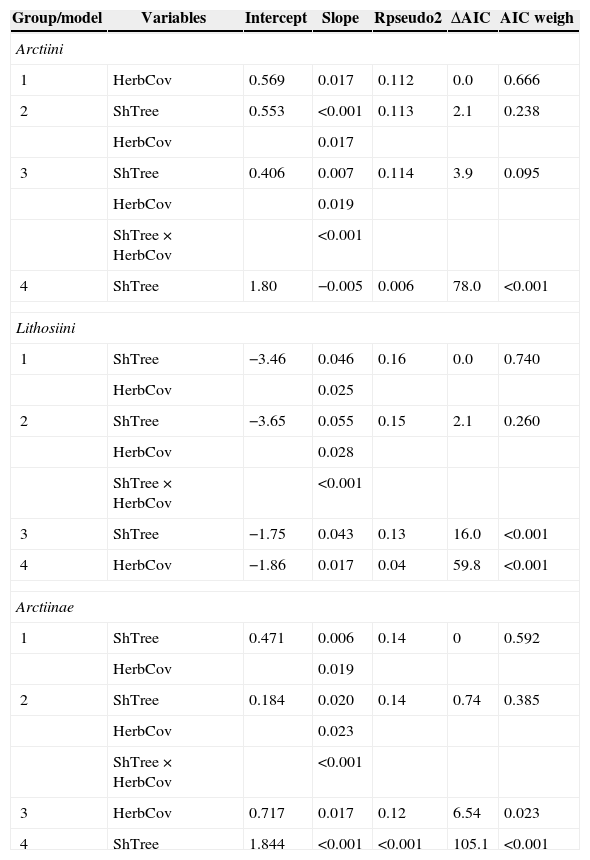
Moth individual density sampled in savanna-like vegetation was higher than in semideciduous forest (F3,36=4.49; p<0.009). Grassland had the highest number of singletons followed by savanna, woodland savanna and semideciduous forest (Table 1). After controlling for the effects of potentially confounding variables we found that both shrub and tree density and herbaceous cover were positively related to moth density (Table 2).
Results of GLMM predicting the response of Arctiini, Lithosiini and Arctiinae individual density to two measures of habitat complexity. ShTree is density of shrubs and trees in each plot; HerbCov is the herbaceous cover in each plot; ShTree×HerbCov is the interaction among the variables. We reported only marginal R2 (Rpseudo2) that represents the variance explained by fixed factors. Models are placed in order of importance according Akaike Information Criterion (from best to worst), for each taxonomic group.
| Group/model | Variables | Intercept | Slope | Rpseudo2 | ΔAIC | AIC weigh |
|---|---|---|---|---|---|---|
| Arctiini | ||||||
| 1 | HerbCov | 0.569 | 0.017 | 0.112 | 0.0 | 0.666 |
| 2 | ShTree | 0.553 | <0.001 | 0.113 | 2.1 | 0.238 |
| HerbCov | 0.017 | |||||
| 3 | ShTree | 0.406 | 0.007 | 0.114 | 3.9 | 0.095 |
| HerbCov | 0.019 | |||||
| ShTree×HerbCov | <0.001 | |||||
| 4 | ShTree | 1.80 | −0.005 | 0.006 | 78.0 | <0.001 |
| Lithosiini | ||||||
| 1 | ShTree | −3.46 | 0.046 | 0.16 | 0.0 | 0.740 |
| HerbCov | 0.025 | |||||
| 2 | ShTree | −3.65 | 0.055 | 0.15 | 2.1 | 0.260 |
| HerbCov | 0.028 | |||||
| ShTree×HerbCov | <0.001 | |||||
| 3 | ShTree | −1.75 | 0.043 | 0.13 | 16.0 | <0.001 |
| 4 | HerbCov | −1.86 | 0.017 | 0.04 | 59.8 | <0.001 |
| Arctiinae | ||||||
| 1 | ShTree | 0.471 | 0.006 | 0.14 | 0 | 0.592 |
| HerbCov | 0.019 | |||||
| 2 | ShTree | 0.184 | 0.020 | 0.14 | 0.74 | 0.385 |
| HerbCov | 0.023 | |||||
| ShTree×HerbCov | <0.001 | |||||
| 3 | HerbCov | 0.717 | 0.017 | 0.12 | 6.54 | 0.023 |
| 4 | ShTree | 1.844 | <0.001 | <0.001 | 105.1 | <0.001 |
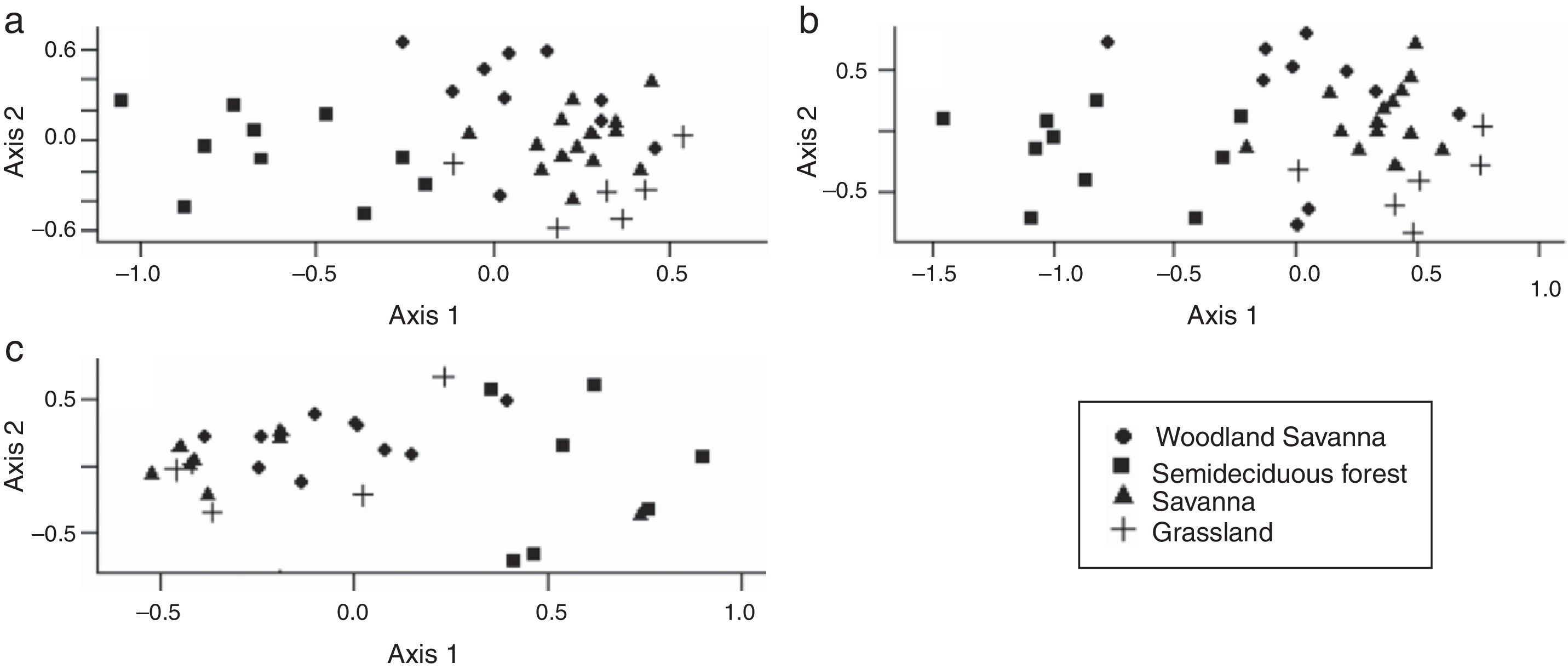
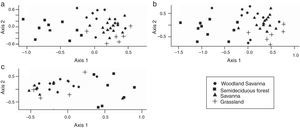
Approximately 25% of the species occurred in all vegetation types, 19% occurred in three and 20% in two types (Table S1). The first two dimensions of the NMDS showed a separation between the Arctiinae fauna of the semideciduous forest and savanna-like vegetation (Fig. 2). Moreover, we observed that the Arctiinae and Arctiini species followed the gradient of decreasing wood plant density (woodland savanna, savanna and grassland; Fig. 2a and b). However, this pattern was not evident for Lithosiini (Fig. 2c and Table S2).
Analysis of the non-metric multidimensional scaling ordination of the Arctiinae fauna (Lepidoptera: Erebidae) sampled in the 40 plots in the Emas National Park, based on Bray–Curtis distances. Stress=20.4 for the Arctiinae, 20.7 for Arctiini and 22.4 for Lithosiini. Arctiinae (a), Arctiini (b) and Lithosiini (c).
More structurally complex vegetation types had more rarefied tiger moth species supporting the habitat heterogeneity hypothesis. However, the same pattern was not recorded for the extrapolated and observed richness. This contrasting result was expected because each richness measure had different metrics. Because the number of plots sampled was higher in the savanna, this vegetation type had higher number of individuals (three times higher than the abundance of the forest) and this contributed to the higher observed and extrapolated richness recorded in savanna than forest. Therefore, we believe the rarefied richness is that best fits to our data because it standardises the number of individuals. In another study in the Cerrado biome, Ribas et al. (2003) found a positive relationship between arboreal ant richness and tree richness and between arboreal ant richness and tree density. A positive relationship between animal species richness and the habitat complexity is the pattern commonly found in the literature (Tews et al., 2004). The mechanism behind the habitat heterogeneity hypothesis is that more structurally complex areas provide more niches, allowing the coexistence of more species (Bazzaz, 1975). Specifically, for Arctiinae adults, the more complex vegetation types provide a greater number of distinct oviposition sites, food sources and shelters against natural enemies and against desiccation from the marked dry season that occurs in the Cerrado.
The semideciduous forest showed a lower individual moth density than the other vegetation types. We can consider that this result may be due to the lower density of shrubs and trees than woodland savanna and the lower herbaceous cover compared to other vegetation types. Other authors also found a lower individual density of these moths in forests formations. Kitching et al. (2000) and Hawes et al. (2009) suggested that the lower abundance found in the forest formations are due to the smaller radius of attraction of light traps in areas with dense vegetation. However, we do not believe that the smaller radius of attraction of light traps in our forest plots is the main factor responsible for the low density of individuals. We suggest that the lower density of Arctiinae individuals in the semideciduous forest might be due to other two reasons. The first is that the area of the semideciduous forest is small and isolated. It is well known in the literature that small areas can accommodate a small number of individuals and that isolation reduces the immigration rate of new individuals (Fahrig, 2003). The second reason relates to sampling bias. Our light traps were suspended 1.5m above the ground in all plots, however, Brehm (2007) noted that the Arctiinae fauna of the canopy are more diverse than the understory Arctiinae fauna. Therefore, the lower individual density of Arctiinae in the semideciduous forest might be due to not catching moths that are active in higher strata of vegetation. However, as other studies with Arctiinae found no difference in abundance between the understory and canopy (Schulze et al., 2001) and as we do not know the canopy fauna at our study area, it is still an opened question whether the trap height biased our findings in the forest.
Moth species composition in the forest was dissimilar from that of other vegetation types. This was expected because these forests have distinct soil types, microclimate and plant species composition when compared to the savanna-like vegetation (Oliveira-Filho and Ratter, 2002). Such dissimilarity was already observed to beetles (Almeida and Louzada, 2009) and mammals (Rodrigues et al., 2002). Moth species composition also differed among the three vegetation types of the savanna-like vegetation (following a tree density gradient) for the Arctiinae and Arctiini, but not for Lithosiini. This contrasting pattern for Lithosiini might be due to the lower individual density sampled and to the life history of this taxon that feeds on algae, lichen and bryophytes during the larval stage (Wagner, 2009). Although most host plants of Arctinii in temperate regions are herbaceous species (Wagner, 2009), most Arctiini host plants records in the Cerrado are woody plants (Diniz et al., 2001). For example, from the 12 Emas National Park tiger moth species (all of the Arctiini tribe) for which we can find records of host plants, only three feed on herbaceous plants (two of which are also recorded on trees). Therefore, a relationship between Arctiini composition and woody plant density is expected. Other studies have also noted the importance of vegetation patterns for the richness and beta diversity of Arctiinae (Ferro and Diniz, 2007; Hawes et al., 2009), as well as other groups of moths (Brown and Gifford, 2002; Hawes et al., 2009). However, it is known that the presence and individual density of Lepidoptera in a particular location depends on other factors such as weather (Brown and Gifford, 2002), fires that occur in Cerrado (Mistry, 1998), and intra- and inter-specific interactions (Chase et al., 2002). These other important variables that can influence the structure of the Arctiinae communities in the Cerrado should be analysed in future studies.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Vanessa Grandolfo, Taynara Nascimento (in memorian), Luiz Rezende, Carolina Caiado, Adriano Jaskulski and Murilo Menezes for help with moth sampling. We also thank Lívia Laureto, Leandro Maracahipes and Edmar Almeida for plant sampling. Carolina Moreno and Luciano Sgarbi received a student fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Marcus Cianciaruso received a productivity grant awarded by CNPq (#306843/2012-9). This research was funded by Site 13 (Parque Nacional das Emas) of the Brazilian Long Term Ecological Research Network (CNPq, 558187/2009-9).