Livestock is promoting the global collapse of mammal populations. The discovery of the best management practices that reconcile conservation with production is urgently needed. We evaluated the effect of cattle grazing on the occupation of three rodent species (Akodon azarae, Oligoryzomys flavescens and Oxymycterus nasutus). We collected habitat covariates and sampled rodents, using live traps and tracking tunnels, in 20 paddocks subjected to different grazing pressures, from two research stations, across four seasons. We applied single-season occupancy modeling to determine whether rodent detection and occupation varied as a function of the covariates describing sampling occasions and grazing intensity. We ran sensitivity analyses to evaluate the effect of the differential sampling effort we applied across research stations. All species had higher detection probabilities during the winter. O. nasutus showed a higher detection probability under tall vegetation. A. azarae reached a higher occupation probability in ungrazed areas, although it also had a low probability of occupation in highly grazed paddocks. O. flavescens occupation seemed constant across the grazing gradient. O. nasutus reached a higher occupation probability in ungrazed areas. Decreasing stocking rates and maintaining ungrazed areas might compose the best management practices for small mammal conservation in the grasslands of Southern Brazil.
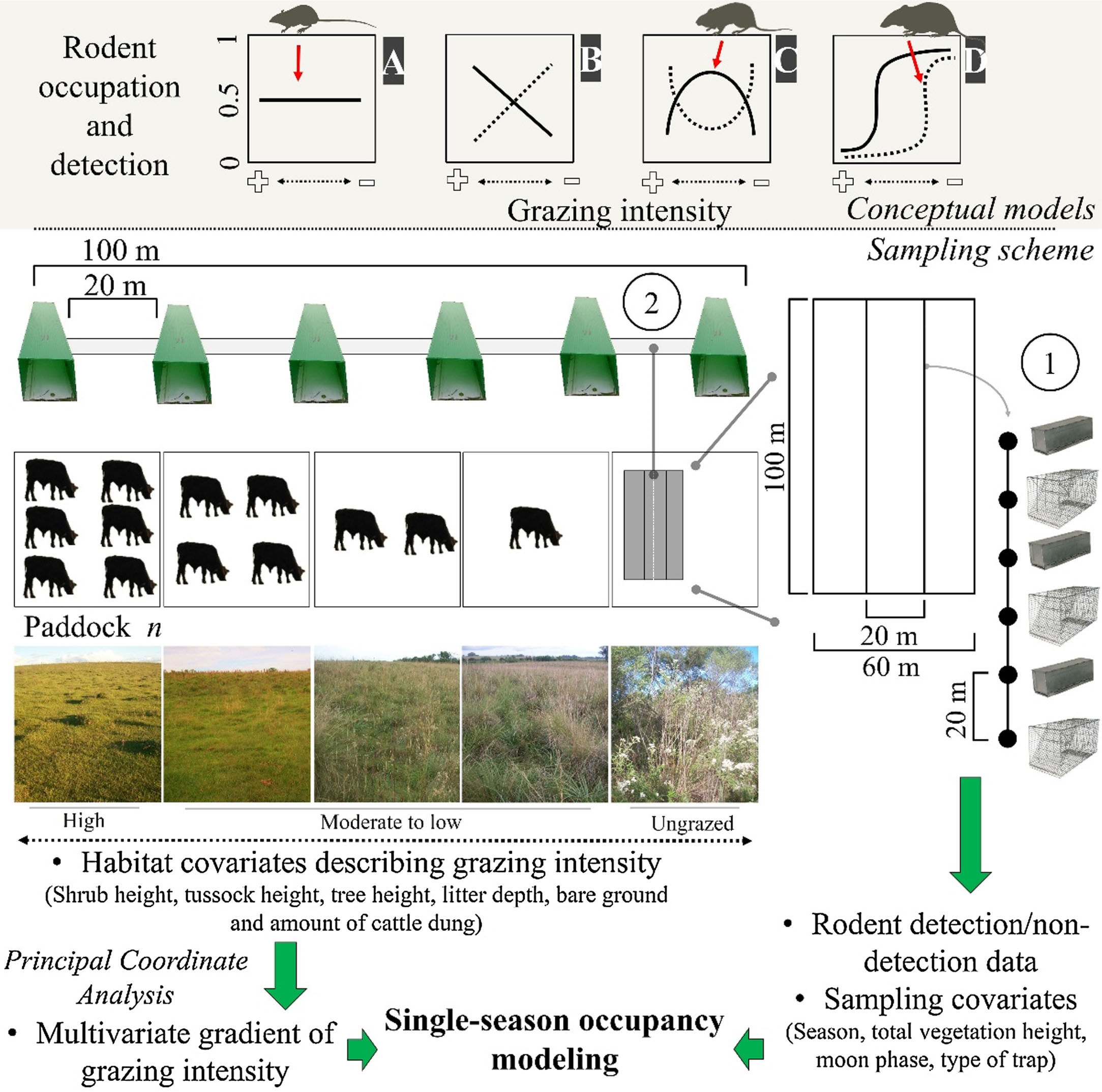
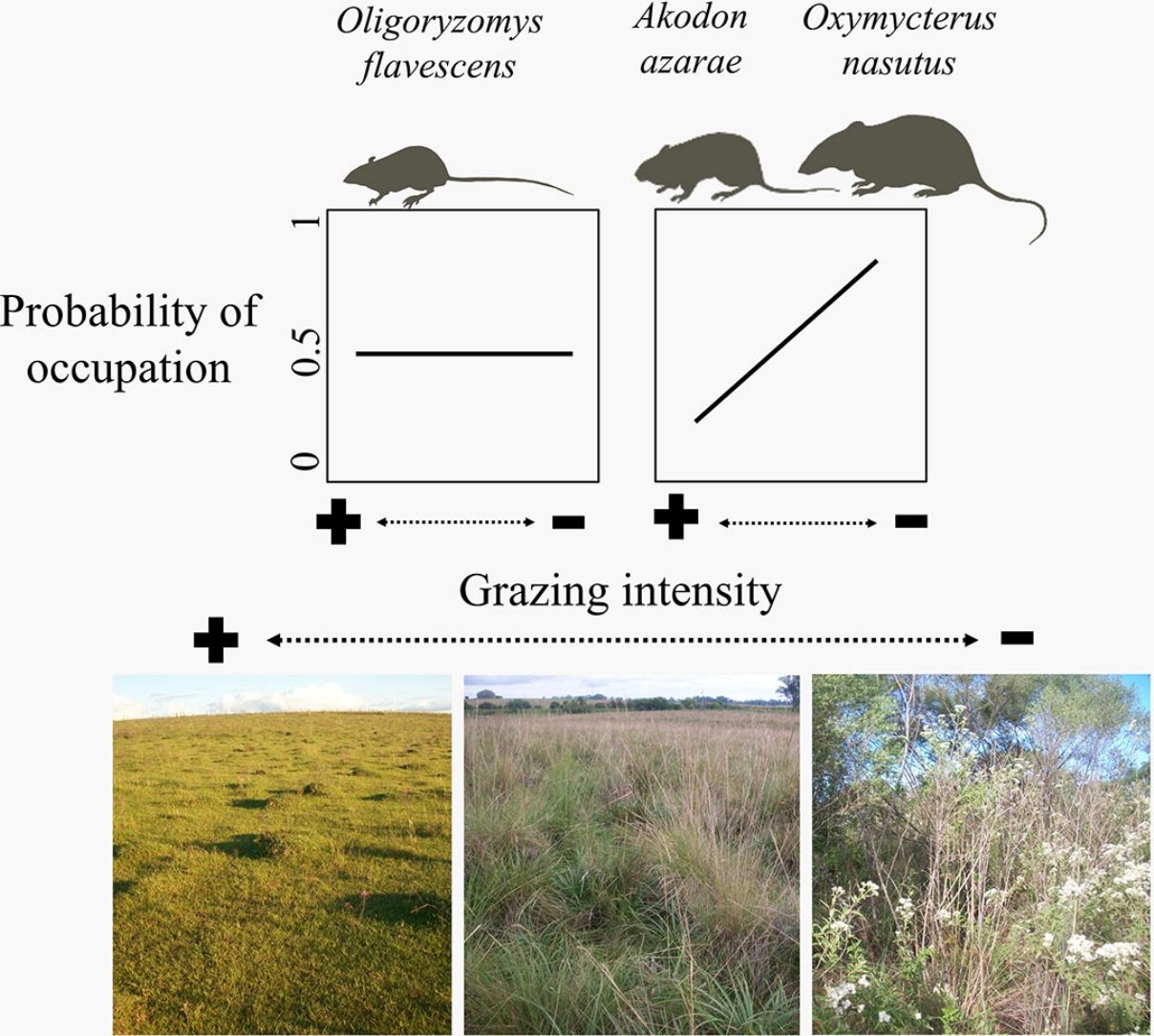
The biomass of humans and their livestock far outweighs the biomass of extant or extinct mammals (Smith et al., 2016). Around 26% of the Earth's surface is dedicated to livestock farming (Ripple et al., 2014; Robinson et al., 2014), resulting in habitat conversion, overgrazing, soil erosion, high water wastage, high disease transmission risk and high emissions of greenhouse gases (Ripple et al., 2014, 2015; Phalan et al., 2016). Alteration of habitats for livestock is promoting the collapse of mammal populations globally (Ripple et al., 2015), and finding systems and practices that reconcile conservation with production are urgently needed (Phalan et al., 2016). Beef production in naturally growing pastures seems more environment-friendly than other alternatives (e.g., feedlots), as the adaptations of grassland plants and animals suggests coevolution with ungulates (Overbeck et al., 2007; Bond and Parr, 2010). This implies that grazing does not affect (Fig. 1A) and may even favor wildlife (Fig. 1B, dotted line). Here, we are concerned with the population-scale processes underlying the negative relationship between livestock grazing and the diversity of mammal communities in the Pampa biome (Pedó et al., 2010; Luza et al., 2016a), which might invalidate the neutral and positive responses of rodents to grazing (Fig. 1A and B, continuous line).
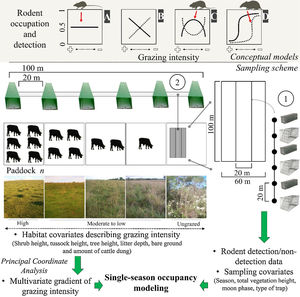
Conceptual models (upper) and sampling scheme (lower) used in the study. Continuous or dotted lines in the conceptual models describe neutral (A), positive/negative linear (B), unimodal/bimodal (C) and logistic (D) relationships between rodent occupation/detection and grazing intensity. We sampled rodents using: (1) live trap grids and (2) tracking tunnel transects. We set grids and transects in ten paddocks subjected to different grazing intensities in each research station. We measured habitat covariates at each trapping point and sampling covariates for each sampling day, and related them to rodent detection using single-season occupancy modeling. Pictures show the variation in habitat structure across the gradient of grazing intensity (Pictures: A. L. Luza).
Grazing by large ungulates can directly (food competition, shelter/nest trampling) or indirectly (vegetation foraging) influence small mammals (Keesing, 1998; Matlack et al., 2001). Ungulate foraging and trampling alters vegetation density, height and cover, as well as the formation and maintenance of litter cover and soil permeability (Matlack et al., 2001; Fox et al., 2003). While overgrazing promotes the growth of disturbance-tolerant plants (rhizomatous and stoloniferous) and the consumption of disturbance-intolerant plants, low/no grazing promotes the accumulation of flammable biomass and the growth of tussocks, shrubs and trees, which cover rhizomatous and stoloniferous plants (Duarte et al., 2006; Overbeck et al., 2007). Grassland habitat structure varies seasonally, because vegetation growth is slower during the winter, which influences cattle density (Nabinger et al., 2009; Trindade et al., 2012) and probably affects the occurrence of rodents (Pedó et al., 2010; Vieira and Paise, 2011). Experimentally manipulating the horizontal (e.g., distance between vegetation patches) and vertical (e.g., height) structure of the vegetation provides a valuable opportunity to evaluate the effect of changes in grassland structure on both beef production and wildlife (Nabinger et al., 2009; Trindade et al., 2012).
Tussock grasses, shrubs and trees assure the high abundance and resilience of small mammal populations in grasslands and grassland-forest ecotones (e.g., Pedó et al., 2010; Luza et al., 2016a). Grazing might exert a negative effect on animal populations when management regimes misuse environmental carrying capacities, potentially subjecting specialist and lightweight species requiring tall/dense vegetation to high mortality and low recruitment rates (Keesing, 1998; Moenting and Morris, 2006). Small mammals requiring tall/dense vegetation are able to colonize a disturbed area that has been abandoned (Fox et al., 2003). In contrast, only opportunist and non-resident species occupy continuously and intensively grazed habitats (Fox et al., 2003). Overgrazing is becoming increasingly common in South Brazilian landscapes, because the government and economy demands the intensification of beef production in grassland remnants, which are becoming smaller and more isolated due to their conversion into crop fields and tree plantations (Carvalho and Batello, 2009; Azpiroz et al., 2012). Thus, an analysis of the occupancy of rodent species with different life histories, in grasslands which are subjected to different grazing intensities, may aid the formulation of the best management practices for the South Brazilian grasslands.
We aimed to evaluate the relationship between cattle grazing and paddock occupancy by three rodent species (Fig. 1). Rodents are conspicuous inhabitants of grasslands and human-modified habitats due to their morphological and behavioral adaptations to diverse environmental conditions (Vieira and Paise, 2011; Sponchiado et al., 2012; Luza et al., 2016a). Studied species consist of the insectivores-omnivores Azara's grass mouse (Akodon azarae [Fischer 1829]) and long-nosed hocicudo (Oxymycterus nasutus [Waterhouse 1837]), and the herbivore-granivore yellow pygmy rice rat (Oligoryzomys flavescens [Waterhouse 1837]). To evaluate rodent occupancy we took the possibility of imperfect detection into account, because a species can be undetected even when occupying a given site (MacKenzie et al., 2002; Guillera-Arroita, 2017).
We expected that the scansorial and bipedal O. flavescens would not be influenced by the grazing gradient (Fig. 1A), because the species is extremely agile and able to exploit exposed habitats (Taraborelli et al., 2003). Conversely, we expected that the semi-fossorial O. nasutus should have both the highest detection in tall grasslands and the narrowest distribution across the grazing gradient (Fig. 1D – dotted line), because it is the less-vagile and has the largest body of the studied species. We expected unimodal detection and occupation probabilities for the cursorial A. azarae (Fig. 1C – continuous line), with its activity concentrated in areas of moderate grazing intensity due to both the high availability of green leaves and insects and its small body size which allows it to move under a thin litter layer (Bilenca and Kravetz, 1998).
Material and methodsStudy areasWe conducted the study from March 2016 to February 2017 on 16 grazed and 4 ungrazed paddocks located in two research stations within the Pampa biome in South Brazil. In Bagé, the eight grazed and two ungrazed paddocks were located in an experimental grassland (31.301170°S, 53.950588°W) of ≈300ha belonging to the Brazilian Agricultural Research Corporation (EMBRAPA). In Eldorado do Sul, the eight grazed and two ungrazed paddocks were located in an experimental grassland (30.103136°S, 51.684382°W) of ≈80ha belonging to the Universidade Federal do Rio Grande do Sul (EEA). As the use of high stocking rates (i.e., keeping a high density of cattle within an area) is a widespread practice on South Brazilian farms (Carvalho and Batello, 2009), we chose these stations because they included the few sites where grazing manipulation has allowed the development of vegetation patches under several grazing levels, including ungrazed areas. Mean temperature throughout the study was 17.93±5.83°C, with a mean precipitation of 0.85±5.65mm/day (Fig. S1.1).
Study speciesThe studied species predominantly occur in open-habitats and are among the most abundant species in the South Brazilian grasslands (Pedó et al., 2010; Vieira and Paise, 2011; Sponchiado et al., 2012; Luza et al., 2016a). A. azarae (mean±sd adult weight of 30.16±9.02g., according to our data) is a cursorial, opportunist and competitively aggressive species that occurs mainly in grasslands but uses crop fields when they provide high resource availability (Bilenca and Kravetz, 1998; Bilenca et al., 2007; Fraschina et al., 2009). O. flavescens (18.90±6.48g) is able to exploit low-cover habitats due to its ability to efficiently flee from predators by suddenly changing its direction (Taraborelli et al., 2003). O. nasutus (61.79±16.72g) is the largest of the study species and seems the most sensitive to changes in habitat structure, since it notably avoids habitats with low vegetation cover (Pedó et al., 2010; Luza et al., 2016a). Other species that could be detected in the study areas include Reithrodon typicus, Ctenomys torquatus, Calomys laucha, Cavia aperea and Scapteromys aquaticus.
Non-volant small mammal samplingA trapping campaign was conducted in each season (from autumn 2016 to summer 2017, Fig. S1.1) at both research stations. At each station we placed ten grids of Sherman (25×8×9cm) and Tomahawk (47×17.5×15cm) traps in the center of ten paddocks subjected to different grazing intensities (Fig. 1; further details in Appendix S1). Grids were distanced at least 100m from each other and covered ≈80ha in EMBRAPA and ≈100ha in EEA. Each grid had 12 Sherman and 12 Tomahawk traps alternatingly placed at 6 points spaced 20m apart along four transects (Fig. 1). Traps were left for five days in each paddock, which was considered sufficient for rapid population assessments (Fraschina et al., 2009; Vieira and Paise, 2011). Traps were revisited twice a day (morning and afternoon) to capture diurnal individuals and to avoid potential rodent mortality due to adverse weather conditions and predation. Total live-trapping effort was 4800 trap/nights in EMBRAPA and 1200 trap/nights in EEA (only one live-trapping campaign, see below); net live-trapping effort (i.e., discounting the unavailable traps) was 4967 trap/nights.
We placed one transect of tracking tunnels (50×10×10cm) within each grid (Fig. 1). We placed tunnels 20m apart and monitored them across two nights per season. We reset the paper sheet and the ink of the tracking tunnels if rainfall occurred during the first night of sampling. Total tracking tunnel effort was 480 tunnel/nights. Since the autumn live-trapping campaign in EEA yielded no captures, any further live-trapping effort would unlikely have resulted in enough captures to make a difference in the models. Therefore, we only used tunnels to monitor paddocks in this site.
We took morphological measurements and ear tissue samples of all captured individuals and marked them with ear tags (∼7mm). To acquire a reference footprint collection (Palma and Gurgel-Goncalves, 2007), we placed live-trapped individuals in a box with a paper sheet and ink to collect their footprints. We released trapped individuals at the point of capture. We baited traps with strong-smelling bait composed of bananas, peanuts, sardines, cod-liver oil, vanilla essence and corn meal. We identified footprints through geometric morphometrics (Palma and Gurgel-Goncalves, 2007). Finally, we measured habitat covariates at each trap/tunnel point to characterize the vegetation of the paddocks (Figure 1; Tables S1.1 and S1.2). Permanova and Betadisper analyses of habitat covariates revealed between-paddock differences in habitat structure (Appendix S1), where highly grazed and ungrazed paddocks composed the two extremes of the grazing gradient (Fig. S1.2; Table S1.3).
Data analysisWe examined the relationship between rodent occupancy and grazing intensity using hierarchical single-season occupancy modeling (MacKenzie et al., 2002). Hierarchical models involve Bernoulli regressions to model the probability of site occupation (ψ) and species detection (p); ψ is the expected occupation state value of the site z after accounting for p. The probability that a site is occupied by a species is ψ, and the probability that it is unoccupied by the species is 1−ψ. When a species is not occupying a site the species cannot be detected (p=0), but when a species is present at a site the species is detected with probability p>0. Some species are imperfectly detected due to differential trap type, abiotic conditions such as the season, and biologic aspects that alter the species activity, for example predator avoidance (MacKenzie et al., 2002; Guillera-Arroita, 2017).
We obtained the final detection data by combining the data obtained from the sequence of five days of live trapping per season and adding the data from the one additional day of tunnel tracking (the day in which we removed the tunnels). Overall, matrices for each rodent species contained 20 paddocks and 24 sampling occasions (days) in EMBRAPA (20 days [all seasons] plus 4 tunnel tracking days [all seasons]), and 9 sampling occasions in EEA (five live trapping days [only autumn] plus four tunnel tracking days [all seasons]). We accommodated the lack of live-trapping sampling in EEA by setting the detections to NA (MacKenzie et al., 2002). We used single-season occupancy modeling as the assumptions of site-closure to colonization and independence among sites seemed plausible for our single-year sampling (MacKenzie et al., 2003). We checked the sensitivity of the results using the complete dataset, which contained data obtained from sites sampled with different sampling efforts, by analyzing the 24 sampling occasions of the EMBRAPA site (Appendix S2).
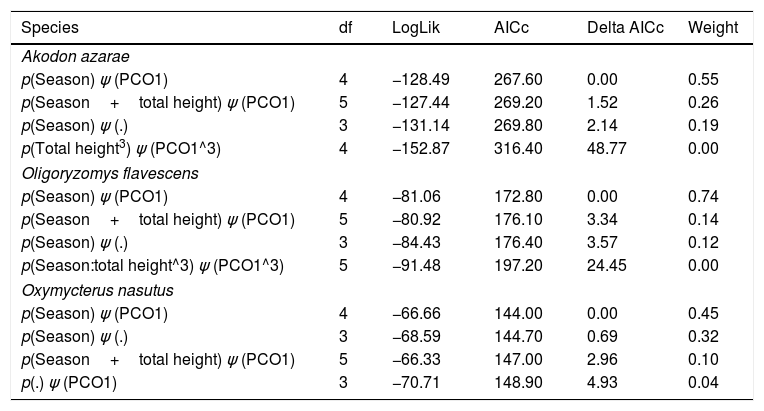
We constructed an a priori set of candidate models to evaluate whether grazing intensity affects the occupation probability (Table S1.4). The models also composed concurrent hypotheses to assess whether season, total vegetation height (including linear, quadratic and cubic terms to represent the conceptual models of Fig. 1), the interaction and additive effect of season and total vegetation height, trap type (the use of live-traps or tracking tunnels) and the moon phase influences the probability of detection. Our data did not allow the inclusion of a site effect in the models because the low number of species records in the EEA site caused perfect model separation. The sample size of 20 paddocks prevented the testing of several interaction terms. To avoid wasting degrees of freedom when estimating the parameters for categorical covariates, we used the paddock centroid extracted from the Principal Coordinate Analysis as the occupation covariate in the candidate models (Appendices S1 and S2). Paddock centroid represents the point that minimizes the among-season differences in habitat characteristics within a given paddock (Anderson, 2006), and is a good representation of the between-paddock variation in grazing intensity.
We used the Akaike Information Criteria, corrected for small sample sizes (AICc), to select the models that presented the most detection and occupation information while using the fewest parameters (Burnham and Anderson, 2002). AICc weights (w) indicate the empirical support for each model, relative to others in the candidate set. We considered that models with Delta AICc>4 units had low support (Burnham and Anderson, 2002). We estimated model parameters using maximum likelihood, and the goodness-of-fit of the best models was estimated through parametric bootstrapping (Fiske and Chandler, 2011). The same tests were used in the sensitivity analyses (Appendix S2). Analyses were conducted using functions implemented in ‘vegan’, ‘MuMIn’ and ‘unmarked’ R packages (R Core Team, 2017).
ResultsA net effort of 4967 trap/nights resulted in the capture of 88 individuals of the three study species. A net effort of 480 tunnel/nights yielded 57 detections of the three study species (Table S1.5). A. azarae, O. flavescens and O. nasutus were detected in both research stations, although in EEA they were only found in ungrazed areas (Table S1.5). We detected A. azarae and O. nasutus in all seasons, while O. flavescens was not found in the spring. We recorded 55 recaptures of 30 different individuals (Table S1.5); most recaptures occurred within the same season (n=52), with only three recaptures taking place in different seasons. Twenty-five recaptures occurred in the same trap that caught the individual the first time, 20 occurred within 20m of the trap responsible for their first capture and 16 occurred within 40m. Only four recaptures occurred in traps 60m away from the trap that caught the individual for the first time. We recorded only one movement between grids (one A. azarae individual moving more than 150m between traps), which should not influence the results because it was not a new detection of the species. We recorded 13 afternoon captures during winter, one in autumn, one in spring and one in the summer.
Rodent detection and paddock occupationParametric bootstrapping showed that all of the best-ranked models fit the data well. Either season or season and total vegetation height were the detection covariates used in the best-ranked models for the three rodents (Table 1; complete results in Tables S1.6, S1.7 and S1.8). Detection of the three species was at least three times higher in the winter than in the non-winter months (Tables 2, S2.3, S2.5 and S2.7). Using EMBRAPA data, we found that season explained the detection of A. azarae and O. flavescens (Tables S2.2 and S2.4). Season and total vegetation height were the detection covariates used in the best-ranked models of O. nasutus (Table 1 and S2.6). The highest detection probabilities of O. nasutus were found in the winter months and under tall vegetation (Table 2; Table S2.7).
Model-selection table with candidate models ranked according to their AICc. p=detection probability; ψ=occupation probability. PCO1=gradient of grazing intensity (Fig. S1.2).
| Species | df | LogLik | AICc | Delta AICc | Weight |
|---|---|---|---|---|---|
| Akodon azarae | |||||
| p(Season) ψ (PCO1) | 4 | −128.49 | 267.60 | 0.00 | 0.55 |
| p(Season+total height) ψ (PCO1) | 5 | −127.44 | 269.20 | 1.52 | 0.26 |
| p(Season) ψ (.) | 3 | −131.14 | 269.80 | 2.14 | 0.19 |
| p(Total height3) ψ (PCO1^3) | 4 | −152.87 | 316.40 | 48.77 | 0.00 |
| Oligoryzomys flavescens | |||||
| p(Season) ψ (PCO1) | 4 | −81.06 | 172.80 | 0.00 | 0.74 |
| p(Season+total height) ψ (PCO1) | 5 | −80.92 | 176.10 | 3.34 | 0.14 |
| p(Season) ψ (.) | 3 | −84.43 | 176.40 | 3.57 | 0.12 |
| p(Season:total height^3) ψ (PCO1^3) | 5 | −91.48 | 197.20 | 24.45 | 0.00 |
| Oxymycterus nasutus | |||||
| p(Season) ψ (PCO1) | 4 | −66.66 | 144.00 | 0.00 | 0.45 |
| p(Season) ψ (.) | 3 | −68.59 | 144.70 | 0.69 | 0.32 |
| p(Season+total height) ψ (PCO1) | 5 | −66.33 | 147.00 | 2.96 | 0.10 |
| p(.) ψ (PCO1) | 3 | −70.71 | 148.90 | 4.93 | 0.04 |
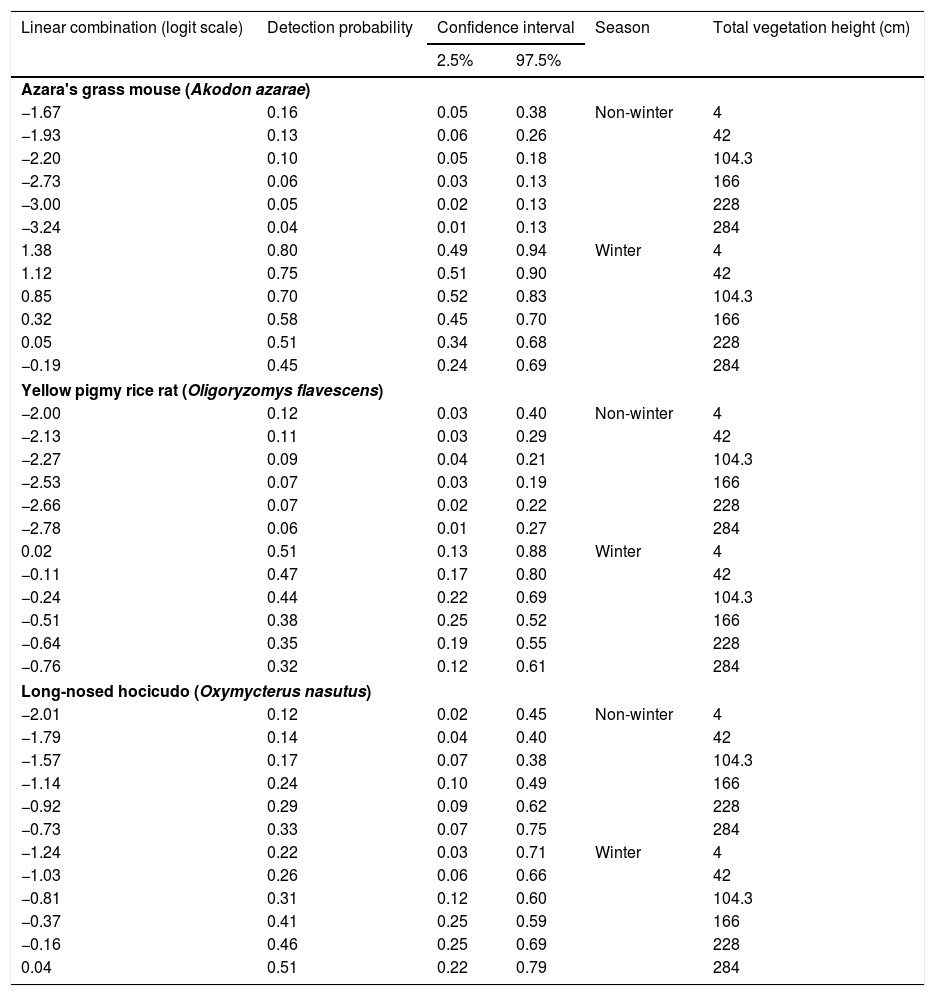
Estimates of detection probability given as a function of the sampling occasion covariates.
| Linear combination (logit scale) | Detection probability | Confidence interval | Season | Total vegetation height (cm) | |
|---|---|---|---|---|---|
| 2.5% | 97.5% | ||||
| Azara's grass mouse (Akodon azarae) | |||||
| −1.67 | 0.16 | 0.05 | 0.38 | Non-winter | 4 |
| −1.93 | 0.13 | 0.06 | 0.26 | 42 | |
| −2.20 | 0.10 | 0.05 | 0.18 | 104.3 | |
| −2.73 | 0.06 | 0.03 | 0.13 | 166 | |
| −3.00 | 0.05 | 0.02 | 0.13 | 228 | |
| −3.24 | 0.04 | 0.01 | 0.13 | 284 | |
| 1.38 | 0.80 | 0.49 | 0.94 | Winter | 4 |
| 1.12 | 0.75 | 0.51 | 0.90 | 42 | |
| 0.85 | 0.70 | 0.52 | 0.83 | 104.3 | |
| 0.32 | 0.58 | 0.45 | 0.70 | 166 | |
| 0.05 | 0.51 | 0.34 | 0.68 | 228 | |
| −0.19 | 0.45 | 0.24 | 0.69 | 284 | |
| Yellow pigmy rice rat (Oligoryzomys flavescens) | |||||
| −2.00 | 0.12 | 0.03 | 0.40 | Non-winter | 4 |
| −2.13 | 0.11 | 0.03 | 0.29 | 42 | |
| −2.27 | 0.09 | 0.04 | 0.21 | 104.3 | |
| −2.53 | 0.07 | 0.03 | 0.19 | 166 | |
| −2.66 | 0.07 | 0.02 | 0.22 | 228 | |
| −2.78 | 0.06 | 0.01 | 0.27 | 284 | |
| 0.02 | 0.51 | 0.13 | 0.88 | Winter | 4 |
| −0.11 | 0.47 | 0.17 | 0.80 | 42 | |
| −0.24 | 0.44 | 0.22 | 0.69 | 104.3 | |
| −0.51 | 0.38 | 0.25 | 0.52 | 166 | |
| −0.64 | 0.35 | 0.19 | 0.55 | 228 | |
| −0.76 | 0.32 | 0.12 | 0.61 | 284 | |
| Long-nosed hocicudo (Oxymycterus nasutus) | |||||
| −2.01 | 0.12 | 0.02 | 0.45 | Non-winter | 4 |
| −1.79 | 0.14 | 0.04 | 0.40 | 42 | |
| −1.57 | 0.17 | 0.07 | 0.38 | 104.3 | |
| −1.14 | 0.24 | 0.10 | 0.49 | 166 | |
| −0.92 | 0.29 | 0.09 | 0.62 | 228 | |
| −0.73 | 0.33 | 0.07 | 0.75 | 284 | |
| −1.24 | 0.22 | 0.03 | 0.71 | Winter | 4 |
| −1.03 | 0.26 | 0.06 | 0.66 | 42 | |
| −0.81 | 0.31 | 0.12 | 0.60 | 104.3 | |
| −0.37 | 0.41 | 0.25 | 0.59 | 166 | |
| −0.16 | 0.46 | 0.25 | 0.69 | 228 | |
| 0.04 | 0.51 | 0.22 | 0.79 | 284 | |
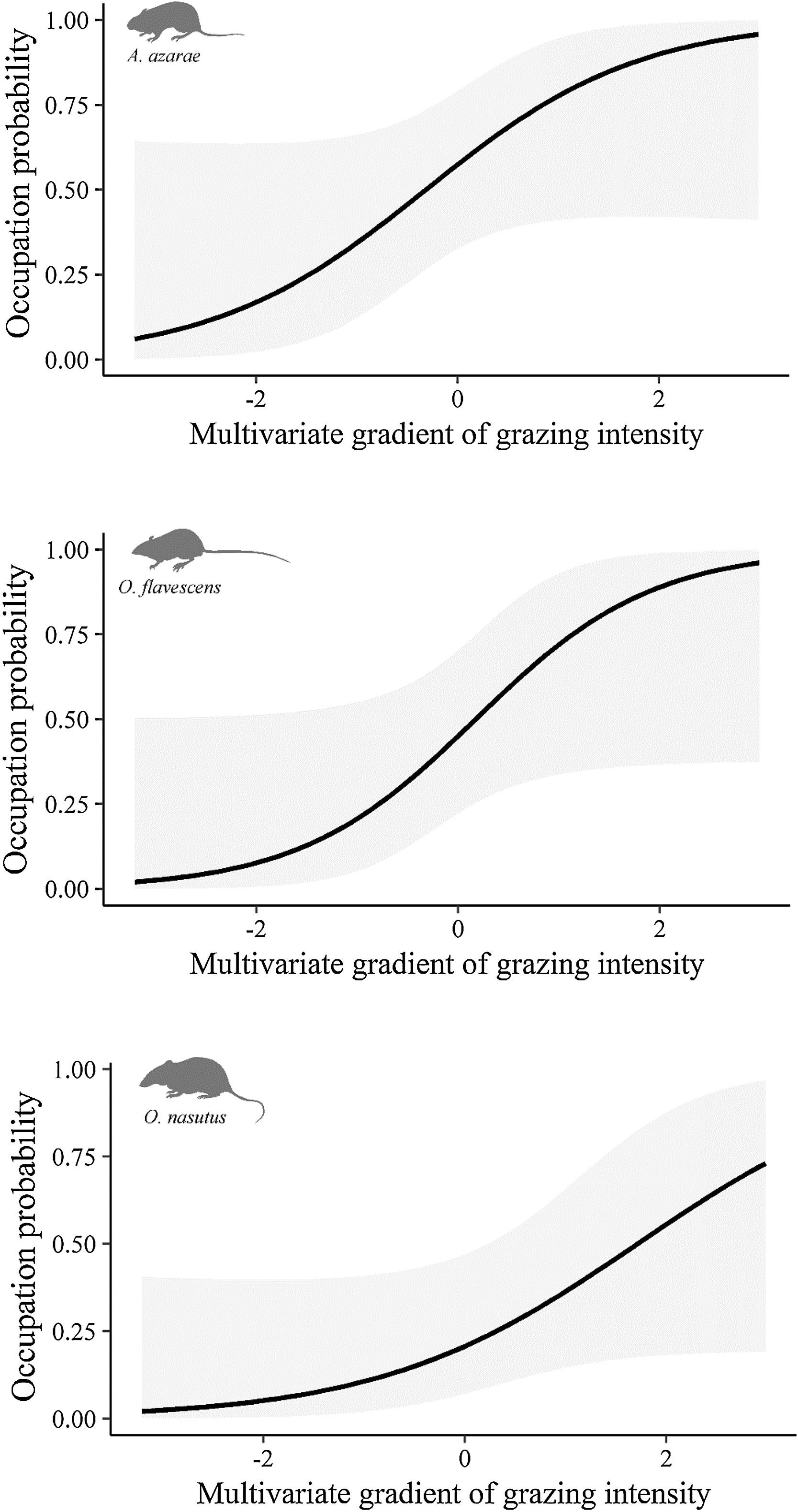
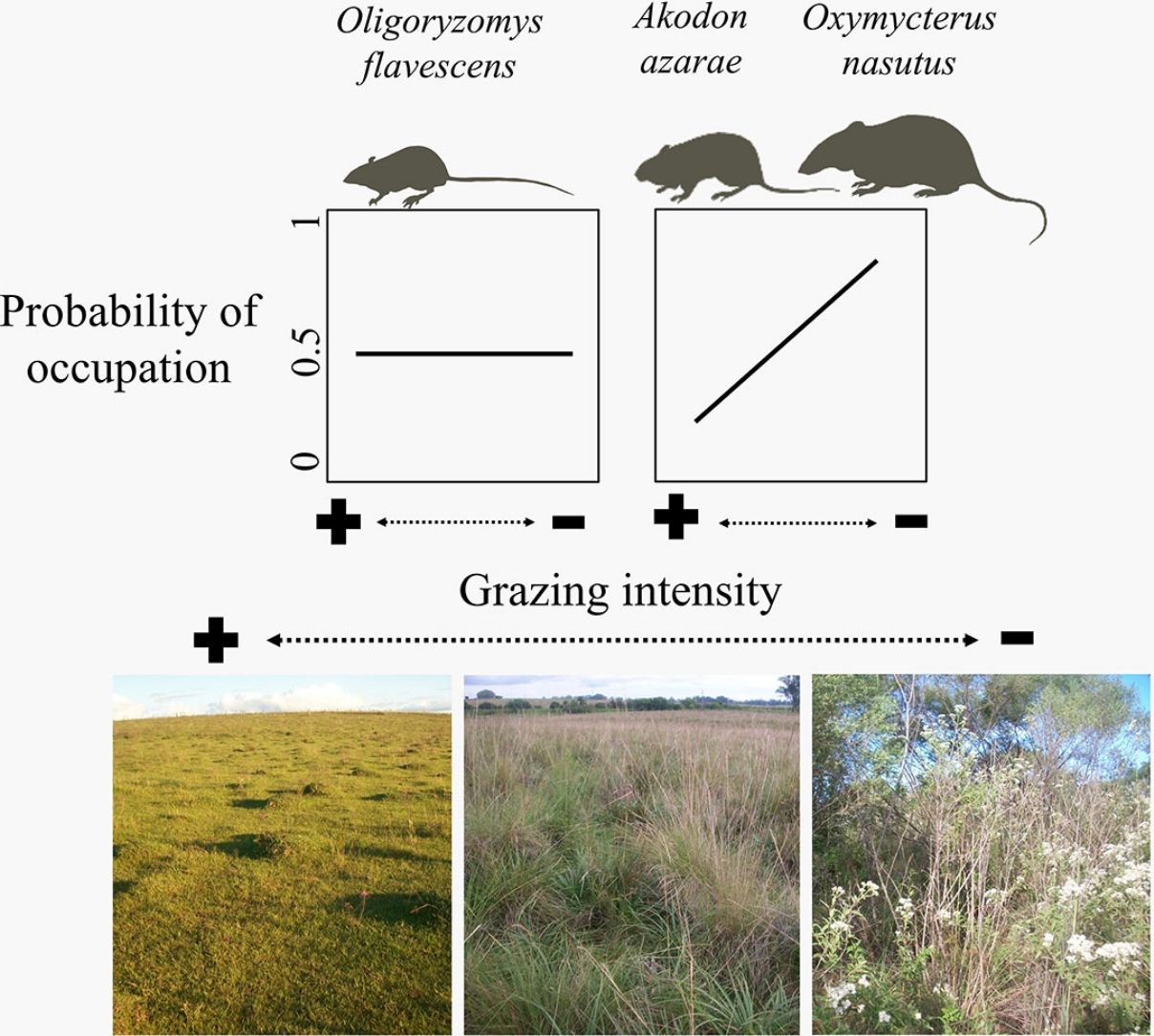
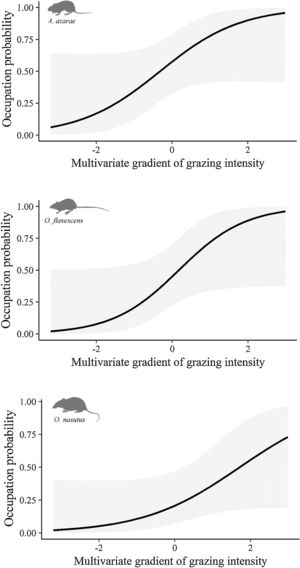
The gradient of grazing intensity (paddock centroid, Fig. S1.2) was included among the most plausible models for the three species (Table 1). Although the occupation estimates had a low precision, the model predictions showed a negative relationship between the probability of paddock occupation and grazing intensity (Fig. 2). A. azarae had the broadest distribution across the grazing intensity gradient, with a probability of occupancy of 0.05 in intensively grazed grasslands; using only the EMBRAPA data, the probability that the species occupied moderately grazed paddocks was 0.55. O. flavescens had both a subtly narrower distribution than A. azarae did, and it had occupation probabilities greater than 0.5 in paddocks subjected to moderate-low grazing intensities (Fig. 2); using the EMBRAPA data, the probability of occupation of O. flavescens was constant across the grazing gradient (Table S2.4). O. nasutus showed the narrowest distribution across the grazing gradient, with its highest occupation occurring in ungrazed areas; this result was consistent using both the complete and the EMBRAPA datasets (Tables S1.8 and S2.6).
Occupation probability as a function of the gradient of grazing intensity. Values of the multivariate gradient of grazing intensity were extracted from Axis 1 of the Principal Coordinate Analysis (Fig. S1.2). The lowest negative values indicate the highest grazing intensities, whereas the highest positive values indicate the absence of grazing.
Livestock contributes to the food security of almost a billion people, but its effects on wildlife and world climate deserves attention as cattle biomass and the amount of land dedicated to livestock farming exceeds any other land use (Ripple et al., 2014; Robinson et al., 2014; Phalan et al., 2016). We evaluated rodent detection and occupation in grasslands hosting species thought to be resilient to grazing (Overbeck et al., 2007; Bond and Parr, 2010). Since we used different sampling procedures and efforts, we used both hierarchical occupancy modeling and sensitivity analyses to estimate rodent occupation. Overall, rodent detection was influenced by season and total vegetation height, whereas rodent occupation was negatively influenced by the increased intensity of cattle grazing.
Grassland structure varies seasonally (Trindade et al., 2012), influencing cattle management and rodent detectability. Grassland managers generally decrease the stocking rates in winter because vegetation grows slowly due to the lower photoperiod and colder weather; thus, the production of seeds and green leaves decreases considerably (Trindade et al., 2012; Nabinger et al., 2009). To meet their minimum energetic requirements rodents overlap and shorten their activity periods during winter, in response to food shortage, low photoperiod and cold nights, heavy and constant rain, and low activity of predators (Fraschina et al., 2009; Vieira and Paise, 2011; Maestri and Marinho, 2014). These factors may increase bait attractiveness and detectability in the winter (Vieira and Paise, 2011). Correspondingly, the probability of detection of the three rodent species was higher in the winter. O. nasutus, the less vagile of the species, had a higher probability of detection during winter months in tall grasslands. The detection probability of A. azarae and O. flavescens was more dependent on season than on vegetation height. Thus, decreasing stocking rates in the winter may be a good management practice since rodents are subjected to low food availability and high climatic stresses.
Ungulate foraging and trampling influence rodent habitats by altering the vegetation density, height and cover, as well as the formation and maintenance of litter cover and soil permeability (Matlack et al., 2001; Fox et al., 2003). Species not influenced by cattle grazing prosper in human-modified landscapes, while intolerant and less-vagile species may be restricted to fewer sites with a low or absent grazing intensity (Medan et al., 2011; Azpiroz et al., 2012; Luza et al., 2014). Occupancy models showed that cattle grazing did not influence the occupation of O. flavescens, which is due to the ability of this bipedal species to exploit habitats with different degrees of vegetation cover (Taraborelli et al., 2003; Luza et al., 2016a). In contrast, occupancy models showed that the probability of occupation of A. azarae and O. nasutus increased with decreasing grazing intensity. Paddocks with dense and heterogeneous vegetation, composed of tussock grasses, tall shrubs and scattered trees, ensure safe foraging for plant leaves, seeds and invertebrates below and inside the layers of dense vegetation (Moenting and Morris, 2006). Although A. azarae is considered an opportunistic species (Bilenca et al., 2007), its preferred conditions and resources are found in ungrazed grasslands. O. nasutus had the narrowest distribution along the grazing gradient and was the species most sensitive to grazing. This could be due to its large body size, low vagility and semi-fossorial nature (Pedó et al., 2010; Luza et al., 2016a). Ungrazed areas might provide favorable habitats and support larger rodent populations (Keesing, 1998). For example, Keesing (1998) found that ungrazed savannas harbored a herbivorous rodent with a density twice that of grazed areas. Thus, populations of many species that are in need of conservation rely on ungrazed areas for reproduction and shelter (Pedó et al., 2010; Azpiroz et al., 2012; Luza et al., 2016b).
The differences we found in the sensitivity analyses by comparing the complete dataset (both EMBRAPA and EEA sites) with the EMBRAPA dataset of O. flavescens can be explained by the species occurrence only in the ungrazed areas of EEA. The failure to obtain any detection of the studied species in the grazed areas of EEA might be due to factors such as the high degree of grassland conversion into tree plantations and crop fields in the surrounding landscape. This is a more probable explanation than the difference in species detections being due to differential sampling techniques and effort, because we did not (1) record more detections in autumn when we used both tracking tunnels and live-traps, (2) record more detections in tunnels when obtaining a higher live-trapping success in EMBRAPA (Table S1.5) and (3) find that the model considering the effect of trap type was among the best-ranked models for the three species (see Results). Despite the high uncertainty in the estimates of occupation probability, resulting from the low number of species detections, we showed that the occupation of A. azarae and O. nasutus increased with a decreasing grazing intensity.
Our results support the idea that the main problem related to livestock is overgrazing, which occurs when too many cattle continuously graze in the same area. Increasing the number of cows in an area is erroneously used in South Brazilian grasslands to increase beef production (Carvalho and Batello, 2009), which has a negative effect on rodents that are sensitive to changes in habitat structure. We advocate for spare ungrazed areas, with the simultaneous decrease of the stocking rate to avoid overgrazing in grasslands used for beef production. We determine that this constitutes the best management strategy for conserving large rodent populations in the grassy landscapes of South Brazil. Low-intensity management that considers the carrying capacity of a grassland (i.e., potential grass growth) is the most productive strategy regarding beef production for these grasslands (Nabinger et al., 2009; Trindade et al., 2012), and may provide the minimal habitat requirements for rodent occurrence. Therefore, the land sparing approach, which embraces both the maintenance of ungrazed habitats while intensifying beef production in the remaining landscape (Phalan et al., 2016), could be an alternative for South Brazilian grassy landscapes if beef production respects the carrying capacity of grasslands.
FundingThe fieldwork was supported by EMBRAPA CPPSUL (project “Best management practices in native grasslands subjected to cattle grazing”, Project No. 04.16.00.005.000), Universidade Federal do Rio Grande do Sul (PROPG-UFRGS, Process No. 01/2015) and CNPq (Project No. 304820/2014-8).
Data statementData can be requested directly from the corresponding author.
The authors acknowledge the contribution of Clodoaldo Leites Pinheiro, Leandro Volk and André Coelho (EMBRAPA CPPSUL). Paulo C.F. Carvalho and the Grazing Ecology Research Group (UFRGS/UFSM) also supported the study. S.M.H., L.D.S.D. and R.M. received research fellowships from CNPq. A.L.L. received a doctoral fellowship from CAPES. The study was conducted according to the regional and national laws of animal capture and manipulation (Licence SISBIO/ICMBio No. 52650-1). We thank Vinicius Galvão Bastazini for providing the function to perform post-hoc contrast analysis of Permanova, and Alexandre R.T. Palma (UFPB) for providing details about tracking tunnel sampling. We thank Fernanda Brum (UFPR), Maria João Pereira (UFRGS), Cristian Dambros (UFSM) and two anonymous reviewers for the helpful comments on the manuscript.