Different causal mechanisms have been suggested to explain species decline in fragmented landscapes, mainly those related with the amount and configuration of habitat for species (habitat availability), and those related with the habitat patch quality. Here we quantify the effects of habitat availability and quality on the abundance of three small mammals in a landscape at the Brazilian Atlantic Forest. We compared species with different habitat preferences and dispersal abilities (Nectomys squamipes, Marmosa paraguayana and Didelphis aurita). The most sensitivity species to fragmentation (N. squamipes) was affected by habitat quality variables only, while the least sensitive species (D. aurita) did not suffer any effect of habitat quality and availability. M. paraguayana, a species with an intermediate degree of sensitivity, responded to both habitat quality and availability. We recommend combining information on both habitat availability and quality to unravel species persistence in fragmented landscapes.
The process of habitat loss and fragmentation causes profound alterations in the amount, configuration and quality of habitat in landscapes, potentially leading to biodiversity decline (Fahrig, 2003). Different causal mechanisms have been suggested to explain species decline in fragmented landscapes, mainly those related with the amount of habitat available for species (hereafter habitat availability) (e.g. amount of habitat remaining in the landscape, and the size and isolation of habitat patches; Fahrig, 2003), and those related with the habitat patch quality (e.g. vegetation structure; Pardini et al., 2005). However, there is a shortfall to construct a general model that considers different species responses. Thus, a central challenge is to understand how species with different habitat preferences and dispersal abilities respond to habitat loss and fragmentation.
Habitat availability depends on the habitat amount and configuration (Saura and Pascual-Hortal, 2007; Crouzeilles et al., 2014). It tends to increase as habitat patch area increases, but it generally decreases as habitat fragmentation increase. One alternative to mitigate the effects of habitat fragmentation is to maintain or improve connectivity between fragments (Crouzeilles et al., 2014). Thus, species with higher dispersal abilities may reach more habitat patches in the landscape (Crouzeilles et al., 2014), consequently improving connectivity and persistence.
On the other hand, species persistence is also affected by alterations in the vegetation structure (i.e. quality) in habitat patches (Murcia, 1995). The primary consequence of fragmentation is the creation and/or increase of edge effects, a set of abiotic and biotic alterations that occurs in habitat patches (Murcia, 1995). Abiotic alterations are related to increase in solar radiation, wind disturbance and air temperature, while biotic alterations are related to changes in species composition. For example, after fragmentation, arboreal species can be vanished from the edges or be extinct locally, while scansorial species may be favored by an increase in the amount of lianas and fast-growing pioneer tree species (Vieira and Monteiro-Filho, 2003). Although some studies focused on habitat use by species (e.g. Kajin and Grelle, 2012), few considered the effects of habitat quality on species persistence in fragmented landscapes (e.g. Pardini et al., 2005; Delciellos et al., in press). Thus, it is key to understand the effects of habitat availability and quality on species persistence.
Here we quantify the effects of habitat availability and quality on the abundance of three small mammals in a landscape at the Brazilian Atlantic Forest. We compared three abundant small mammals species with different habitat preferences and dispersal abilities inhabiting our study area (Nectomys squamipes, Marmosa paraguayana and Didelphis aurita). Our main hypothesis is that these species respond differently to habitat loss and fragmentation, with N. squamipes affected mainly by habitat quality because it is highly dependent on a specific habitat type (high habitat preference); M. paraguayana is affected by habitat availability and quality because it has an intermediate habitat preference and low/intermediate dispersal ability, i.e. connectivity in the landscape depends on the habitat configuration; and D. aurita is not affected by habitat availability and quality because it has low habitat preference and high dispersal ability, i.e. connectivity in the landscape do not depend on the habitat configuration (see Section ‘Studied species’).
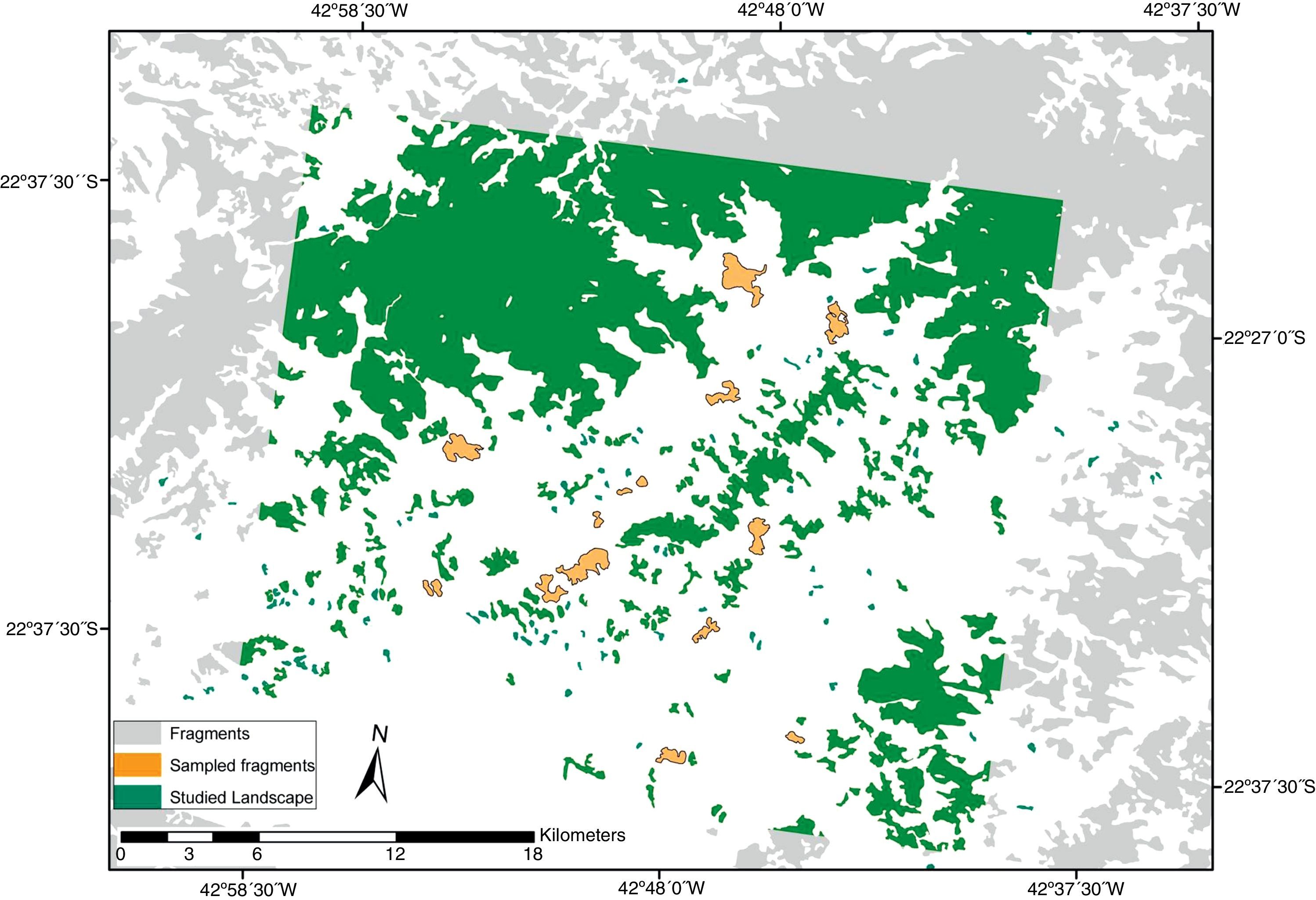
MethodsStudy areaThe study area was located in the counties of Guapimirim (2220S, 42,590W), Cachoeiras de Macacu (22280S, 42,390W), and Itaboraí (22440S, 42,510W), in the state of Rio de Janeiro, southeastern coast of the Brazilian Atlantic Forest. The sampling sites were located into a fragmented landscape, with fragments close to the basis of a continuous forest strictly protected (Serra dos Órgãos National Park), and surrounded by a matrix of pasture and crop plantations (Fig. 1). The vegetation of the fragments is disturbed to various degrees, with a relatively open understory and canopy.
Data collectionSmall mammals were sampled in 15 fragments during five consecutive days between January and September in 2008. The species abundance is not influenced by climatic (dry and wet) seasons in the study area, thus each fragment was sampled only once. In each fragment, four transects were set radially according to the four cardinal directions (N, S, W, E), with 280m long from the center to the edge. Transects had fifteen trap stations set 20m apart; each trap stations had two live traps (Tomahawk and Sherman) on the ground and one additional live trap (Tomahawk and Sherman alternated) set from one to three m above the ground in six trap stations. The live traps above the ground were set in places with connection between tree canopies to capture small mammals with arboreal and scansorial habitats. The total sampling effort was 720 trap-nights per fragment.
Traps were checked every morning and baited with a mixture of oat, banana, peanut butter, and bacon. Each trapped animal was removed from the study area during the trapping sessions to avoid a decrease in the number of traps available every night, and also avoiding underestimation of species abundance. Animals were housed in individual plastic cages, fed ad libitum, and released at their original fragment at the end of the trapping session.
Studied speciesWe studied three small mammal species: N. squamipes (Brants, 1827), M. paraguayana (Tate, 1931), and D. aurita (Wied-Neuwied, 1826). N. squamipes is a medium-sized semiaquatic rodent (250g), strongly associated to streams, ponds and marshes in forested areas (Ernest and Mares, 1986). It is most captured near water courses (up to 10m), and rarely captured in grassland matrices (Pires et al., 2002). M. paraguayana is a small marsupial (130g) with arboreal habits, captured in both understory and canopy, and with few captures on the forest ground (Grelle, 2003). It tends to be more abundant in fragmented landscapes (Pires et al., 2002). D. aurita, the largest marsupial studied (1300g), is a generalist species with scansorial habits, occasionally using the canopy (Cunha and Vieira, 2002). It is tolerant to fragmentation effects due to its high dispersal ability (Pires et al., 2002). Dispersal ability for N. squamipes and M. paraguayana is up to 300m, and 1000m for D. aurita (based on the review of Crouzeilles et al., 2010). In the same fragmented landscape studied here, the perceptual ability (i.e., the ability of animals to perceive the landscape) is 100m for of M. paraguayana and 200m for D. aurita (Forero-Medina and Vieira, 2009).
Quantifying habitat availabilityWe measured three variables to quantify habitat availability for each sampled fragment: (i) habitat patch size (ha); (ii) isolation to continuous forest (Serra dos Órgãos National Park), which was measured as the shortest Euclidean distance (m) and represents the potential connectivity between a source of species and the sampled fragments; and (iii) component size (ha), the sum of the area of all habitat patches within species dispersal ability. For example, the component size is larger when the landscape is highly connected and/or when the species has a large dispersal ability. This metric was quantified using the Probability of Connectivity index (see Saura and Pascual-Hortal, 2007 for further details). We used fragment size as a patch attribute, shortest Euclidean distance between two habitat patch boundaries as a connection attribute and a dispersal value corresponding to a probability of 50% of direct dispersal between fragments. We used two different dispersal values for each species, which was based on the species perceptual and dispersal abilities. There is no information on perceptual ability of N. squamipes, thus we used the same value of perceptual ability of M. paraguayana because both species have the same dispersal ability (see Section ‘Studied species’).
Forest fragment data were gathered from SOS Mata Atlântica and INPE (2010), derived from TM/Landsat 5, ETM+/Landsat 7 or CCD/CBERS-2 images, available at a scale of 1:50,000, and delimiting remnants >3ha. All geographic information system data were converted to UTM projection to assure accurate area and distance calculations. We used the software ArcGis 9.3 (ESRI, 2008) and Conefor Sensinode 2.5.8 (Saura and Pascual-Hortal, 2007) to conduct the analyses.
Quantifying habitat qualityWe measured seven variables to quantify habitat quality for each sampled fragment: (i) canopy cover, (ii) understory cover, (iii) lianas, (iv) Cecropia sp. (tree), (v) Astrocaryum aculeatissimum (palm tree), (vi) fallen logs, and (vii) water course. Background knowledge of the study species were taken into account in the choice of these variables. Canopy and understory cover relates to the degree of shadow from trees and exposure of the lower strata of the forest to the sun. Closed canopies and/or understories increase humidity in the forest floor, and connectivity between tree branches, allowing a higher mobility of arboreal and scansorial species between forest strata (Cunha and Vieira, 2002). Lianas also provide connections among trees and are important components of forest structure in secondary stages. Cecropia sp. and A. aculeatissimum are proxies for habitat degradation since they are more abundant in forest fragment edges and gaps, which are places with high light incidence on-the-ground (Grelle and Garcia, 1999; Delciellos et al., in press). Fallen logs provide habitat for a suite of invertebrate species, an important food resource for insectivores and omnivores small mammals (Astúa de Moraes et al., 2003). Water courses increase local humidity and are the main habitat of the water rat (Ernest and Mares, 1986).
All variables were collected within a 3m radius circle from the center of each trapping station (see Section ‘Data collection’) and at the same period which small mammals were sampled. All variables were categorized as present (1) or absent (0), except for canopy and understory cover, that were categorized as opened (0 for 0–30%), semi-opened (1 for >30–70%), and closed (3 for >70%). We summed these values for each variable in each trap station in each fragment and then we converted it to a percentage measure. For example, if liana was found in all 60 trap station within a fragment, received a value of 100%. Thus, we have a quantitative data instead of a presence/absence data for variables of habitat quality.
Data analysisWe used an information theoretic approach (Burnham and Anderson, 2002) to quantify the effects of habitat availability and quality on the abundance of small mammals. We performed separate analyses of habitat availability and quality for each species, totaling six analyses (two for each species). We fitted generalized linear models to contrast multiple candidate models that could additively include up to three and seven variables for habitat availability and habitat quality analysis, respectively. We excluded all competitive models containing correlated variables to avoid multicollinearity (R2>0.5); liana and A. aculeatissimum or Cecropia sp. or water resources (habitat quality), and patch size and 100 or 300m component size, and 100 and 300m component sizes for M. paraguayana and N. squamipes, and patch size and 200 or 1000m component size for D. aurita (habitat availability). We modeled species abundance assuming a Poisson distribution, but when the assumption of overdispersion was violated, a Negative Binomial distribution was used. We compared models with the corrected version of Akaike's Information Criteria for small samples (AICc), ΔAICc (=AICci–minimum AICc) and the Akaike weight (wi), which indicates the probability that the model i is the best model within the set (Burnham and Anderson, 2002). Models were considered equally plausible with ΔAICc≤2. The relative importance of each variable was obtained by summing the wi of all models containing the variable (Burnham and Anderson, 2002). All analyses were carried out in the R 2.12 environment (R Development Core Team, 2010).
ResultsWe captured a total of 145 individuals, 93 of D. aurita, 36 of M. paraguayana and 16 of N. squamipes in the 15 sampled fragments. The three species responded differently to the effects of habitat quality and availability (Tables 1 and 2). N. squamipes and M. paraguayana were affected by habitat quality variables, while only M. paraguayana was affected by habitat availability variables.
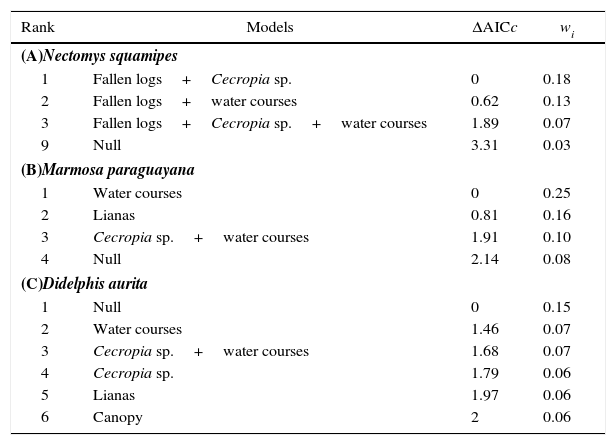
Comparison of the most plausible models (ΔAICc≤2) predicting the effects of habitat quality on abundance of Nectomys squamipes, Marmosa paraguayana and Didelphis aurita in 15 sampled fragments of Atlantic Forest. The null model was inserted for comparison purposes. ΔAICc=AICci–minimum AICc, wi=Akaike weight.
| Rank | Models | ΔAICc | wi |
|---|---|---|---|
| (A)Nectomys squamipes | |||
| 1 | Fallen logs+Cecropia sp. | 0 | 0.18 |
| 2 | Fallen logs+water courses | 0.62 | 0.13 |
| 3 | Fallen logs+Cecropia sp.+water courses | 1.89 | 0.07 |
| 9 | Null | 3.31 | 0.03 |
| (B)Marmosa paraguayana | |||
| 1 | Water courses | 0 | 0.25 |
| 2 | Lianas | 0.81 | 0.16 |
| 3 | Cecropia sp.+water courses | 1.91 | 0.10 |
| 4 | Null | 2.14 | 0.08 |
| (C)Didelphis aurita | |||
| 1 | Null | 0 | 0.15 |
| 2 | Water courses | 1.46 | 0.07 |
| 3 | Cecropia sp.+water courses | 1.68 | 0.07 |
| 4 | Cecropia sp. | 1.79 | 0.06 |
| 5 | Lianas | 1.97 | 0.06 |
| 6 | Canopy | 2 | 0.06 |
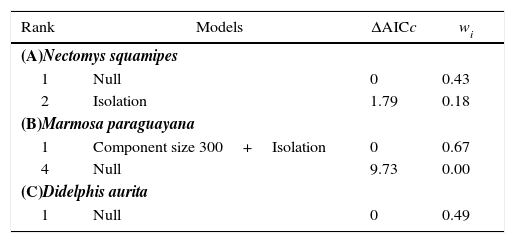
Comparison of the most plausible models (ΔAICc≤2) predicting the effects of habitat availability on abundance of Nectomys squamipes, Marmosa paraguayana and Didelphis aurita in 15 sampled fragments of Atlantic Forest. The null model was inserted for comparison purposes. Isolation=isolation to continuous forest, ΔAICc=AICci–minimum AICc, wi=Akaike weight.
| Rank | Models | ΔAICc | wi |
|---|---|---|---|
| (A)Nectomys squamipes | |||
| 1 | Null | 0 | 0.43 |
| 2 | Isolation | 1.79 | 0.18 |
| (B)Marmosa paraguayana | |||
| 1 | Component size 300+Isolation | 0 | 0.67 |
| 4 | Null | 9.73 | 0.00 |
| (C)Didelphis aurita | |||
| 1 | Null | 0 | 0.49 |
For N. squamipes, three models were considered equally plausible (Table 1(A)). Fallen logs was the variable with the highest relative importance (Σwi=0.66), followed by water courses (Σwi=0.41), and Cecropia sp. (Σwi=0.35), and these three variables were positively related with the abundance of N. squamipes. For M. paraguayana, three models were considered equally plausible (Table 1(B)). Water courses was the variable with the highest relative importance (Σwi=0.30), followed by lianas (Σwi=0.25) and Cecropia sp. (Σwi=0.12). The abundance of M. paraguayana was negatively related with water courses, and positively related with lianas and Cecropia sp. For D. aurita, the null model was the best-ranked model (Table 1(C)).
Habitat availabilityFor M. paraguayana, the only plausible model had positive effects of component size (300m) and isolation to continuous forest on abundance (Table 2(B)). Isolation to continuous forest was the variable with the highest relative importance (Σwi=0.94), followed by component size (300m) (Σwi=0.67). For D. aurita and N. squamipes, the null model was the best-ranked model (Table 2(A) and (C)).
DiscussionOur results supported our main hypothesis that the three species respond differently to habitat loss and fragmentation, with N. squamipes affected by habitat quality, M. paraguayana affected by habitat quality and availability, and no effects for D. aurita. The most sensitivity species to fragmentation (N. squamipes) was affected by changes in the forest structure only, while the least sensitive species (D. aurita) did not suffer any effect of changes neither in forest structure nor in the habitat amount and/or configuration in the landscape. M. paraguayana, a species with an intermediate degree of sensitivity, responded to both forest structure and habitat amount and configuration in the landscape. Thus, we highlight the importance of combining information on both habitat availability and quality to unravel species persistence in fragmented landscapes.
The strong relationship between N. squamipes abundance and variables that describe habitat quality, i.e., fallen logs, water and Cecropia sp., reveal that this species is highly dependent on habitat characteristics to persist in forest fragments. The presence of fallen logs can benefit the water rat by ensuring food availability (Bowman et al., 2000), since its diet comprise fungi, invertebrates, and small vertebrates (Ernest and Mares, 1986), and places for shelter and nest construction (Briani et al., 2001). Nests are frequently near water bodies and small streams, places which this semi aquatic rodent has a clear preference and use for vital functions (Ernest and Mares, 1986). In addition, this species is more abundant in secondary forests, which tends to have a high abundance of Cecropia sp. An additional and new result we present in this study is the apparent impossibility of the water rat to disperse among disrupts habitats, stressing the potential effects of habitat loss and fragmentation on the species persistence. Thus, the current highly fragmented situation of the Brazilian Atlantic Forest is potentially problematic for this species.
The persistence of M. paraguayana in a highly fragmented landscape occurs because this marsupial is able to make use of secondary forest, and to cross the matrix reaching other forest fragments (Pires and Fernandez, 1999). M. paraguayana is an arboreal species that prefer highly connected understory and closed canopy, moving along branches and lianas (Kajin and Grelle, 2012). This marsupial is rarely captured on the forest floor and, in fragmented landscapes, it seems to prefer to cross forested areas than the matrix, even when other forest fragments are within its dispersal range (Dalloz, 2013). Nonetheless, M. paraguayana still cross different matrix types (Pires et al., 2002), and can exist as systems of metapopulations (Pires and Fernandez, 1999). In our study landscape, the dispersal ability of M. paraguayana (300m; Crouzeilles et al., 2010) connects fragments, increasing the amount of habitat available, and decreasing the distance to the continuous forest. Thus, individuals are able to reach and explore other forest fragments. It is important to note that the connection between fragments depends on the habitat configuration in the landscape. Thus, the habitat configuration is of serious concern for this marsupial persistence.
Diet- and habitat-generalist species usually resist to a wide variety of conditions and changes in the environment, including habitat fragmentation. The medium-sized opossum D. aurita is the classic example of a generalist species (omnivores; Astúa de Moraes et al., 2003), which feed on many different food items (including garbage), and live on a variety of habitats, from primary and secondary forests to urban areas. They are also more abundant in small, disturbed areas when compared to larger, intact forests (Fonseca and Robinson, 1990), gaining advantage over changes in forest structure and community composition. D. aurita is also able to move long distance in the matrix (1000m; Crouzeilles et al., 2010). Therefore, the lack of relationship between both habitat quality and availability in our study confirm our expectations over this generalist species. However, this does not mean that other variables are not determinants of its abundance.
Here we confirmed that the three studied species respond differently to habitat loss and fragmentation. Future studies aiming to unravel the effects of habitat loss and fragmentation on populations should conduct species-specific analysis, since species from the same taxonomic group can respond differently to changes in the environment. It remains to understand the determinants of D. aurita population in these fragmented landscapes; these populations can be maintained by frequently immigration or be driven by intraspecific factors. In addition, efforts should be focused to construct general models that consider different species responses. Finally, we demonstrated how important is to combine information at different spatial scales to unravel how species can persist in highly disturbed and fragmented landscapes.
Conflicts of interestThe authors declare no conflicts of interest.
We thank students and staff of the Laboratório de Vertebrados for collecting data in the fieldwork, particularly to Ana C. Delciellos, Natalie Olifiers, Vanina Z. Antunes, Antonio A. Menezes, and Luis R. Bernardo. Support was provided by FAPERJ/CAPES/PAPD to RC, CAPES to MSF, CNPq (research productivity fellowship) and FAPERJ (JCE) to CEVG, and PDA/MMA, FAPERJ, CNPq, CAPES, Instituto BioAtlântica, PROBIO I/MMA/GEF and PROBIO II/MCT/MMA/GEF.