Designing protected areas capable of supporting organism flow is critical for species persistence across wide scales. Here, we evaluated the capacity and protection level of forest fragments to sustain connectivity for carnivores in the Atlantic Forest. We simulated potential dispersal considering species spatial demands and quantified the importance of patches with different sizes in sustaining species movement. We found that patches smaller than species home-range size represented more than 95% of the patches used during species dispersal. These small remnants play a key role in upholding connectivity for carnivores, especially for species capable of moving long distances. Although the forest structure did support most species movements, our results showed that some species must cross matrices composed by crops and pasture to complete their trajectories. Moreover, between 29% and 70% of the area around the species' movement paths, which could act as potential corridors, overlap with human activities. Current established protected areas – mainly targeted for sustainable use – cover only 15% of the entire extension of small remnants enrolled in species dispersal. We reinforce that conservation efforts should not overlook the potential of small forest fragments to improve connectivity. Integrating key fragments with forest restoration and matrix management would benefit long-term species persistence.
Human-induced changes have already impacted more than 70% of the ice-free land surface of Earth (Luyssaert et al., 2014) and have profoundly marked contemporary tropical forests (Lewis et al., 2015; Taubert et al., 2018). Because these biomes concentrate most species on the planet, habitat disturbance has a devastating impact on tropical forests’ biodiversity, with estimated decline in the global richness reaching 65% for some species groups (Alroy, 2017). If the dynamic of current threats does not change, in 50–100 years from now, tropical forests are condemned to diminish even more in size, be more restricted to steeper areas at high altitudes, in addition to presenting more simplified ecological communities (Edwards et al., 2019).
Besides habitat loss, the pressure on tropical forests is further enhanced by hunting, invasive species and fragmentation (Lewis et al., 2015). Land-use changes affect not only the amount of native vegetation, but also the spatial configuration and quality of the remaining forest through habitat fragmentation (Fischer and Lindenmayer, 2007). Breaking habitat continuity can result in smaller, more isolated and more exposed habitat patches to the effects of human interference in the matrix (Bennett and Saunders, 2010). Long-term experimental studies in multiple biomes have shown that fragmentation is responsible for a decrease in species richness of 13% to 75% (Haddad et al., 2015). Fragmentation also causes degradation of ecosystems, reduction in nutrient retention and in trophic dynamics (Haddad et al., 2015).
Increasing unsuitable land covers surrounding forest patches also drives changes in movement behavior (Ramos et al., 2020; Tucker et al., 2018), affecting the degree to which the landscape allows species to access the remaining fragments of resources, i.e., the landscape connectivity (Taylor et al., 1993). Consequently, the persistence of populations in fragmented landscapes may depend on individuals capable of crossing hostile matrices to reach suitable habitats (Watts et al., 2015). A global study using an extensive GPS-tracking database found that terrestrial mammals have shorter displacements in human-modified landscapes than in areas with lower human influence (Tucker et al., 2018). The decrease in species movement may occur in response to structural dissimilarity between the matrix and the original cover (Eycott et al., 2012; Prevedello and Vieira, 2010). Restricting animal movements and landscape connectivity jeopardizes not only the persistence of populations and species, but also adversely affect ecosystem functioning (Bauer and Hoye, 2014; Lundberg and Moberg, 2003).
Whereas fragmentation has progressed towards natural landscapes around the world, maintaining the integrity of movement routes can be especially critical for wide-ranging species such as mammalian carnivores (e.g., Crooks et al., 2011; Khosravi et al., 2018; Ashrafzadeh et al., 2020). Large area requirements and low densities make carnivores particularly vulnerable to habitat loss and fragmentation, mainly larger-bodied ones (Cardillo et al., 2004; Crooks, 2002). The interaction between intrinsic biological susceptibility and other anthropogenic threats (e.g., habitat degradation, persecution, depletion of prey, roadkill and sport hunting) has resulted in population decline and geographic range contraction of many species worldwide (Cardillo et al., 2004; Ripple et al., 2014; Wolf and Ripple, 2017). In the Atlantic Forest, for example, many carnivores experience a forest distributed in little core habitat with a high level of isolation (Crooks et al., 2011). Among forest fragments, these species are faced with a matrix composed by crops, pasture and human settlements that cover about 70% of the original extension of the biome (Rezende et al., 2018).
A landscape management strategy under study designed to mitigate the effects of habitat fragmentation and degradation in the Brazilian tropical forests consists of establishing biodiversity corridors (Ayres et al., 2005; MMA et al., 2006). These regional planning units comprise large areas of high biological importance formed by a mosaic of protected areas (PAs) interspersed with areas occupied by different land uses (Ayres et al., 2005). The integrated management in a biodiversity corridor aims to ensure the maintenance of large-scale ecological and evolutionary processes via connectivity for individuals and genes, while providing for the development of a regional and sustainable economy (Ayres et al., 2005). Therefore, to guide conservation efforts in biodiversity corridors, it is important to assess whether the remaining forest structure can promote ecological connectivity for vulnerable species, such as carnivorous mammals. For this, we simulated potential dispersal routes for four species of carnivores with different movement abilities in the Serra do Mar Biodiversity Corridor to answer the following questions: (i) What is the contribution of forest fragments with different sizes to maintain connectivity for carnivores? (ii) Are the currently established protected areas in Atlantic Forest capable of promoting habitat connectivity for these species? Because carnivores can be good umbrella species (Di Minin et al., 2016) and in the Atlantic Forest they experience a high level of habitat fragmentation (Crooks et al., 2011), our study has important implications for connectivity conservation planning in one of the most imperiled tropical forests in the world.
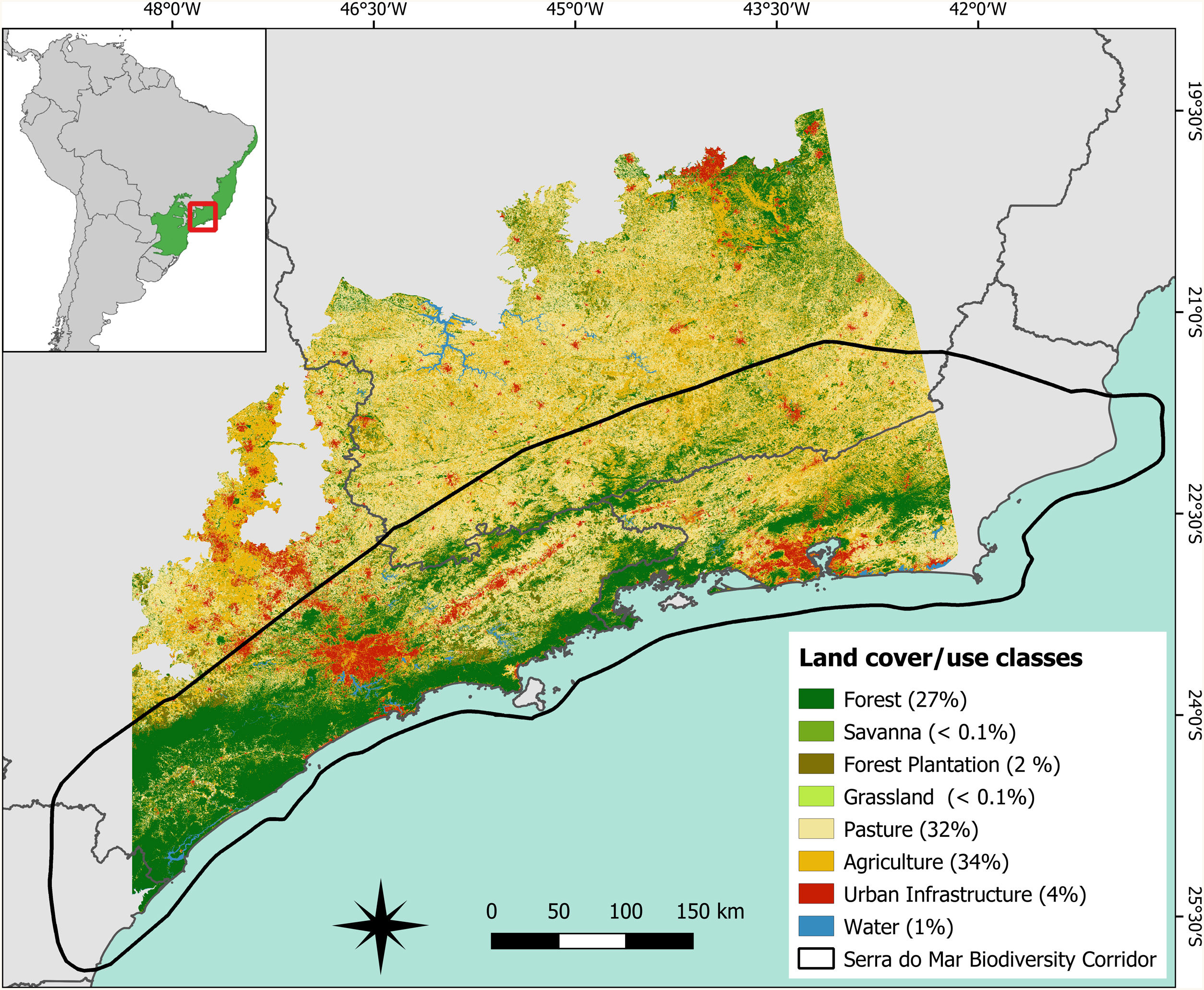
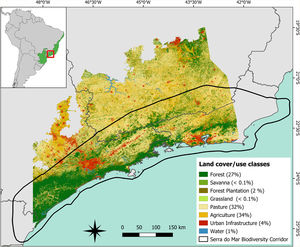
Materials and methodsStudy areaWe assessed the importance of forest fragments in maintaining regional connectivity for the target species in a wide area of the Atlantic Forest (∼207,024 km2; Fig.1) covered mainly by the Serra do Mar Coastal Forests and Upper Paraná Atlantic Forest ecoregions (Olson et al., 2001). According to MapBiomas (MapBiomas, 2019), 27% of our study area is covered by native forest, distributed in 214,361 fragments ranging from less than 1 ha to 2,025,063 ha, 66% by agriculture, which is represented in the region mainly by temporary crops, and pastures (Fig.1). These regional characteristics reflect the patterns found across the Atlantic Forest biome (Rezende et al., 2018). The study area covers almost the entire Serra do Mar Biodiversity Corridor (Fig.1), one of the two biodiversity corridors planned for the Atlantic Forest (MMA et al., 2006). This biodiversity corridor holds the largest extension of continuous forest in two of the strongest economies of the country (São Paulo and Rio de Janeiro states) and contains important PAs of considerable size. Because of its enormous biological diversity, the Serra do Mar region plays a key role in the conservation of endemic and threatened species in southern Atlantic Forest (Ayres et al., 2005), in addition to providing significant ecosystem services for urban centers in its surroundings such as reduction of temperature as well as qualitative and quantitative water supply (Starzynski et al., 2018).
Spatial distribution of land cover and use classes in the study area (∼207,024 km2) located in the Brazilian Atlantic Forest. The figure in the upper left corner shows the location of the Atlantic Forest in the Brazilian territory and the location of the study area within the biome (in red). The area delimited by the black outline in the main figure refers to the limits of the Serra do Mar biodiversity corridor. The values in parentheses indicate the percentage of the study area occupied by each land cover and use class computed using the 2018 land cover/use map provided by MapBiomas database (MapBiomas, 2019).
We selected the margay (Leopardus wiedii), ocelot (Leopardus pardalis), cougar (Puma concolor) and tayra (Eira barbara) as our target species. These carnivores show different levels of dispersal ability, matrix use, habitat requirements and sensitivity to human disturbance (Appendix A Table S1). Although all target species are widely distributed, their populations throughout the Atlantic Forest are imperiled mainly by habitat loss due to agricultural activities (ICMBio/MMA, 2018). Moreover, the cougar and the margay are part of the red list of 110 mammals officially threatened in Brazil (ICMBio/MMA, 2018).
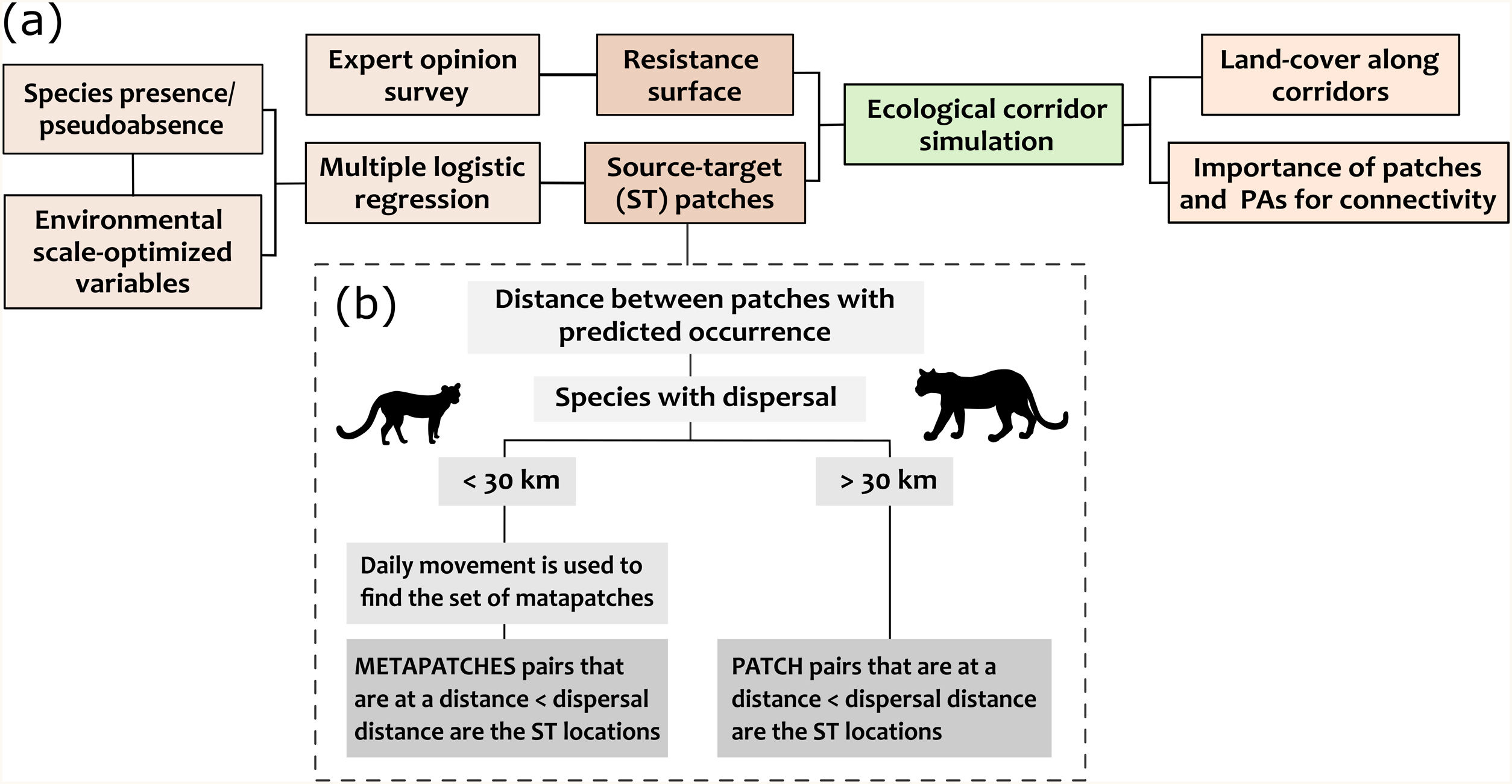
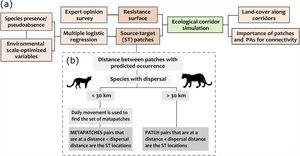
Simulating dispersal routesThere are many frameworks to quantify the importance of landscape elements for species dispersal (Diniz et al., 2019). Here, we used specific species demands related to habitat and movement to simulate potential dispersal paths and assess the role of habitat fragments in maintaining connectivity from the perspective of each species (Fig. 2a).
Schematic representation of the methodological framework applied to assess the importance of forest fragments in sustaining connectivity for four carnivores in the Atlantic Forest (a). Each step was applied individually to the target species and the selection of ST locations (i.e., forest patches between which the dispersal routes were simulated) was performed according to the species movement capacity (b).
We used the software LandScape Corridors (LSCorridors) to simulate multiple movement paths for each target species (Ribeiro et al., 2017). LSCorridors is a free software package accessed via GRASS GIS that identify ecological corridors based on the movement responses of organisms to landscape structure (Ribeiro et al., 2017). Here, we used these simulated corridors as a proxy for the species dispersal routes across the study area. The algorithm of LSCorridors is based on the modification of the classical least-cost path approach that allows the incorporation of stochastic variation in movement modeling (Pinto and Keitt, 2009). At each simulation, the algorithm selects different source-target points within a particular pair (or set) of patches to be connected (from now on termed ‘ST locations’), thus representing different departure and arrival points of the movement routes (Ribeiro et al., 2017). We applied the MP route simulation method that considers the information for each landscape pixel separately and simulated 30 possible routes per ST location. We maintained the default value of the variability parameter (equal to 2) for all species (Ribeiro et al., 2017).
Regardless of the resistance offered by the landscape elements, the least-cost algorithm always finds a path or a set of paths that represent the solution that minimizes the final accumulated cost between the ST locations (Diniz et al., 2019). Thus, in certain situations, the simulated paths invariably cross areas of great resistance, as there are no less resistant alternative paths. For this reason, after identifying potential dispersal routes, we described the land cover/use along all least-cost paths and within a buffered area around them. The radius of the buffer was defined as that one capable of limiting a circular area equivalent to species home range (Appendix A Table S1).
Simulating movement paths using LSCorridors requires two inputs: (i) a species-specific resistance surface representing how much the landscape facilitates or hampers individual movement of a species and (ii) the identification of ST locations from which dispersal is simulated. Below, we briefly described the approaches used to obtain this input data and provided more details in the Appendix B.
Resistance surfaceA resistance surface is a raster layer representing the cost imposed by landscape elements on species movement. It reflects organism’s differential propensity, physiological costs and/or mortality (Zeller et al., 2012). Ideally, resistance surfaces should be derived from movement data (Zeller et al., 2012). However, we used expert opinion due to the lack of such data for the target species (Appendix A Fig. S1). We created the resistance surfaces by reclassifying the land cover/use map according to the median resistance assigned for each class of land cover/use and for each species by 18 carnivore field and research experts (Appendix A Fig. S1). The reclassification was applied to the land cover/use layer with a spatial resolution of 30 m provided by the MapBiomas database for the year of 2018 (MapBiomas, 2019). The resistance values range from 1 to 100, with higher values indicating higher resistance. Here we used the median of the values because the median is the best centrality measure of a skewed distribution (Appendix A Table S2).
Identifying source-target patchesWe used multiple logistic regressions (binomial distribution, logit function) and model selection to first predict the species occurrence along forest patches and then define between which patches the movement would be modeled (i.e., the ST locations). Although some target species occur in other types of vegetation cover (savanna and grassland), we only predicted their occurrence in forest fragments since the other classes of natural vegetation together accounted for less than 0.04% of the study area. Despite this, all classes of natural vegetation were considered in the resistance surfaces. We selected species presence data from studies carried out between 1999 and 2017 in the Brazilian Atlantic Forest from a database of camera traps records (Lima et al., 2017). We inferred absence points (i.e., pseudoabsences) following a species-specific procedure (see Appendix B).
We defined six explanatory variables describing forest patches and the local landscape structure around them (limited by a buffer): patch area, patch climatic and topographic suitability, distance to urban infrastructure, matrix resistance, immediate forest cohesion and core area percentage. For the scale-dependent variables (the last three), we tested the spatial scale (the size of the local landscape) effect on species, considering species movement capacity, and selected the spatial scale that present the strongest effect on each species. All variables, except climatic and topographic suitability, were extracted from the land use/cover layer corresponding to the year of the occurrence record (MapBiomas, 2019). We compared models through the Akaike Information Criterion corrected for small sample sizes (AICc).
After selecting the best model, we used it to predict the likelihood of species persistence across forest fragments. The final probability map was truncated based on a threshold where the receiver operator curve (ROC) is closest to the perfect fit. We evaluated model performance using AUC and the rate of overlap between patches with predicted presence and the records from an independent dataset (see Appendix B for more details).
After identifying the set of suitable patches (i.e., fragments with predicted occurrence) using the best logistic model, we adopted two different strategies to define the pair of ST locations (Fig. 2b) following Mimet et al. (2016). For species with high capacity of movement (cougar and ocelot), we considered a pair of patches as ST if they were at a Euclidean distance less than or equal to the species dispersal capacity. For species with less movement capacity (margay and tayra), we first clustered patches according to species daily distance to find the potential metapatch populations (Zetterberg et al., 2010) and considered as ST those pairs of metapatch populations located at a distance compatible with species dispersal. Thus, at each dispersal simulation for margay and tayra, the LSCorridor algorithm selected a pixel within any patch belonging to a metapatch population as a start or end of the movement. Therefore, each species presented a different set of ST locations determined according to the predicted occurrence along the forest patches and the application of the distance thresholds described above (Appendix A Table S3).
Importance of habitat patches and protected areas for connectivityAfter simulating the multiple movement routes, we selected those paths with length equal to or less than the species dispersal distance. Although each target species had a specific set of patches as its departure and arrival, we did not assume any restrictions regarding the forest patches that could potentially be used during movement. LSCorridor provides the index RSFI informing the number of corridor or path simulations passed through each landscape pixel. Although we did not use this index, we adopted the same logic to quantify the importance of habitat patches for connectivity. For this, we computed the ‘weighted betweenness’ metric (Freeman, 1978) as a measure of interpatch connectivity. The betweenness index is calculated as the proportion of all least-cost paths in a network that cross a given node, reflecting its potential to act as a stepping stone and thus facilitate species movement.
We grouped all forest fragments crossed by the simulated dispersal routes into size classes using a species-specific perspective (see Appendix A Table S1). A forest fragment was labeled as ‘Small’ when its area was smaller than the species home range; ‘Medium’ fragments presented an area equal to or larger than the home range, but smaller than the minimum area for a viable population; and ‘Large’ fragments have area enough to sustain a minimum viable population. Because there was a different number of routes for each species, we calculated a relative weighted betweenness. We calculated the betweenness index for each fragment within each group and standardize it to vary from 0 to 1. The importance of each size class was setting as the sum of the relative betweenness of all fragments within the class divided by the sum of their respective areas.
Finally, we intersected the map of ST patches for all species as well as the map of patches crossed by dispersal routes to identify all fragments that are important to connectivity. We then quantified the overlap of the extension of these important areas with the Brazilian currently established PAs along the different jurisdictions (municipal, state and federal) and conservation purposes (sustainable use and integral protection). The spatial data of the protected areas were downloaded from the Brazilian Ministry of Environment database. All spatial analyzes were performed in the R 3.5 environment (R Core Team, 2018) using the raster package (Hijmans, 2017).
ResultsPatch-level species occurrenceThe best multivariate model used to predict species occurrence retained different sets of explanatory variables (Appendix A Table S3). The models AUC ranged between 0.58 and 0.86 and the percentage of correct predictions (hit rate), when an independent dataset was used to validate the models, vary from 40% to 87% (Appendix A Table S3). Although the best model for ocelot did not perform well in any of the evaluation measures, we decided to carry out the connectivity analysis for this species because its requirements represent an intermediate scenario among the other carnivores.
In total, 709 forest fragments had predicted occurrence for tayra (mean area = 4,467.6 ha), 773 for margay (mean area = 3921 ha), 6365 for ocelot (mean area =414.2 ha) and 5750 for cougar (mean area =563.3 ha). Considering all fragments, only 43 were shared by all species and, together, they represented 57.8% of the total forest area with expected occurrence for carnivores (see Appendix A Fig. S2).
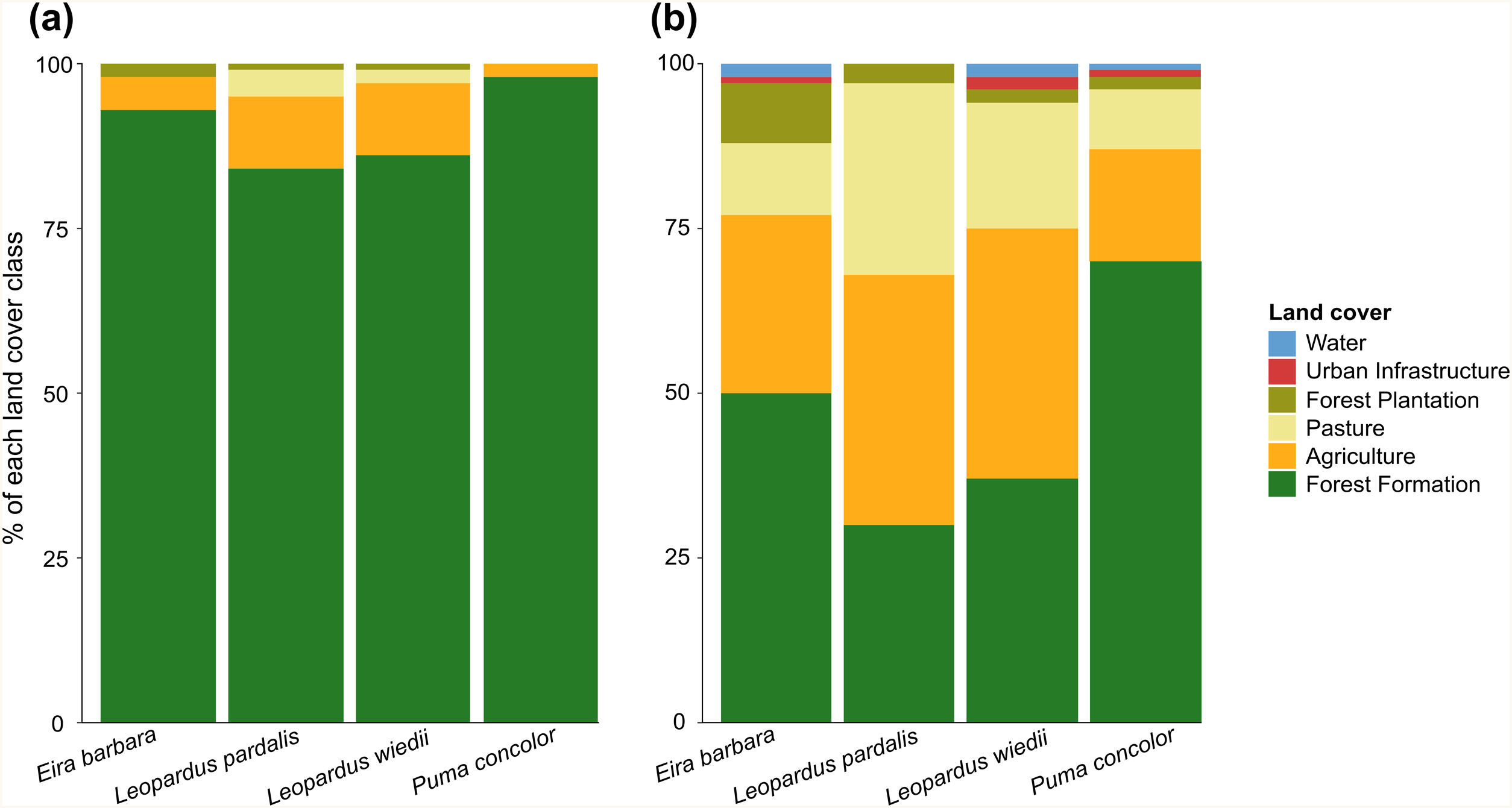
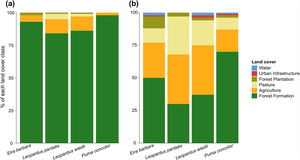
Dispersal routes compositionThe movement simulations showed that species displacements were mainly supported by forest areas (Fig. 3a). Only 2% and 5% of the area used during cougar and tayra movements, respectively, were covered by agriculture, while for ocelot and margay, this land use occupied 11% of the routes. The buffered area around the least-cost paths showed high heterogeneity (Fig. 3b). For the cougar and tayra these areas were covered mostly by forest (70% and 50%, respectively), whereas for the ocelot and margay the buffered areas were dominated by pastures and agriculture, totalizing 67% and 57% for each species, respectively.
The land cover and use composition of the least-cost dispersal routes (a) and the area buffered around them (b). The radius around the multiple least-cost paths was defined as the one capable of limiting a circular area equivalent to the home range of each study species (see Appendix A Table S1).
Species required a different number of fragments to move between ST locations (Appendix A Fig. S2), varying from 427 patches for the tayra to 9360 patches for the ocelot (Appendix A Table S4, Fig. S3). The largest patches in the study area, located in the two major mountain ranges (Serra do Mar and Serra da Mantiqueira), were capable to provide both habitat and connectivity for all species (Appendix A Fig. S2-S3).
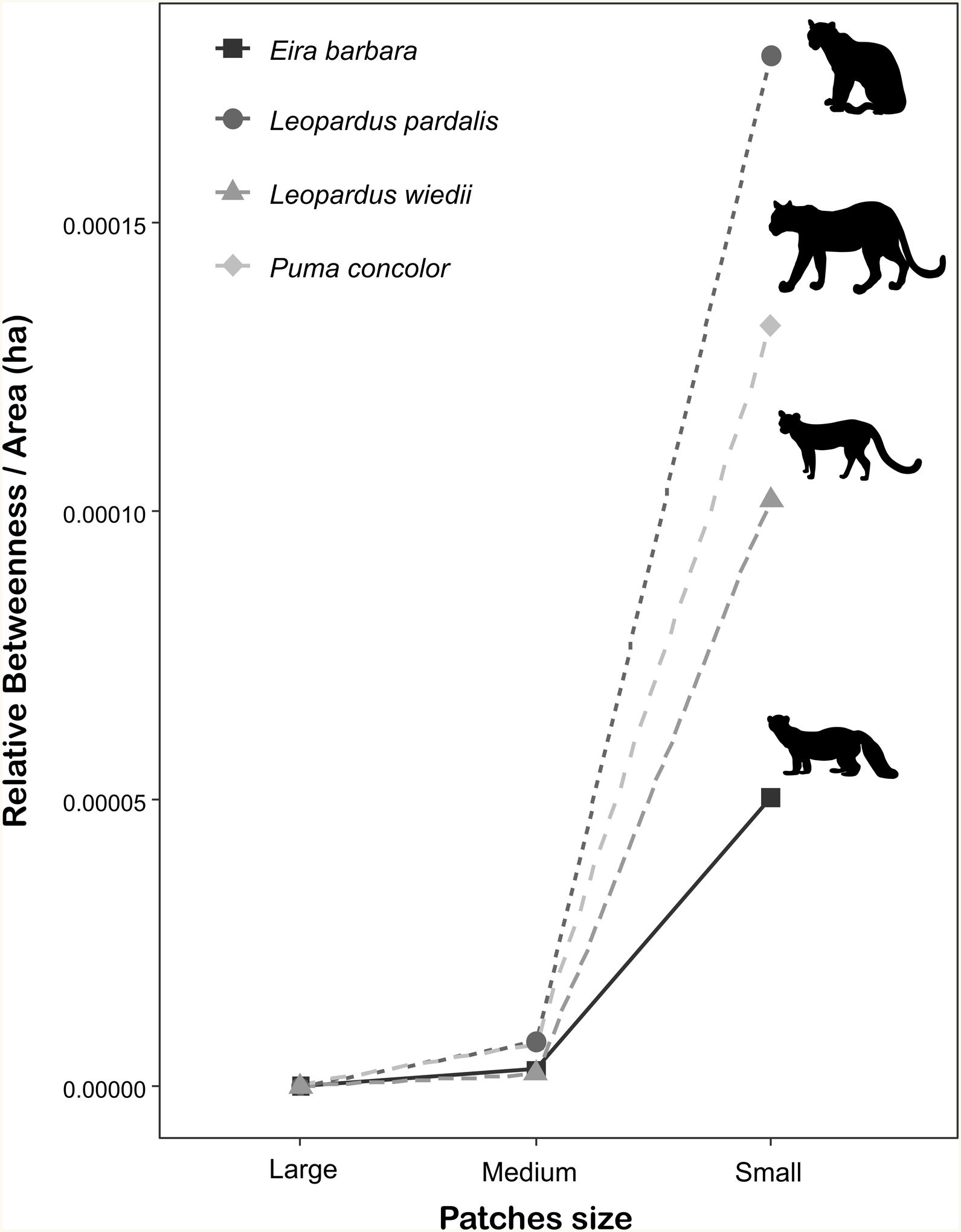
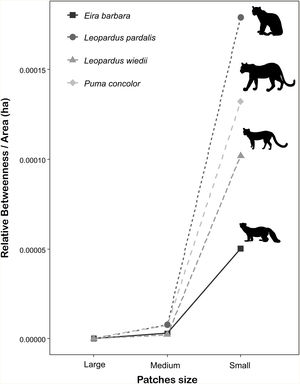
The importance of small patches (i.e., forest fragments smaller than species home range) for landscape connectivity was two to three orders of magnitude greater than the capacity of large and medium ones (Fig. 4), being more pronounced for species with the highest dispersal ability (cougar and ocelot). The total area of all these fragments (576,683.8 ha) represents 2.8 % of the study area. On average, the area of small fragments represented 5.5% (±5.3%) of the total area of all patches used during the simulated routes but 97.7% (±2.0%) of the total number of fragments crossed during the movement (Appendix A Table S4).
Patch importance in sustaining connectivity for each target species. Patch classification in different sizes was made according to the specific spatial requirements of each species. Small patches have a smaller area than the species’ home range. Medium patches have an area equal to or larger than the home range size, but smaller than the minimum area for a viable population. Large patches have area sufficient to hold a minimum viable population. Patch importance was defined as the sum of the relative weighted betweenness of all patches within the size class weighted by the sum of their areas in hectare. Species home-range area ranges from 1266 ha to 5000 ha and the minimum area estimated for a viable population ranges from 8326 ha to 52,989 ha (Appendix A Table S1).
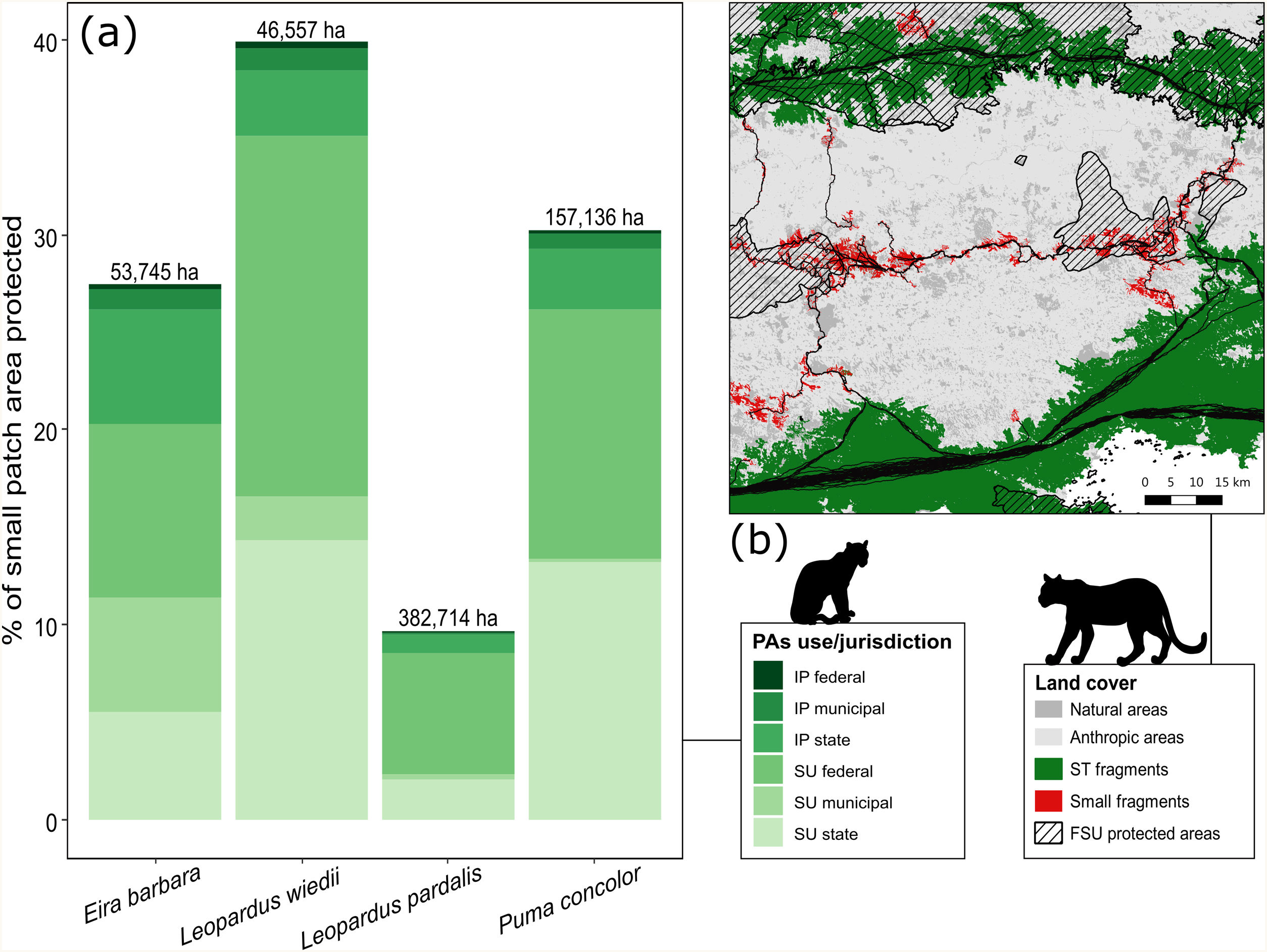
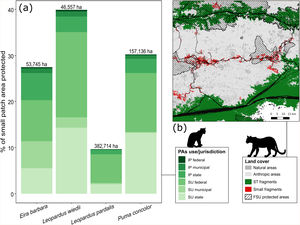
Protected areas covered 20.9% of the entire extension of our study area and about 65% to 78% of the total forest area used in the species trajectories (Appendix A Table S4). Small patches that integrated species paths received less protection than medium and large patches. Considering the whole set of small fragments crossed during species dispersal, only 15% (∼87,397 ha) of their area were under some level of legal protection, but this cover differs among the species (Appendix A Table S4). Among the categories of protected areas, in general, sustainable use PAs under federal and state jurisdiction showed greater effectiveness in protecting the small patches used during the potential dispersal of species (Fig. 5, Table S4). For cougar, for example, 26% of the 30% of the protected extent of these small fragments are within federal and state PAs for sustainable use (Appendix A Table S4).
Percentage of small key fragments (< species’ home range area) for connectivity under legal protection according to protected area use (SU: sustainable use, IP: integral protection) and jurisdiction (a). The number at the top of each bar in (a) indicates the total area occupied by the entire set of small patches (protected and unprotected). The map in (b) shows an example of the small patches (red) used during the simulated dispersal routes (black paths) for the ocelot (Leopardus pardalis) and cougar (Puma concolor) between source-target locations (dark green). The hatched areas show part of the Brazilian set of protected areas for sustainable use under federal jurisdiction (FSU protected areas).
Our results indicate that the forest remnants are still capable of supporting species movement mainly due to the presence of small fragments spread over the human-dominated landscapes in the Atlantic Forest. Despite this conclusion, we also observed that part of the dispersal routes for some species are formed by agriculture and pasture areas, and the environment surrounding them is largely impacted by these land uses. We also found that protected areas do not appropriately protect small fragments, despite small fragments’ value for ecological connectivity.
We showed that patches smaller than species home range have a key role in promoting connectivity for all species. However, prioritizing and assessing protected areas for carnivores often implies overlooking small fragments (e.g., Sollmann et al., 2008; Santini et al., 2016). This is justified due to the negative relationship between patch size and local extinction probability, and because small protected areas have greater edge effect predisposing particularly wide-ranging carnivores to human conflicts (Woodroffe and Ginsberg, 1998). Nevertheless, when located on the right place, small fragments can act as relevant stepping stones or part of semi-continuous vegetation strips, which play a key role in maintaining species movement. While these fragments have not enough extension for a population, they enhance habitat reachability for species that, otherwise, would have their persistence compromised by isolation. Facilitating dispersal and upholding long-distance displacements, stepping stones allow species to successfully colonize new suitable patches and expand their range, which is critical in the face of environmental changes (Saura et al., 2014).
We observed that small patches have a particularly positive effect on the connectivity of far-ranging species (Fig. 4-5b). This is in line with Herrera et al. (2017) who identified that small patches (5−50 ha) are essential as connectors to species that disperse long distances (> 5 km) between grassland patches in Argentina. In Iran, unprotected, small core patches also showed high potential to improve connectivity between protected areas for some carnivores with large dispersal abilities (Ashrafzadeh et al., 2020; Khosravi et al., 2018). Although controversial, some authors believe that habitat patchiness has a positive effect on movement because a greater number of smaller patches scattered over the landscape can increase patch encounter rate, improving functional connectivity (Fahrig, 2017). In the Atlantic Forest, small patches have also proved to be valuable for reptiles (Lion et al., 2016) and for alleviating the isolation of birds depending on matrix composition (Barbosa et al., 2017; Uezu et al., 2008).
However, before advocating stepping stones as an effective and less expensive solution to manage fragmented landscapes, it is essential to bear in mind their limitations (Kramer-Schadt et al., 2011). For example, the nature of the matrix can affect the effectiveness of connectors (Baum et al., 2004). According to Uezu et al. (2008), stepping stones would be more efficient when the matrix offer an intermediate resistance to species. This is because a hostile matrix would prevent any movement and, at the other extreme, a very movement-friendly matrix would make stepping stones obsolete (Uezu et al., 2008). Thus, stepping stones cannot benefit highly sensitive or strictly forest-dependent species, for which corridors with adequate width may serve better (Kramer-Schadt et al., 2011).
Our results revealed that agriculture and pasture dominated the area surrounding forest patches used during the simulated movements of Leopardus pardalis and Leopardus wiedii and constituted about 14% of their dispersal routes (Fig. 3). Although these species can cross these environments without a continuous canopy, this scenario is a particularly unfavorable for them because they are more sensitive to human disturbance than the other carnivores (Cruz et al., 2019; Horn et al., 2020). The tolerance of the cougar to anthropogenic habitats has been evidenced, for example, by an isotope analysis that showed the agricultural matrix (sugarcane) is an important food source for this species (Magioli et al., 2014). For species more sensitive to the matrix, management and the restoration initiatives are essential to guarantee the integrity of movement paths.
There are some circumstances in which stepping stones can negatively impact species persistence. A simulation study showed these connectors only have a positive effect on dispersal and colonization success of the Lynx lynx if they are large enough to allow the production of new dispersers (Kramer-Schadt et al., 2011). This finding is also supported by the Saura et al. (2014). According these studies, very small or low-quality stepping stones can act as sinks and compromise the success of colonization because they can intercept dispersers by the "shadow effect" (Hein et al., 2004), but do not offer enough resources to produce new dispersers (Kramer-Schadt et al., 2011). As we define small fragments as forest areas smaller than the species home range, it is unlikely that animals will settle in stepping stones. We believe that more important than the capacity of producing dispersers, is the topology of these connectors in relation to suitable habitat patches. If stepping stones are not so far from large blocks of forests, they will be used as stopover points, providing just temporary shelter and rest sites. Conversely, if they form a long way to the nearest viable patch, dispersal can be compromised. This can be evaluated, for example, through population dynamic studies (e.g., Kramer-Schadt et al., 2011). Although our work has drawn attention to the potential of small fragments to maintain connectivity, we suggest that all the above considerations should be made before including these areas as components of any conservation effort.
Some movement areas for the cougar and the ocelot between the region of Serra do Mar and Serra da Mantiqueira pointed out by our simulations were also identified as important connectors for the jaguar (Castilho et al., 2015; Diniz et al., 2018). Castilho et al. (2015) showed that the restoration and protection of a 30 m riparian forests could increase the connectivity area linking mosaics of PAs by over 400 % for cougar and 500% for jaguar. Many municipalities inside our study region have high vegetation debts in riparian areas (Rezende et al., 2018) and, therefore, the restoration of these areas would bring a great improvement for the movement of carnivores and many other species, including their prey (Paolino et al., 2018; Zimbres et al., 2018). In addition, our results emphasize the lack of protection of areas able to support carnivore dispersal and the importance of sustainable use protected areas for connectivity conservation as already shown for the jaguar (Diniz et al., 2018).
Only two decades ago, Brazilian environmental policy formally recognized that connectivity should be an aspect considered in the establishment of protected areas (article 25 of the Brazilian law 9985, July 2000). Before that, many Brazilian PAs were created in an opportunistic way, ignoring the spatial arrangement of the existing network. However, ensuring species movement will be decisive for their long-term persistence in the face of current and future scenarios of climate and land-use changes (Heller and Zavaleta, 2009). This brings the need to establish PAs that allow the movement of multiple species, ensuring that the whole system works as a well-connected network and increasing the efficiency of already existing PAs (Albert et al., 2017). We showed that despite small forest fragments (those with area smaller than species home range) played a crucial role in maintaining connectivity for carnivores, they are poorly protected and vulnerable to human impacts. Neglecting the contribution of small fragments (< 250 ha) means giving up more than 42% of the remaining vegetation of the Atlantic Forest (Ribeiro et al., 2009). Therefore, we recommend that decision makers look more closely at the potential of small connectors to integrate restoration and prioritization plans aiming to protect species connectivity in the Atlantic Forest.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
MFD and RL research is funded by CNPq (grant #150488/2019-0 and grant #306694/2018-2, respectively). This paper is a contribution of the INCT in Ecology, Evolution and Biodiversity Conservation founded by MCTIC/CNPq (grant #465610/2014-5) and FAPEG (grant #201810267000023).