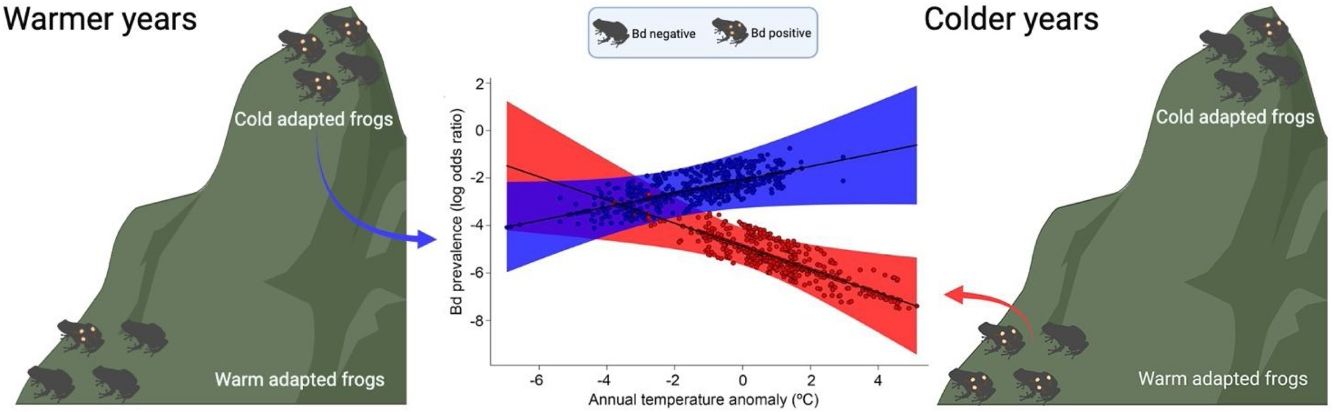
Theory predicts that susceptibility to disease in ectothermic hosts increases as temperatures depart from host’s thermal optima, because pathogens have functionally broader thermal tolerance ranges and acclimate faster than hosts to shifts in temperature. Hence, hosts adapted to cooler and warmer climates should be at greater risk of infection under abnormally warm and cool conditions, respectively. Batrachochytrium dendrobatidis (Bd) is a chytrid fungus that affects amphibians worldwide. In Brazil's Atlantic Forest, Bd outbreaks have been linked to numerous declines in amphibian populations, particularly in cooler high elevation areas. Thus, we hypothesize that years with abnormally warm temperatures could shift the balance in favor of the pathogen, thereby driving the historical declines. We also hypothesize that warm-adapted amphibians from lowland sites could experience elevated Bd infection risk during abnormally cold years. To test whether thermal mismatch (elevation vs. temperature anomaly) drove shifts in Bd prevalence through time we compiled a comprehensive database spanning 50 years, gathered across an elevational gradient within the Atlantic Forest. In agreement with our predictions, cool-adapted hosts had higher Bd prevalence when temperatures were higher than historical averages. In parallel, Bd prevalence in warm-adapted hosts was higher in colder-than-average years, although frogs from higher elevations exhibited an overall higher risk of disease due to disproportionally high infection prevalence. Our study links the thermal mismatch hypothesis with historical shifts in Bd prevalence in Brazilian frogs, indicating that Bd infections, modulated by climate change, may continue to have a negative impact on Neotropical amphibians.
The synergistic effects of anthropogenic climate change and emerging infectious diseases threaten biodiversity and human health (Daszak et al., 2000; Pecl et al., 2017). Besides influencing mean global temperature and precipitation patterns, human interference is also increasing the variability of these factors (Cai et al., 2014; Easterling et al., 2000; Schär et al., 2004), leading to a less predictable climate. Increased climatic anomalies may be one of the main causes of disease outbreaks globally (Cohen et al., 2020, 2019a; Rohr and Raffel, 2010) due to the strong link between climate and host-pathogen dynamics (Altizer et al., 2013; Harvell et al., 2002). However, despite mounting evidence supporting a strong link between climatic anomalies and disease outbreaks in wildlife, few studies have investigated the mechanisms via which such links might shape host-pathogen dynamics (see Cohen et al., 2017; Rohr and Raffel, 2010).
Host-pathogen dynamics can be especially affected by climate extremes in systems involving ectothermic hosts. Ectotherms have a limited capacity to produce metabolic heat and generally depend on external heat sources to maintain their body temperature (Seebacher et al., 2015). Fluctuations in temperature can thus push these animals closer to their thermal limits, negatively affecting their natural behavior and physiological processes (Angilletta, 2009; Huey and Steverson, 1979). Thus, understanding the dynamics between ectothermic hosts and pathogens presents an ideal system for assessing the impact of climate change on disease outbreaks.
Several mechanisms have been proposed to explain how anthropogenic climate change could influence disease dynamics in ectothermic hosts. Recently, Cohen et al. (2017) proposed the thermal mismatch hypothesis, which states that host susceptibility to a pathogen increases as temperatures depart from host’s thermal optima. For instance, the hypothesis predicts that ectothermic hosts adapted to cooler temperatures can experience the greatest susceptibility to pathogen infection in warmer climates and vice-versa (Cohen et al., 2017). The thermal mismatch hypothesis is based on the assumptions that hosts and pathogens are locally adapted, that cool- or warm-adapted hosts and parasites occur near their thermal limits of performance, and that pathogens, being smaller and having a much faster life cycle than their hosts, have broader thermal tolerances and are equipped to adapt or acclimate to new environmental conditions much faster than hosts (Carvalho et al., 2017; Raffel et al., 2006; Rohr et al., 2018). Thus, considering that a pathogen can maintain high performance over a wider range of temperatures compared to the host, disease outbreaks are expected to occur at a temperature range that reduces host performance while that of the pathogen remains relatively high. The thermal mismatch hypothesis was first proposed and supported using amphibians and their pathogenic fungus Batrachochytrium dendrobatidis (Bd) as a study system (Cohen et al., 2017), and it was later corroborated by multiple experiments and field studies using diverse host-pathogen systems (Cohen et al., 2020, 2019a, 2019b; Sauer et al., 2020, 2018). However, the applicability of the thermal mismatch hypothesis to complex megadiverse tropical systems has not been thoroughly tested, providing a significant opportunity to improve its predictive capacity, particularly for highly endemic and endangered species.
Amphibian population declines and extinctions caused by Bd have been reported globally (Fisher and Garner, 2020; Scheele et al., 2019), but the majority of these declines were recorded in seemingly pristine, high-elevation tropical regions (Carvalho et al., 2017; Lips et al., 2008; Scheele et al., 2019). This pattern is potentially due to a greater sensitivity to warming in amphibians at these megadiverse sites (Cohen et al., 2019b), where temperatures are generally constant (Brattstrom, 1968; Navas, 1996), and due to the narrow thermal breadths of endemic hosts adapted to mountain tops (Deutsch et al., 2008; Rohr et al., 2018). Within Brazil's Atlantic Forest, known for its remarkable amphibian biodiversity (Toledo et al., 2021), Bd outbreaks were observed more than a century after the earliest Bd detection in the region (Rodriguez et al., 2014). These outbreaks had a significant impact on multiple amphibian populations, particularly on those adapted to high elevations (Carvalho et al., 2017; Toledo et al., 2023). Such epidemiological patterns led us to hypothesize that climate variability could be an underlying mechanism driving chytridiomycosis in Brazil.
Here, we tested the potential impact of thermal mismatch by assessing the effect of the interaction between elevation and temperature anomaly on Bd prevalence in amphibians from the Brazilian Atlantic Forest. We compiled a 50-year time series dataset of Bd presence/absence in museum-preserved amphibians sampled across Atlantic forest sites with varying elevations and temperatures. Considering the predictions of the thermal mismatch hypothesis, we expected to detect the following patterns: 1) higher Bd prevalence in highland, cool-adapted host populations during warmer-than-average periods, and 2) the opposite pattern for lowland (warm-adapted) host populations – higher Bd prevalence during colder-than-average periods, after accounting for other biotic and abiotic factors. Lastly, because most amphibian declines in Brazil have occurred at high elevations (Carvalho et al., 2017), and because amphibians are expected to experience less thermal fluctuations at high elevations (Brattstrom, 1968; Cohen et al., 2019b; Navas, 1996; also supported by our data), we predict to find a greater effect of thermal mismatch on cool-adapted amphibians.
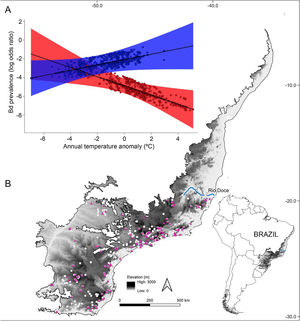
Materials and methodsStudy region and Bd prevalence dataThe Atlantic Forest can be divided into two divergent bioclimatic regions, the Northern and Southern portions (NAF and SAF, respectively), with Rio Doce as the biogeographic boundary between these two regions (Carnaval et al., 2014; Oliveira-Filho and Fontes, 2000; Thomé et al., 2014). Relative to the NAF region, the SAF region (spanning from 19 to 32 degrees in latitude) exhibits several distinct features, including lower mean annual temperature, higher volume of precipitation, and greater variation in elevation, ranging from sea level to about 3,000 m (Carlucci et al., 2021; Grimm, 2003; Lambertini et al., 2021; Lins-e-Silva et al., 2021). Distinct patterns of Bd infection prevalence have been observed between these two regions (Lambertini et al., 2021; Ruthsatz et al., 2020). In addition, in an extensive sampling of museum-preserved specimens from the Atlantic Forest that comprised an 85-year time frame (1930–2015), Carvalho et al. (2017) determined that the majority of the historical samples were from SAF. Hence, by integrating the SAF climatic and topographical features with the Bd dataset, we were able to conduct a powerful "natural experiment" (Körner, 2007) testing whether thermal mismatch could explain temporal patterns of Bd infection prevalence. Our dataset comprised 17,616 museum-preserved tadpoles collected between 1963 and 2013 and visually screened for Bd (Carvalho et al., 2017).
Environmental dataWe extracted historical monthly mean temperatures and total rainfall data from the Hadley Climate Research Unit (Harris et al., 2014), and elevation data from Bioclim (Fick and Hijmans, 2017). To extract the data, we specified the georeferenced location where each tadpole was collected in the field, and used the function extract from the raster package (Hijmans, 2014). Monthly climate and elevation data were available with a resolution of 2.5 min (∼21 km2) and 30 s (1 km2), respectively. Using the historical monthly data across the 50 years period, we calculated temperature and rainfall anomalies for each year in our dataset by (1) averaging monthly mean values of the immediate previous year (12 months prior to sampling), and averaging monthly mean values from the preceding 50 years (600 months prior to sampling), and then (2) by subtracting the historical mean (from the preceding 50 years) from the annual mean of the year of interest.
Statistical analysesTo test our hypotheses, we aimed to determine the effect of temperature anomaly and elevation, and their one-level interaction, on Bd prevalence through time. We fit Generalized Linear Mixed Effect Models (GLMMs) using a binomial error distribution (link logit) and a covariance structure AR(1) to account for temporal autocorrelations across years and seasons, and the models were fit using the glmmTMB function and package (Brooks et al., 2017) in R v. 4.0.2 (R Core Team, 2020). Thermal mismatch was included in the model as an interaction term between elevation and annual temperature anomaly across locations where amphibians were collected. Elevation is a strong proxy for long-term climate adaptation (Sternberg and Thomas, 2014) and is strongly correlated with mean annual temperature in our dataset (r = -0.87), while annual temperature anomaly captures temperature fluctuations departing from historical averages (past five decades). We used environmental variables at an annual time scale due to previous findings indicated that annual temperature variations are a significant factor influencing chytridiomycosis dynamics in tropical amphibian communities (Rohr and Raffel, 2010). Additionally, considering that certain species within our database may spend a year or more in the tadpole stage, it was not possible to accurately identify the exact time at which a tadpole became infected by Bd.
Given our predictions that highland, cold-adapted amphibians would show higher Bd prevalence under warm years and that lowland warm-adapted amphibians would show higher Bd prevalence under cool periods, we predicted that the estimated coefficient of bidirectional thermal mismatch interaction should be positive. To control for the effects of latitude and rainfall anomaly, which have been shown to influence Bd prevalence (Becker and Zamudio, 2011; Kriger et al., 2007; Moura-Campos et al., 2021; Ruggeri et al., 2018), we included latitude (in absolute values) as a random effect in the model and rainfall anomaly as a fixed effect. In addition, latitude accounted for site effects, because all latitude and longitude combinations represent a unique location in our data. While we acknowledge that species identity can greatly influence disease outcomes due to varying levels of susceptibility to Bd, which is driven by a myriad of factors such as developmental mode and habitat use, accurately identifying the tadpoles from our study to the species level posed a significant challenge, mainly because this life-stage for a substantial fraction of the species in the study region is either poorly known or have not been formally described. Still, we directed our sampling efforts towards amphibian species undergoing larval development, deliberately excluding all terrestrial-breeding species with direct development that tend to have less contact with waterborne Bd in the wild. This approach was thus taken to further minimize the known influence of species developmental mode on the likelihood of Bd detection (Mesquita et al., 2017). Additionally, the high occurrence of endemic species in high elevation regions of the SAF limits the ability to compare the same species across different elevations (Toledo et al., 2021). Hence, we did not include species as a random factor in our model.
We performed an automated model selection approach to test the relative importance of each environmental variable and the interaction between elevation and temperature anomaly using the dredge function of the MuMin package (Bartoń, 2020). This function generates models using all combinations of variables, and ranks the models based on the Akaike Information Criterion (AIC). We determined the variance inflation factors (VIF) among variables included in the final model using the multicollinearity function through the performance package (Lüdecke et al., 2021). VIF values were lower than 4 (Hair et al., 2010), ruling out cross-correlation as a potential bias in our analyses. Lastly, in support of our hypothesis that high elevation frogs may be more susceptible to Bd due to thermal mismatches arising from their historical exposure to lower thermal variations compared to lowland amphibians, which is based on the expectation that highland frogs should be less adapted to climatic fluctuations (as indicated by Brattstrom, 1968; Cohen et al., 2019b; Navas, 1996), we carried out a Fligner–Killeen test for homogeneity of variances (fligner.test function). Specifically, we tested whether the variance in mean temperature differed among our focal sampling sites with contrasting low and high elevations. For this, we used data from tadpoles collected from the 10th (centered on 20 m) and 90th (centered on 1248 m) percentiles of the elevational gradient.
The fit of the best model was assessed visually and statistically using a simulation-based approach in the package DHARMa (Hartig, 2020). First, we used the function simulateResiduals to create scaled residuals simulated from the best model (adjusted for 1,000 simulations). Then, we performed Kolmogorov-Smirnov (KS) dispersion and outlier tests to detect overall deviations from the expected distribution using the function testResiduals. Lastly, we tested whether the expected number of zeros based on our fitted model differed from the number of observed zeros, using the testZeroInflation function. The model was robust in all tests (P > 0.05), except for the KS test; although visually we were not able to detect a strong deviation from predicted (Figure S1). According to the authors of DHARMa, achieving a perfect fit in a model that includes a large sample size is unlikely (Hartig, 2020). Lastly, to visually inspect our residuals, we generated partial residual plots using the visreg function and package (Breheny and Burchett, 2017). This function isolates and displays the effects of focal variables while controlling for the effects of other factors included in the statistical model.
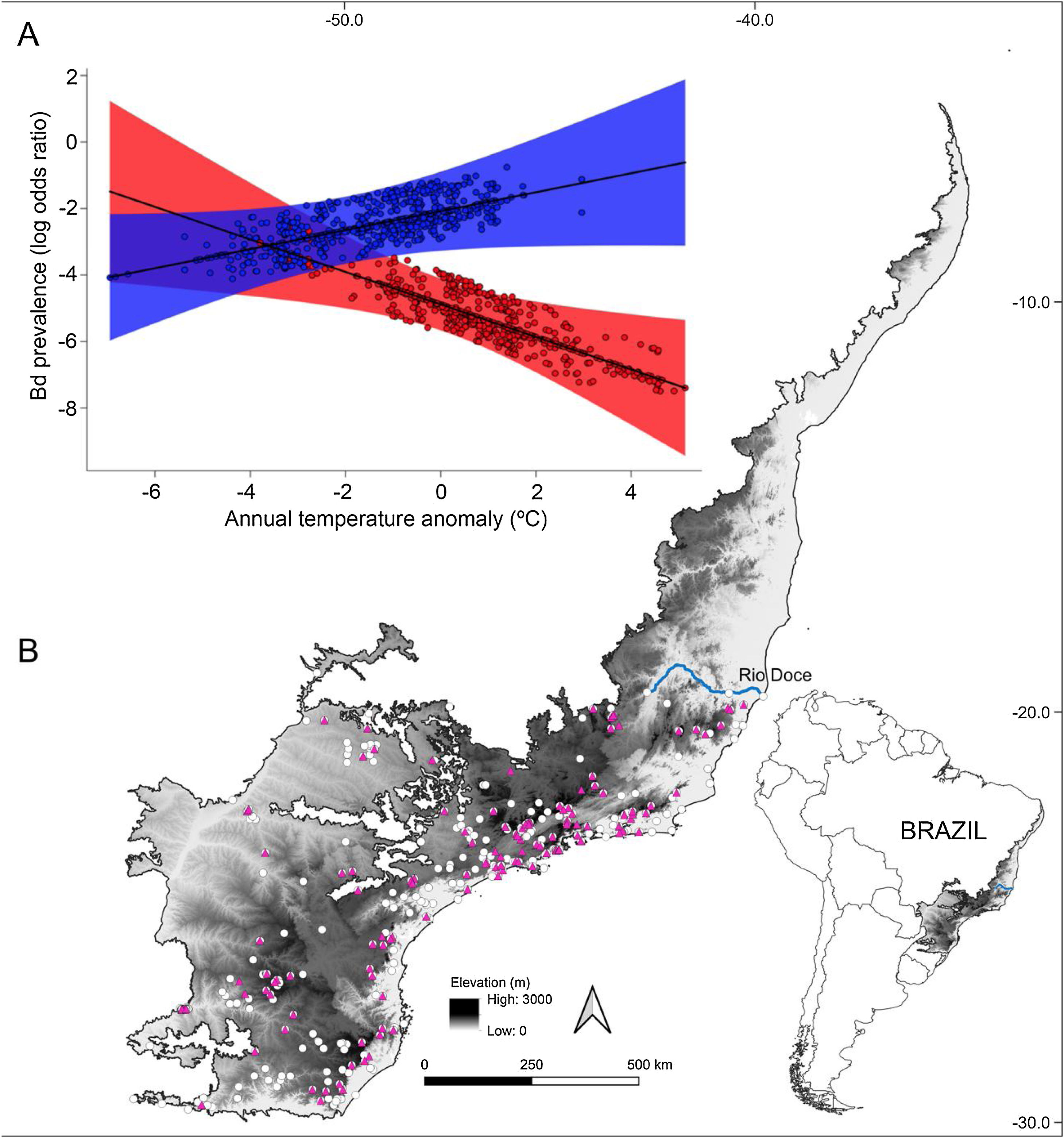
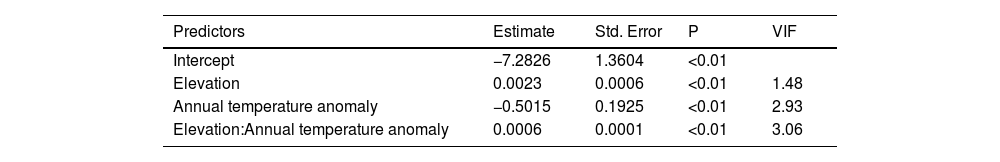
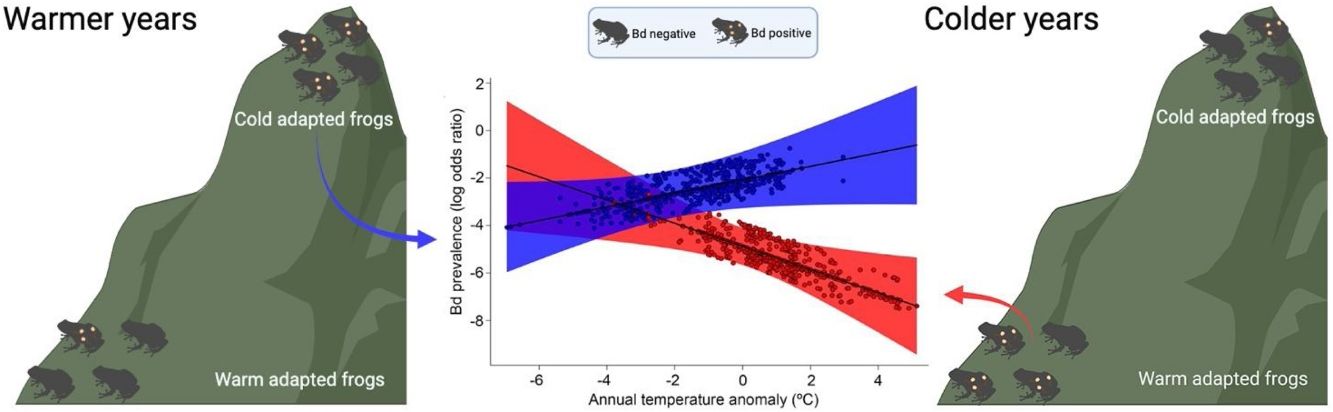
ResultsOur result showed that elevation (β = 0.0023; P < 0.01) and temperature anomaly (β = −0.5015; P < 0.01) are strong predictors of Bd prevalence (Table 1– model with highest Akaike weight). In addition, we also determined that the effect of temperature anomaly on Bd prevalence was modulated by elevation as well, as shown by the significant interaction between these two factors (β = 0.0006; P < 0.01; Table 1; Fig. 1A). Our findings indicate that Bd prevalence increased in highland cool-adapted hosts during warmer years, while it also increased in lowland warm-adapted hosts during cooler years. Furthermore, our result suggests that highland cool-adapted hosts showed the highest levels of infection prevalence regardless of temperature, with Bd prevalence almost doubling during warmer-than normal years (Fig. 1A). Lastly, the Fligner–Killeen test for homogeneity of variances determined that host species at lower elevations in our study experienced greater temperature variability (χ2 = 71.236, df = 1, P < 0.001). However, based on model slopes, both lowland and highland amphibians experienced comparable effects of thermal mismatch (Fig. 1A).
Parameter estimates of the Generalized Linear Mixed Model with the highest Akaike weight. The model accounted for temporal autocorrelations (AR1: year and seasons) to test the effects of environmental variables on Batrachochytrium dendrobatidis prevalence. Parameter estimates are on the logit scale, and can be back-transformed as ex/(1+ex). Variance Inflation Factors (VIF) are also included.
| Predictors | Estimate | Std. Error | P | VIF |
|---|---|---|---|---|
| Intercept | −7.2826 | 1.3604 | <0.01 | |
| Elevation | 0.0023 | 0.0006 | <0.01 | 1.48 |
| Annual temperature anomaly | −0.5015 | 0.1925 | <0.01 | 2.93 |
| Elevation:Annual temperature anomaly | 0.0006 | 0.0001 | <0.01 | 3.06 |
Latitude: Variance = 12.336; Times series (across years): Variance = 4.045, Corr(ar1) = 0.91; Times series (across seasons): Variance = 0.635, Corr(ar1) = 0.13; N = 17,616.
(A) Estimated relationship between thermal mismatch (significant interaction term: ‘elevation:annual temperature anomaly’) and pathogen prevalence. Partial residual plot of Batrachochytrium dendrobatidis (Bd) prevalence showing the estimates from the Generalized Linear Mixed Model with the highest Akaike weight. The color red represents lowland warm‐adapted hosts (10th percentile elevation centered on 20 m) and blue represents highland cool‐adapted hosts (90th percentile elevation centered on 1248 m). Points represent individual hosts screened for Bd, and shading shows 95 % confidence interval. (B) Geographical distribution of Bd-positive (purple triangles) and Bd-negative tadpoles (white dots) collected between 1963 and 2013 in the Southern Atlantic Forest.
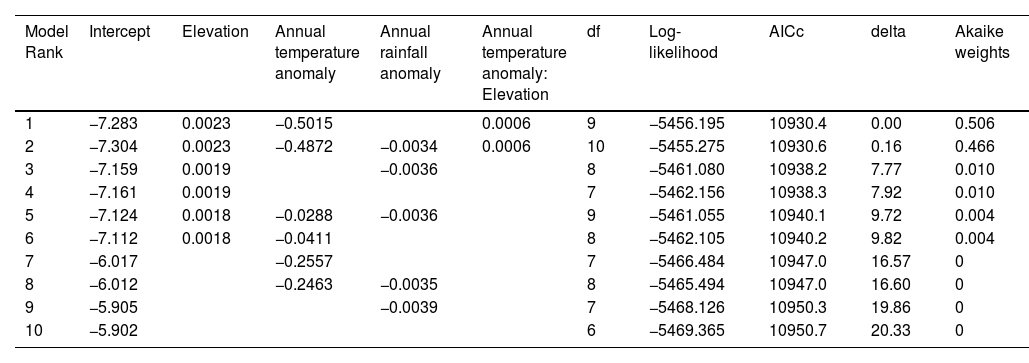
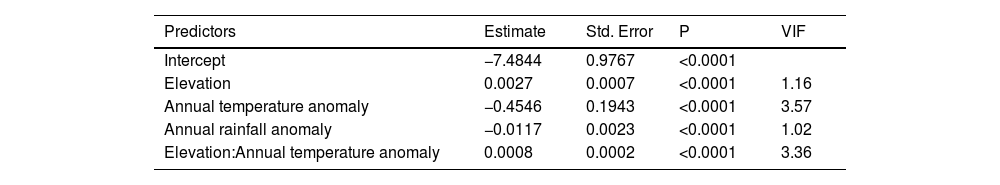
To verify the explanatory power of the full model mentioned above, we determined, using an automated model selection method, that the best two (and most parsimonious) models included the thermal mismatch term (elevation:annual temperature anomaly; Table 2). The top-ranked model included, in addition to the interactive effect between elevation and annual temperature anomaly, the effect of each of these variables alone, while the second-best model also included the effect of rainfall anomaly (Table 2). To better understand why rainfall anomaly did not feature in the top-ranked model, we ran an alternative model excluding ‘seasons’ and determined that the effect of rainfall anomaly was likely diluted when we incorporated the former as a covariance structure AR(1) (Table 3). In addition, to further assess whether our decision to include ‘seasons’ as a covariance structure AR(1) in our global model was the most parsimonious choice, we conducted a likelihood ratio test (function anova) comparing the fit of both models (with and without 'seasons'), and determined that the inclusion of ‘seasons’ did significantly improve model fit (χ2 = 577.64, P < 0001).
Results of automated model selection. Shown are standardized regression coefficients for numeric predictors.
| Model Rank | Intercept | Elevation | Annual temperature anomaly | Annual rainfall anomaly | Annual temperature anomaly: Elevation | df | Log-likelihood | AICc | delta | Akaike weights |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −7.283 | 0.0023 | −0.5015 | 0.0006 | 9 | −5456.195 | 10930.4 | 0.00 | 0.506 | |
| 2 | −7.304 | 0.0023 | −0.4872 | −0.0034 | 0.0006 | 10 | −5455.275 | 10930.6 | 0.16 | 0.466 |
| 3 | −7.159 | 0.0019 | −0.0036 | 8 | −5461.080 | 10938.2 | 7.77 | 0.010 | ||
| 4 | −7.161 | 0.0019 | 7 | −5462.156 | 10938.3 | 7.92 | 0.010 | |||
| 5 | −7.124 | 0.0018 | −0.0288 | −0.0036 | 9 | −5461.055 | 10940.1 | 9.72 | 0.004 | |
| 6 | −7.112 | 0.0018 | −0.0411 | 8 | −5462.105 | 10940.2 | 9.82 | 0.004 | ||
| 7 | −6.017 | −0.2557 | 7 | −5466.484 | 10947.0 | 16.57 | 0 | |||
| 8 | −6.012 | −0.2463 | −0.0035 | 8 | −5465.494 | 10947.0 | 16.60 | 0 | ||
| 9 | −5.905 | −0.0039 | 7 | −5468.126 | 10950.3 | 19.86 | 0 | |||
| 10 | −5.902 | 6 | −5469.365 | 10950.7 | 20.33 | 0 |
Parameter estimates of the Generalized Linear Mixed Model without considering 'seasons' in the model. The model accounted for temporal autocorrelation (AR1: year) to test the effects of environmental variables on Batrachochytrium dendrobatidis prevalence. Parameter estimates are on the logit scale, and can be back-transformed as ex/(1+ex). Variance Inflation Factors (VIF) are also included.
| Predictors | Estimate | Std. Error | P | VIF |
|---|---|---|---|---|
| Intercept | −7.4844 | 0.9767 | <0.0001 | |
| Elevation | 0.0027 | 0.0007 | <0.0001 | 1.16 |
| Annual temperature anomaly | −0.4546 | 0.1943 | <0.0001 | 3.57 |
| Annual rainfall anomaly | −0.0117 | 0.0023 | <0.0001 | 1.02 |
| Elevation:Annual temperature anomaly | 0.0008 | 0.0002 | <0.0001 | 3.36 |
Latitude: Variance = 13.438; Times series (across years): Variance = 3.346, Corr(ar1) = 0.8; N = 17,616.
Our study indicates that peaks in Bd prevalence in preserved specimens of Neotropical amphibians sampled over a 50-year period could be linked to temperature deviations from historical climatic conditions. Specifically, highland, cool-adapted amphibians showed higher Bd prevalence in the warmer-than normal years, while lowland, warm-adapted populations showed higher Bd prevalence in the coolder-than normal years, supporting the thermal mismatch hypothesis for Neotropical amphibians (Cohen et al., 2017). Interestingly, we detected a significant and consistent rate of increase in Bd prevalence in lowland, warm-adapted hosts, during colder years, even though they likely evolved under conditions of higher temperature variability compared to cool-adapted amphibians. However, highland amphibians still showed a relatively higher risk of Bd infection, indicating the thermal mismatch as another potential mechanism that, synergistically with the direct physiological effects of accelerated global warming and variability, may drive several high-elevation amphibian species to extinction. Lastly, we found comparable effects of thermal mismatch (regression slopes) for both lowland and highland amphibians even though highland amphibians experienced less climate variability.
High-elevation amphibians have been disproportionately impacted by Bd, both in Brazil (Carvalho et al., 2017; Toledo et al., 2023) and elsewhere (Fisher and Garner, 2020; Scheele et al., 2019). This suggests a potential synergistic link between climatic anomalies and the presumed disease-induced amphibian declines throughout the Atlantic Forest of Brazil after Bd had been already enzootic for many decades. The earliest detection of Bd in the region dates back to the late 1800s (Rodriguez et al., 2014), contrasting with other parts of the globe where Bd emerged and almost immediately decimated naive amphibian populations. Our findings indicate that accelerated global change driving climate variability could cause recurrent Bd outbreaks in amphibian communities that have already adapted to and rebounded from historical Bd outbreaks.
The evidence that climatic anomalies could also influence the severity of chytridiomycosis in highly diverse lowland amphibian communities in the SAF shows that global climate change could also favor Bd outbreaks in regions spared from recent population crashes (Carvalho et al., 2017; Toledo et al., 2023). Colder temperatures are known to reduce the metabolic rate of warm-adapted species, thereby compromising various behaviors, biochemical pathways, and physiological processes (Angilletta, 2009; Huey and Stverson, 1979). For instance, as the amphibian immune function (including innate and adaptive immune responses) depends on external heat sources, low temperatures can cause reductions in peripheral leukocyte levels, lymphocyte (T and B cells) proliferation, macrophage endocytosis, secretions of antimicrobial skin peptides (Carey et al., 1999; Maniero and Carey, 1997; Matutte et al., 2000; Raffel et al., 2006), and shifts in antimicrobial metabolites produced by amphibian skin bacteria (Medina et al., 2017), potentially increasing the risk of infection following a weakened immune response (Raffel et al., 2006). However, until now, lowland amphibians have been mostly unaffected by Bd, with no reported Bd-induced population declines and extinctions (Carvalho et al., 2017; Scheele et al., 2019; Toledo et al., 2023). This could be attributed to the fact that the adverse effects of extreme cold snaps on the immune system of warm-adapted amphibians, which could trigger Bd-related declines, are often counterbalanced by the overall global trend of increasing average temperatures. Thus, the observed increasing temperatures above Bd’s optimal range are probably reducing the likelihood of infections and disease outbreaks in tropical lowlands. However, abnormally cool weather events are more likely to occur with the predicted increases in temperature variability (Cai et al., 2014; Easterling et al., 2000; Schär et al., 2004), which could still intensify disease pressure on lowland amphibian species through mechanisms associated with thermal mismatch.
Overall, our findings suggest that the effect of climatic anomalies on Bd dynamics varies along the studied elevational gradient. Based on the observed results, we expect that warmer years ahead of us might lead to heightened Bd pressure in tropical amphibian populations at higher elevations. Although many studies have found Bd prevalence to be positively associated with elevation (Brem and Lips, 2008; Gründler et al., 2012), others have observed a negative (Catenazzi et al., 2011) or no relationship with Bd (Kriger and Hero, 2008; Lambertini et al., 2016; Zornosa-Torres et al., 2021), or have identified confounded effects of elevational and land cover change (Becker and Zamudio, 2011). Therefore, we propose that incorporating information about host thermal mismatch into modeling approaches could serve as a more practical strategy for developing more targeted disease management programs.
ConclusionsWe hereby provide support for the thermal mismatch hypothesis in tropical amphibian populations historically impacted by the chytrid fungus Batrachochytrium dendrobatidis, and highlight the need to consider elevational breadths and optimum temperature ranges of amphibians while modeling Bd dynamics in the wild. Our study also suggests that a synergistic interaction between climatic anomalies and Bd may have triggered post-enzootic outbreaks of chytridiomycosis, leading to several observed historical amphibian declines in Brazil. By understanding the underlying mechanisms driving host-pathogen dynamics in amphibians, we can better direct our efforts towards preventing declines and extinctions in other vertebrate groups facing the challenges of an increasingly unpredictable climate.
Data availability statementThe data and full code for all statistical tests are available at dryad (https://doi.org/10.5061/dryad.69p8cz98k).
Conflict of interestThe authors have declared no conflicts of interest.
We thank Gabriel Schubert Ruiz Costa for technical assistance throughout data compilation. Grants and fellowships were provided by São Paulo Research Foundation (FAPESP #2016/25358-3; #2019/18335-5; #2022/09659-4), the National Council for Scientific and Technological Development (CNPq #302834/2020-6), and by the Coordination for the Improvement of Higher Education Personnel (CAPES - Finance Code 001).