Differently from commonly used forest conservation strategies, the absence of disturbance in non-forest-ecosystems can result in loss of biodiversity. Grassy ecosystems characterize extensive areas in all biomes in Brazil, offering great contribution to biological diversity and providing ecosystem services on which society depends. Palaeoecological evidence indicates these ecosystems evolved under the influence of grazing and fire, and these disturbances have been essential for controlling the dominance of woody vegetation. While the need for fire to maintain grassy biomes has been recently discussed, grazing as a management tool for conservation is still little accepted by the local scientific community and by decision-makers. Here we provide a comprehensive analysis of the current literature on grazing management and its effects on grassy ecosystems in Brazil. Based on a review of the role of grazers prior and after European colonization, and a synthesis on grazing effects across different systems, we call for the evaluation of grazing as a management tool for biodiversity conservation in Brazil’s grassy systems. Grazing should be an interesting management strategy especially in Legal Reserves with grassy vegetation types, as it can ally conservation with livestock production. To achieve these potentially complementary objectives, a research agenda needs to be developed to devise adaptive management strategies for Legal Reserves. In this, relevant stakeholders should be included and both the ecological conditions of the ecosystem in question and the socioeconomic determinants considered.
Grassy ecosystems are characterized by a continuous layer of herbaceous species (i.e., dominated by graminoids and forbs), and include grasslands without trees, savannas and open woodlands (Parr et al., 2014; Veldman et al., 2015). In Brazil, remnants of these non-forest ecosystems occupy ca. 120 million hectares and occur in all biomes (Overbeck et al., 2015). As in other regions of the world, mostly in the tropics and subtropics, these ecosystems have often been misunderstood as degraded systems produced by tree clearing followed by burning and grazing, or as systems in secondary succession (Lehmann and Parr, 2016; Veldman et al., 2015). However, palaeoecological data indicate, for large parts of South America, the past presence of open environments characterized by frequent disturbances such as fire and herbivory (Bond, 2015; MacFadden, 2005). These large extensions of grass-dominated habitats developed about 18 million years ago (Strömberg et al., 2013). Approximately 7 million years ago, C4 grasses, the main diet for ungulates, became the dominant group of plants in tropical and subtropical regions (Strömberg, 2011). Around the world, the long period of coexistence and coevolution of plants and large ungulates, associated with the presence of fire and together with considerable climatic and soil variability formed ecosystems with high plant species diversity and often high levels of endemism (Veldman et al., 2015), and this is also true for South America (Overbeck et al., 2007; Strömberg, 2011).
At present, a range of ecosystem services benefitting millions of people living in Brazil rely on grassy ecosystems (Asner et al., 2004; Overbeck et al., 2015). Conservation of these systems and their benefits thus should be an important issue in conservation policy. However, conservation and restoration in Brazil currently still do not consider the need to maintain the disturbance regimes that grassy ecosystems have coevolved with and that is necessary for their structure and biodiversity (Buisson et al., 2018; Durigan and Ratter, 2016; Overbeck et al., 2018; Pillar and Vélez, 2010). In grassy biomes, suppression of disturbances such as fire and grazing promotes strong effects on many aspects of the ecosystem, such as drastic changes in the grassy plant community (Altesor et al., 2006; Overbeck et al., 2005), tree and shrub encroachment (Geiger et al., 2011; Griffith et al., 2017; Oliveira and Pillar, 2004) , decreasing species diversity (Abreu et al., 2017; Ferreira et al., 2020), thus modifying ecosystem processes (Müller et al., 2012; Veldman et al., 2015) as well as services and benefits that derive from them (e.g. Honda and Durigan, 2017).
The Brazilian Native Vegetation Protection Law from 2012 (Law 12.651/2012) establishes the protection or restricted use of natural vegetation in every rural property by means of the ‘Legal Reserve’ requirement. The purpose of the Legal Reserve is the conservation of biodiversity and the maintenance of ecosystem services, while also allowing for sustainable use of natural resources in rural properties (Metzger et al., 2019). Legislation requires at least 20% of the farm area set-aside as Legal Reserve, and in the Legal Amazonia region the requirement increases to 35% for savanna vegetation and to 80% for forests. Considering the fact that large proportions of grassy ecosystems are in private land, and are part of the Legal Reserve network in Brazil, it seems important that their conservation can be integrated into the current land use and land tenure system. Altogether, based on the requirements of the Native Vegetation Protection Law from 2012 (n. 12.651/2012), almost 1.1 million km2 of non-forest vegetation, most of it grass-dominated, is to be included in Legal Reserves and in ecologically critical areas designated as Permanent Protection Areas (Overbeck et al., 2015). Additionally, protected areas with presence, or at least dominance, of grassy ecosystems are in urgent need of appropriate management strategies (see e.g. Carlucci et al., 2016; Overbeck et al., 2016).
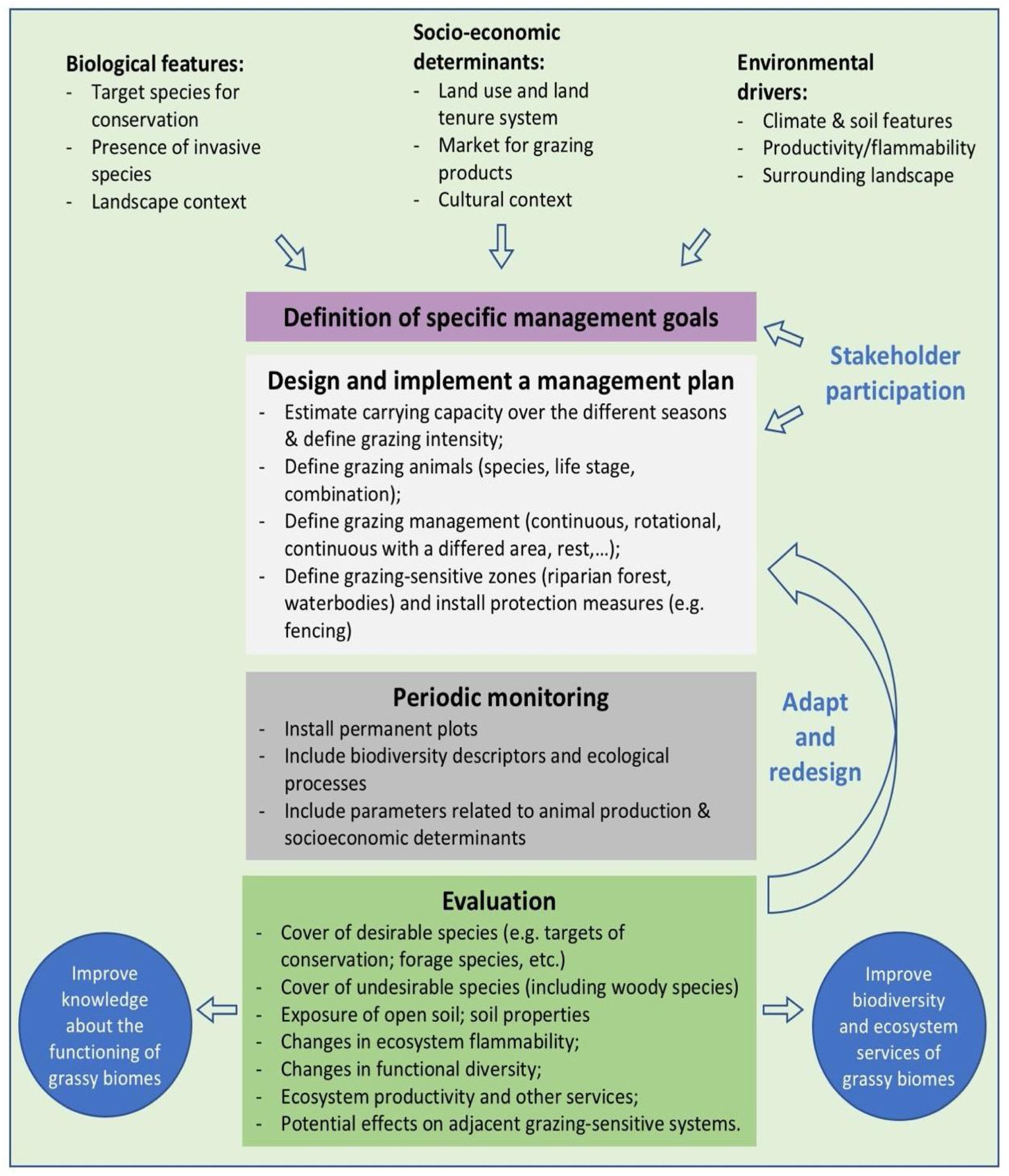
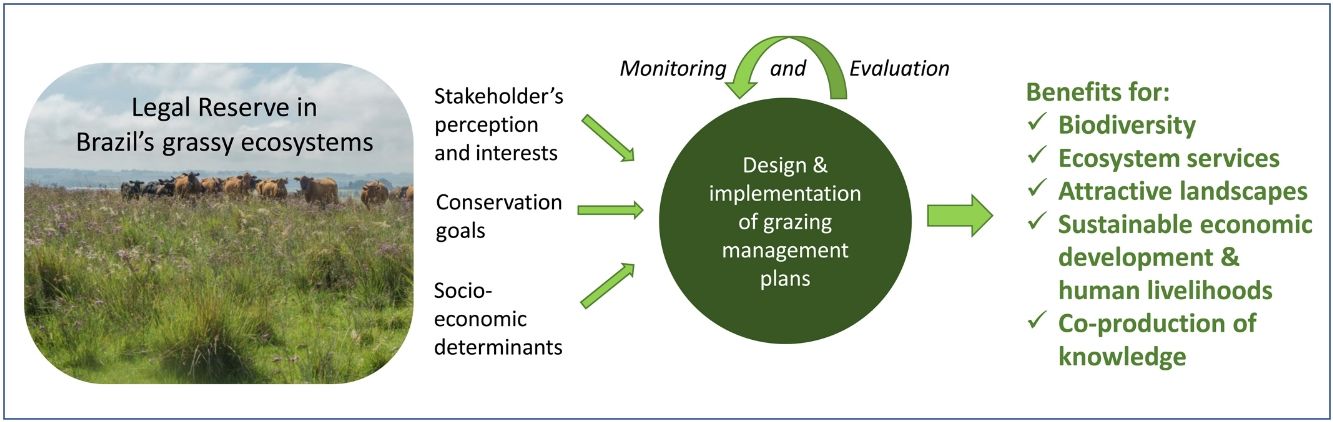
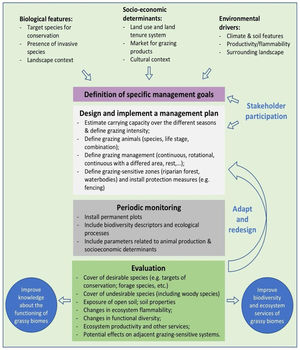
In the Legal Reserve, sustainable exploitation is allowed, providing that a management plan is authorized by the environmental agencies. However, when the Law explicitly refers to sustainable management (Articles 20–24), grassy biomes are ignored: all regulations refer to forest. Therefore, the question ‘how can grassy ecosystems in the Legal Reserve be used in a sustainable manner’ remains unresponded. As most grassy ecosystems remnants are privately owned (Overbeck et al., 2015), building partnerships with farmers in the design and monitoring of management strategies in Legal Reserves may be crucial for conservation. With this paper, we aim to advance in the debate on whether managing disturbances can contribute to biodiversity conservation in Brazil. We focussed on the possibilities offered by management with grazing animals, still often considered a taboo for conservation in Brazil (Carvalho, 2014; Pillar and Vélez, 2010). To this end, here we summarize recent findings and present a framework (Fig. 4) for the decision-making process in specific situations across Brazil.
The palaeodynamics of grassy biomes and the role of large grazersThe present configuration of Brazil’s ecosystems originates from climatic fluctuations during the late Quaternary, with expansion of open ecosystems in detriment of forests during glacial and the opposite in interglacial periods (Ratter et al., 1997). The evidence suggests that the drivers for these dynamics include changes in environmental properties, such as insolation, concentration of carbon dioxide in the atmosphere, temperature and pluviometric regimes, as well as changes in disturbance regimes, especially those related to fire and herbivory by megafauna (Alizadeh et al., 2015; Doughty et al., 2016; Rull et al., 2015). Until the end of the Pleistocene and early Holocene, large herbivores existed throughout South America, and theirmain diets have been inferred by carbon and oxygen isotope analyses (Omena et al., 2020). These herbivores included species of the genera Hippidion (mixed-feeder of C3 and C4 plants) and Equus (predominantly C4 grazer), Notiomastodon (C4 grazer and mixed-feeder), Toxodon (C4 grazer and mixed-feeder) and Macrauchenia (mixed-feeder) (França et al., 2015; MacFadden, 2013; Omena et al., 2020; Lopes et al., 2020). Based on the range of occurrence of these large grazers and their diets, the Caatinga and Cerrado biomes were characterized by a mixed vegetation and domination by C4 plants, that is, grasses (Omena et al., 2020). Further, palaeoecological records of spores produced by the coprophilous fungus Sporormiella have been used as a proxy of large herbivore presence and abundance (Raczka et al., 2016). Records from the Lagoa Santa region in SE Brazil (Raczka et al., 2018) indicate that by the end of the Pleistocene the concentrations of spores were about three orders of magnitude larger than during the Holocene after the extinction of large herbivores, and at similar levels of present-day concentrations observed in other sites with high domestic cattle use (Raczka et al., 2016).
During the Late Pleistocene, two processes occurred concomitantly to climatic changes: the arrival and establishment of human populations in South America and the massive extinction of 59 megafauna species (Sandom et al., 2014). There is evidence that different species of the now-extinct large grazers coexisted with early human populations (Lima-Ribeiro and Diniz-Filho, 2013). The lack of disturbances caused by large herbivores after their extinction combined with increased rainfall in the mid to late Holocene may have been key factors for the expansion of forests in detriment of open savannas and grasslands in some regions (Doughty et al., 2016; Gill, 2014; Mayle et al., 2009). Yet, palaeopollen data show that throughout these periods the dominant grassy vegetation and their species remained and now characterize open ecosystems in Brazil (e.g., Behling, 2002; Salgado-Labouriau et al., 1997).
From European colonization to presentDuring the late Holocene (last ~2000 years BP), the current environmental conditions consolidated with increasing summer rainfall, which favoured the expansion of forest species over grassy ecosystems, mainly in the South Brazilian grassland and the Cerrado, and expanding the areas occupied by Atlantic and the southern Amazonian forests. This process of forest expansion was likely restrained in some regions with the introduction of cattle (Bos taurus and Bos indicus) and the reintroduction of horses (Orlando et al., 2008) by European colonizers in the 16th and 17th century. In north-eastern Brazil, while coastal forests were opened for sugar cane plantations, livestock production developed in the semi-arid interior, associated with a nomadic pastoral culture of Sertanejos in response to the availability of resources for animal production (forage), among other socio-cultural issues (Claudino, 2016; Vaz, 2013). In the Pampa region to the east from the Uruguay river, livestock was introduced by the Spanish Jesuits in the 17th century and rapidly spread over the Pampa grasslands of southern Brazil and Uruguay, forming vast herds of feral animals (Osório, 2015), which were later introduced in the grasslands of the highland plateau further north.
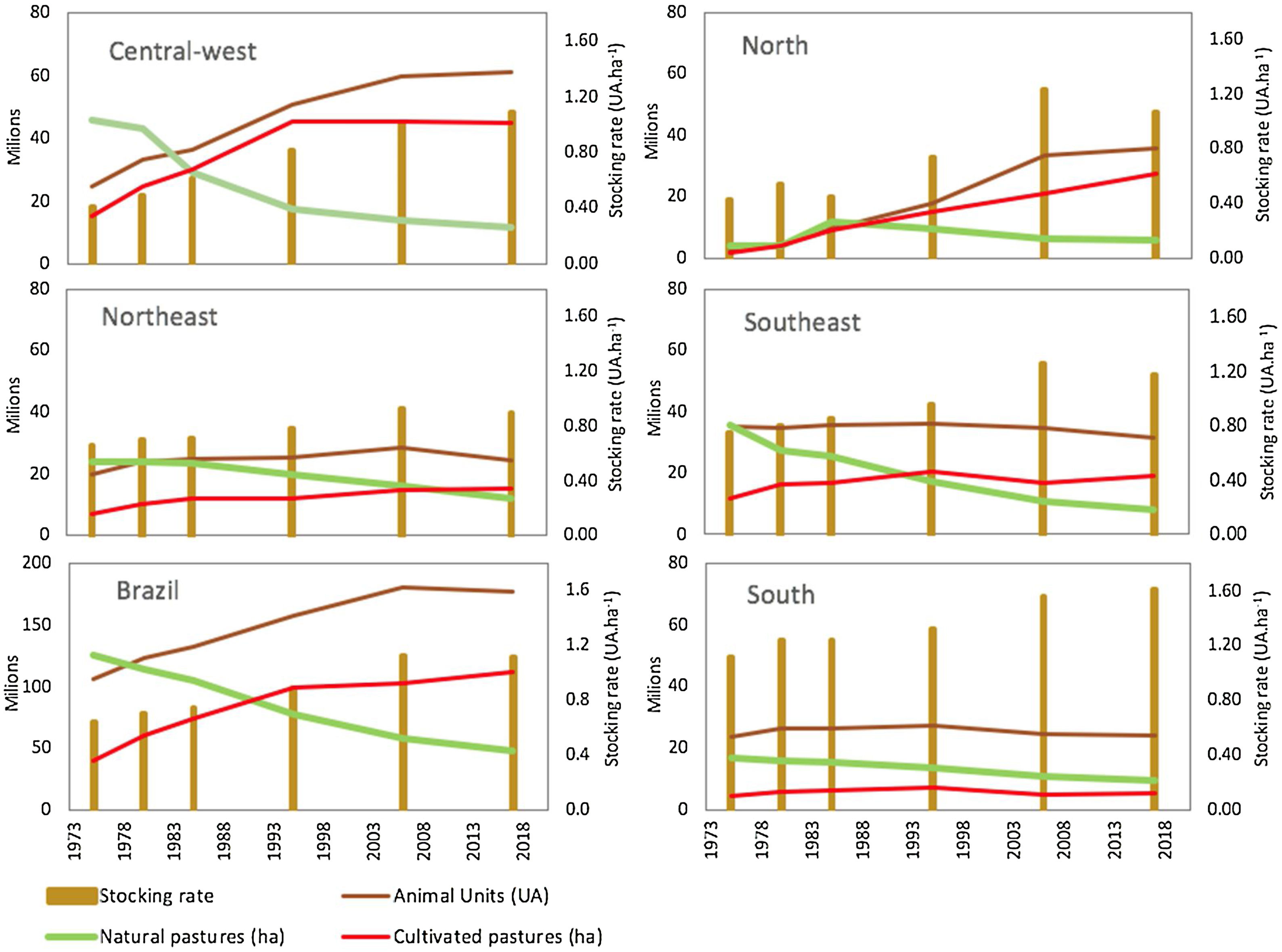
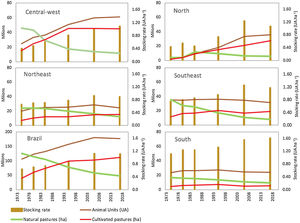
Scientific studies from the 1970s and 1980s indicate widespread low-intensity livestock production in natural grassy ecosystems of the Cerrado, Amazon and Pantanal regions before the intensification of grazing lands and the planting of exotic forage species. Indeed, until the 1970s, these natural pastures were the main forage source for Brazilian livestock (Fig. 1; Claudino, 2016; Silva et al., 2012; Serrão and Falesi, 1977; Saturnino et al., 1977; Serrão, 1986). Serrão and Falesi (1977) provide information about the forage potential of native species and physiognomies in the Cerrado, Pantanal and Amazon regions. Saturnino et al. (1977) describe in detail the production systems on natural pastures across the country and compare their economic viability with intensive systems based on the introduction of exotic forage species. The management of campos limpos, campos sujos and campos cerrados involved burning during the dry season and the use of the regrowth as a pasture under a stocking rate between 10 to 4 ha per animal unit (AU) (Serrão, 1986). In the state of Minas Gerais, Saturnino et al. (1977) also describe a livestock production system associated with the extraction of wood for charcoal production, in a cycling process. While the low productivity of livestock systems under natural pastures may be attributed to the low quality of dominant plant species, management practices lead to low quantity and quality of available forage (Saturnino et al., 1977; Serrão and Falesi, 1977; Serrão, 1986).
Temporal changes in grazing lands (natural pastures and cultivated pastures) and livestock in different regions of Brazil. Data from six agricultural censuses carried out from 1975 to 2017 by the Brazilian Institute of Geography and Statistics (IBGE). Natural pastures are areas of native grassy ecosystems (grassland, savannas) used for grazing. Animal Units (AU) correspond to the total number of sheep and goat divided by 6 plus the total number of cattle and buffaloes regardless of age and race. The stocking rate is the number of Animal Units divided by the total area of cultivated pastures and native grasslands.
Exotic forage species were initially introduced by planting, but from the 1960s on, their expansion was accelerated due to the import, production and use of seeds (Fonseca et al., 2010). In the 1970s, the area occupied by only a few species, mainly from the Brachiaria genus, reached more than 20 million hectares in central-west Brazil (Fig. 1). This process was strongly encouraged by Brazilian research institutions through programs such as the Pasture Improvement Project for the Legal Amazon – PROPASTO (Serrão, 1986; Townsend et al., 2012) and the Cerrados Development Program – POLOCENTRO (Saturnino et al., 1977). The conversion of native vegetation into cultivated pastures started in the southeast and central-west regions and later expanded to the north of Brazil, allowing for the intensification of livestock production by increasing the stocking rate to a much higher level ranging from 1 to 1.3 AU/ha (Fig. 1).
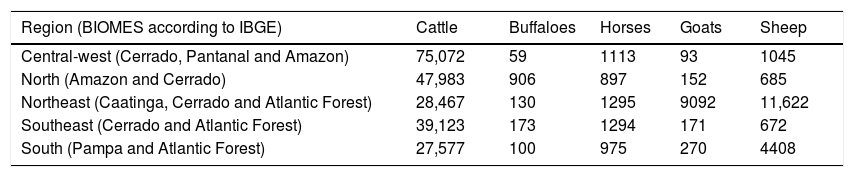
Although the animal species predominantly used in Brazilian pastoral systems to date are bovine cattle (Bos taurus, Bos indicus), there are also significant herds of sheep (Ovis aries), goats (Capra aegagrus), horses (Equus ferus) and buffaloes (Bubalus bubalis) (Table 1). The vast majority of these animals are at present kept in cultivated pastures with exotic grasses (Batista et al., 2020), except in the Pampa, Pantanal, and Caatinga, and in the Marajó island, where grazing natural vegetation is still prevalent. In the Pampa grasslands and in the highland grasslands of the southern part of the Atlantic Forest region, there is a predominance of cattle of European breeds (Bos taurus), and sheep, adapted to the more temperate conditions (Pillar et al., 2009). Elsewhere, Indian breeds (Bos indicus) are preferred, which are more adapted to the hot climate. While in the Cerrado and Amazon Forest, cattle production is mostly based on pastures with exotic grasses (Batista et al., 2020), in the Pantanal, with its singular hydrological dynamics, natural grasslands are the principal basis for cattle grazing. In recent years, however, the use of exotic forage species has increased in the Pantanal (Aquino et al., 2017). Large numbers of goats and sheep stand out specifically in the semiarid north-eastern Brazil, which is linked to their ability to consume woody species present in the Caatinga as well as to survive under the semi-arid condition. In the north, especially in Marajó island, in the state of Pará, large herds of buffaloes are adapted to the consumption of coarser native grassy vegetation as well as wetlands to maintain their thermal comfort.
Herds, in thousands of animals for the year 2016, in different regions of Brazil (IBGE 2016).
| Region (BIOMES according to IBGE) | Cattle | Buffaloes | Horses | Goats | Sheep |
|---|---|---|---|---|---|
| Central-west (Cerrado, Pantanal and Amazon) | 75,072 | 59 | 1113 | 93 | 1045 |
| North (Amazon and Cerrado) | 47,983 | 906 | 897 | 152 | 685 |
| Northeast (Caatinga, Cerrado and Atlantic Forest) | 28,467 | 130 | 1295 | 9092 | 11,622 |
| Southeast (Cerrado and Atlantic Forest) | 39,123 | 173 | 1294 | 171 | 672 |
| South (Pampa and Atlantic Forest) | 27,577 | 100 | 975 | 270 | 4408 |
Currently, grazing with different combinations of cattle, buffaloes, sheep, horses and goats is practiced in grassy ecosystems in all Brazilian biomes, in different production systems that vary, for example, in terms of grazing intensity, depending on the environmental conditions and also on regional traditions. Whether the current effects of domesticated grazers on grassy ecosystems is similar to the past effects from the now-extinct large grazers is an open question (Lorimer et al., 2015). First, grazing animals, including domestic ones, differ in their grazing behaviour and effects. Second, due to climatic changes, as discussed above, the current grassy ecosystems differ in aspects such as productivity and seasonality from those found in the past. Nonetheless, we have clear evidence that exclusion of grazing animals, combined with fire suppression and a wetter climate, cause strong and rather fast changes in vegetation structure, especially encroachment of woody vegetation and losses of characteristic species of open ecosystems, as much documented for grasslands in southern Brazil (Boldrini and Eggers, 1997; Ferreira et al., 2020; Müller et al., 2012) and adjacent grasslands to the south (Altesor et al., 2006; Lezama et al., 2014). Likewise, strong changes in vegetation cover and biodiversity under exclusion of disturbances have been shown also for the Cerrado (Abreu et al., 2017; Durigan et al., 1987).
Major grassy ecosystems across Brazil present similar plant genera composition in the herbaceous stratum. Examples for dominant genera are Paspalum, Axonopus, Andropogon, Leersia, Desmodium and Trachypogon, which occur from the Pampa in the south to the grasslands and savanna of the Amazon (Boldrini, 2009; Klink and Joly, 2006; Rocha et al., 2014; Santos et al., 2002). Most of the species forming the herbaceous stratum in grassy ecosystems have traits that allow rather quick regeneration after disturbances such as fire and grazing (Archibald et al., 2019), including protected meristems (i.e. rhizomatous and rosette species), clonality, presence of bud banks and below-ground storage organs (Pilon et al., 2020; Overbeck and Pfadenhauer, 2007; Fidelis et al., 2010). However, grazing itself influences the functional characteristics of the plant communities: heavier grazing will lead to the selection for plant species with the capacity to quick recovery of their aboveground biomass, i.e., resource-acquisitive species, while low grazing intensities, or even absence, will lead to the selection of resource-conservative species, such as tussock grasses (Cruz et al., 2010). Needless to say, the great variability of rainfall distribution and volume (from less than 300 mm to over 1800 mm per year) and soil types (variable combinations of texture, fertility and depth) across Brazil’s grazing systems, which interact with existing biota, also condition the intensity of grazing that each ecosystem can support.
Linked to the past megafauna extinctions, initiatives for ‘rewilding’ have developed on almost all continents. The common goal of these initiatives – despite a variety of approaches and concepts – is the maintenance or the enhancement of biodiversity and ecological processes, reducing the impact of present and past human interventions through the reintroduction of populations of specific animal species (Lorimer et al., 2015; Malhi et al., 2016). The development of rewilding projects initially took into account the expectation of returning to different historical benchmarks for each continent (predominantly between the Middle Pleistocene and Early Holocene). We recognise the contribution of rewilding research to the understanding of ecological dynamics in the pre-human world and the ecological and evolutionary consequence of the megafauna absence (Lorimer et al., 2015). However, here we do not propose the reintroduction and maintenance of “de-domesticated” or wild populations with minimal intervention by humans, as done, for example in Oostvaardersplassen nature preserve on Netherlands, Pleistocene Park in Siberia and Yellowstone National Park in US. Instead, we argue that well-managed grazing of natural grassy ecosystems by large domestic mammals, in private land and with economic returns, does not jeopardize the biodiversity and functioning of these ecosystems but can, in contrast, provide an economically feasible way to conserve grassy ecosystems.
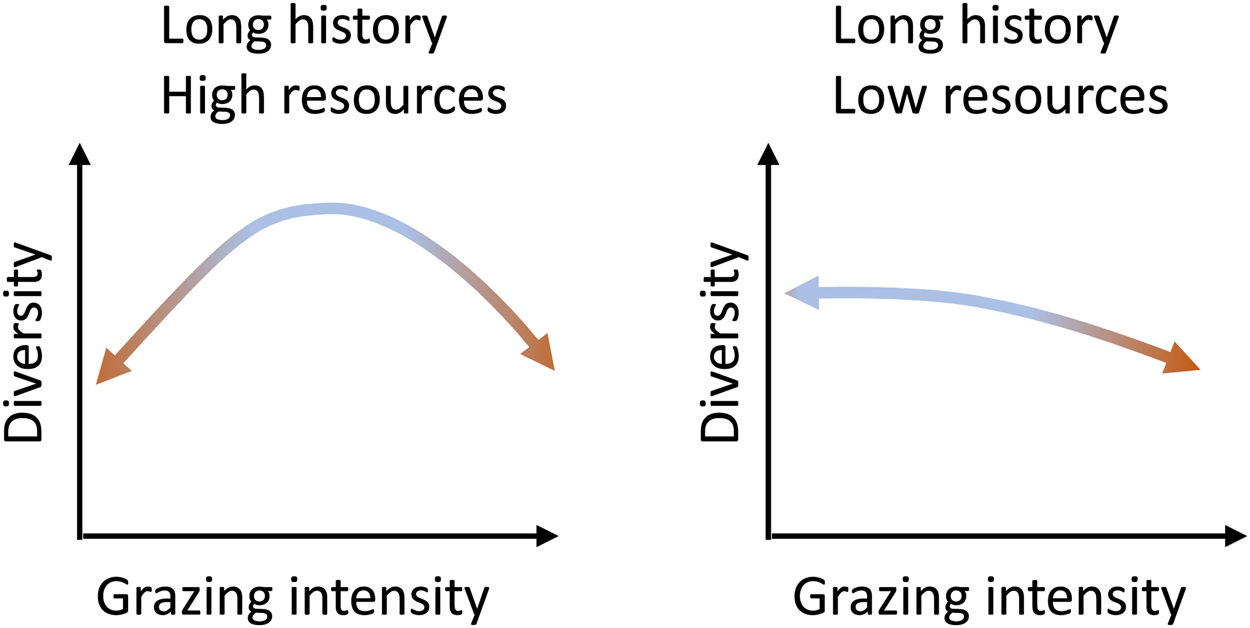
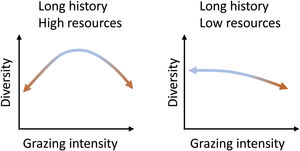
What do we know about grazing impacts on Brazil’s grassy ecosystems?Grazing is a complex process and can both induce changes to and depend on ecosystem structure (McNaughton, 1984). Cingolani et al. (2005), based on earlier conceptual models of the relationship between grazing and plant diversity (including Milchunas et al., 1988), proposed models on the response of ecosystems diversity to grazing intensity, based on evolutionary history of grazing in the system and (climatically defined) resource availability. Brazil’s grassy biomes, with their long evolutionary history of grazing, can be expected to fall either into their “long history - high resources” and “long history - low resources” categories (Fig. 2): The greater the abiotic limitations of the system, the more sensitive to grazing intensity the system should be. Where productivity is high, both the absence of grazing and excessive grazing should lead to diversity losses, and diversity will be maximized at intermediate levels. Where productivity is low, increasing grazing pressure will lead to diversity losses. Therefore, as diversity may be reduced at grazing level extremes, not all organisms that evolved in these environments are well adapted to grazing (Cingolani et al., 2005).
Equilibrium curves for two combinations of resource availability and long evolutionary history of grazing. They represent situations in which there is still the possibility of reversing the changes imposed by the intensity of grazing. (Adapted from Cingolani et al., 2005 and Milchunas et al., 1988).
Despite the economic importance of livestock production across Brazil’s regions, the literature on the effect of grazers on natural ecosystems is overall scarce. Published studies mostly are from the South Brazilian grasslands (Fedrigo et al., 2017; Lezama et al., 2014; Pillar et al., 2009) and the Pantanal (Santos et al., 2011). The available evidence supports the relevance of grazing for the maintenance of biodiversity and ecosystem processes in the grasslands of Pampa and in the southern part of the Atlantic Forest (Ferreira et al., 2020). Indeed, South Brazilian grasslands, almost completely under grazing, hold a very high biodiversity (Andrade et al., 2018; Menezes et al., 2018). For the other regions, we find some, and sometimes rather old, studies on the forage potential of species and ecosystems (Saturnino et al., 1977; Serrão, 1986; Townsend et al., 2012; Pereira-Filho et al., 2013), the productivity of these ecosystems with grazers (Townsend et al., 2012; Pereira-Filho et al., 2013), the use of fire on productivity and forage quality (Souza Filho et al., 1999) and on the degradation of these ecosystems. Among the negative effects addressed in these studies is overgrazing in the Pampa (Wagner et al., 2013) and in the Caatinga (Rito et al., 2017; Sousa, 2018; Schulz et al., 2019), and the promotion of Melinis minutiflora invasion by cattle in campo rupestre (Alves et al., 2014). Although these studies do not answer directly the question on how far native species and ecosystems are adapted to grazing, they give us indication of the potentials and limits for grazing management.
One relevant question is the grazing intensity that the different grassy ecosystems can support. The answer for each ecosystem will depend on the management used, the specific features of local biodiversity and the fluctuation of productivity throughout the year. Based on long-term studies carried out in the Pampa (Barbieri et al., 2014; Fischer et al., 2018), on the description of the livestock systems that existed until the 1970’s in the Cerrado, Pantanal and Amazon (Saturnino, 1977; Serrão, 1986), on the studies about the savannas of the Amazon (Townsend et al., 2012) and the Pantanal (Santos et al., 2011), some initial recommendations can be made. For example, in campos limpos and campos sujos of the Cerrado (Saturnino et al., 1977; Serrão, 1986) and in campos de terra firme and savannas of the Amazon (Serrão, 1986) on low-fertility soils with a well-defined dry season, a stocking rate between 3 to 10 ha per AU can be indicated. For the Pantanal, Santos et al. (2011) propose a higher carrying capacity (~0.5 AU/ha) for the open grasslands and lowlands than for carronal and savannas, with coarser vegetation, which supports a lower stocking rate (~0.4 AU/ha). For grasslands in the lowland areas of the Amazon River and in the grasslands of the Pampa and the highland region of southern Brazil, the potential is much higher. The grasslands of the lowland areas of the Amazon river receive a precipitation of about 2500 mm/year, the soils present high levels of nutrients, and the primary productivity lies between 5 to 18 Mg dry matter/ha including species of high forage quality. For these conditions, Townsend et al. (2012) suggest approximately 1 AU/ha. With rainfall well distributed throughout the year and the main limitation for primary productivity in the cold season, the grassy ecosystems of southern Brazil can support, on average, almost 1 AU/ha (Nabinger et al., 2009), and, depending on the management used, values of more than 2 AU/ha can be reached under specific conditions (Barbieri et al., 2014).
The debate on grazing as a conservation tool often focuses on supposed negative impacts of grazing. However, given that – as shown above – carrying capacities of grazing animals that are compatible with biodiversity of different systems can be determined, it appears that the dilemma “to graze or not to graze” (Bakker et al., 2003) is solved and that the question now is “how to graze?”. Potential negative effects can occur, but these will be consequences of specific – in this case, unsuitable – grazing strategies adopted (usually, too high grazing intensity). Given the potential of grazing to align biodiversity conservation with economic gains and thus rural livelihoods, the evaluation of grazing impacts and studies allowing for the definition of grazing strategies that promote major conservation objectives are urgently needed. These can be conducted in light of the experiences developed in similar ecosystems around the planet such as in the North-American (Fuhlendorf et al., 2012; Sayre et al., 2012) and African grassy ecosystems (Stevens et al., 2016; Hempson et al., 2017).
Towards best practices of grazing managementFor the design of grazing management strategies with conservation purposes, a crucial issue is what spatio-temporal distribution of grazers and intensity of grazing would be adequate to maximize conservation goals in each ecosystem type. Some of the frequently reported problems caused by grazing in ecosystem management are overgrazing, the impact on riparian ecosystems and water quality, and the damages to adjacent forest or other grazing-sensitive vegetation that domestic grazers may access. Studies also have demonstrated that uneven grazing distribution can increase soil erosion and may lead to changes in habitat conditions of endangered species, at both lower and higher grazing intensities (Fuhlendorf and Engle, 2001). However, these unwanted effects can easily be circumvented by help of artificial structures such as fences, water points away from riparian vegetation, artificial shading structures and resource attractors (Bailey, 2004). These strategies for controlling grazing heterogeneity have been recommended for more than 45 years in rangeland management for conservation in arid and semi-arid ecosystems in the western United States, but knowledge gap and the costs required for implementation have limited their adoption by livestock farmers (Briske et al., 2008). Therefore, implementation of management strategies involving grazing for grassy biomes in the different biomes in Brazil should include building partnerships with farmers to develop the most appropriate practices. Such practices shall consider conservation objectives in grassy biomes and adjacent systems as well as economic returns and thus overall feasibility of the grazing system.
The choices of domestic herbivores and grazing intensity in the different grassy ecosystems should take into account specific characteristics of each ecosystem such as the dynamics of primary production, vegetation structure, biomass quality (e.g., C/N ratio) at the different strata, the effects not only on vegetation but also on the native fauna and other ecosystem processes. Different herbivores have distinct patterns of feeding in response to seasonality and plant functional groups (for example, warm season grasses, cool season grasses, warm season forbs, cool season forbs and shrubs). For example, cattle diet is predominantly composed of grasses, while sheep, the smallest of the commonly used domestic grazers, have a more selective diet that, besides grasses (both C3 and C4), includes a large proportion of forbs (Schwartz and Ellis, 1981). However, other studies point out to variations in cattle diet due to the availability of plants with higher protein content (Senft et al., 1985), physiological demands (Aharoni et al., 2009) and in response to different management systems (Hart et al., 1993). Among the species predominantly used in Brazil, buffalo is the grazer that develops even under a diet with a predominance of more fibrous grasses (Lapitan et al., 2008). Horses feed essentially on grasses and their digestive physiology is adapted to consume larger amounts of roughage forage than cattle (Fleurance et al., 2012). The past role of horses in natural ecosystems and possibly also their link to the history of humans has led to numerous reintroduction initiatives such as free-roaming horses in more than 18 countries across almost all continents (Beever et al., 2019). In the grasslands found within the Cerrado and Atlantic Forest domains, grass species with lower forage quality are often dominant (Lacsano, 1991; Serrão, 1986). In such cases, grazing by horses and buffaloes may be an interesting option, also depending on socioeconomic context. A specific challenge in grassy ecosystems is woody encroachment control. Woody encroachment can be avoided or even reversed by management strategies using different domestic and wild herbivores (grazers and browsers) (Augustine et al., 2011), controlling the intensity of herbivory by browsers (Pontes et al., 2012) and combining herbivory by browsers and controlled fire (Ascoli et al., 2013). It seems, thus, that in ecosystems where shrub encroachment threatens biodiversity and productivity, mixed grazing by sheep, cattle, buffaloes and goats could be used as a management option to improve biomass consumption and encroachment control due to their complementary grazing behaviour (Osoro et al., 2013).
The choice of the grazing management strategy should depend on the dynamics of the ecosystem and the desired structure to build. A consistent example of the effect of management choices on shaping ecosystems was developed in a long-term experiment (LTER) at the experimental station of the Federal University of Rio Grande do Sul. More than 30 years of maintenance of different grazing intensities for the same category of grazers (young beef cattle), under a continuous management in a natural grassland of the Pampa biome, produced different types of grasslands (Fig. 3, pics 5a–c). While the high grazing intensity treatment formed short plant communities that were structurally more uniform, adapted to fast regrowth, protection of meristems and greater palatability, grasslands submitted to low grazing intensity formed structurally more complex plant communities: low grazing pressure allowed the development of a vegetation dominated by tall slow growing species with high investment on strong and long-lived leaves, lower palatability and traits related to defence from herbivory (Fischer et al., 2018). Medium grazing intensity created less grazed patches, with vegetation structure similar to that found in low grazing, interspersed by intensely grazed patches, with reduced canopy height and induced development of tillers, leading to a dense and prostrate canopy (Fig. 3, pic 5c). Such intensely grazed patches are known as grazing laws, which can be considered a proxy of the long evolutionary history of grassy ecosystems (McNaughton, 1984; Hempson et al., 2015). Importantly, the grassland structure developed in response to the different grazing intensities affects the grazing behaviour. Depending on the spatial distribution of forage resources in the grazed paddocks, in qualitative and quantitative terms, young beef cattle need to spend more or less effort to meet their nutritional demands (Carvalho et al., 2015). Overall, the experiment has provided clear evidence that grazing effects on vegetation vary considerably with the adopted grazing management, in this case, the regular adjustment of the stocking rate according to the available forage (see also Fedrigo et al., 2017).
Cerrado - (1a) with cattle, (1b) four years without cattle, (1c) nine years without cattle and (1d) ten years without cattle and after fire; Pantanal - (2a) typical floodplain physiognomy (2b) Moderate grazing intensity, with greater presence of Axonopus purpusii, (2c) Campo Limpo physiognomy with the dominance of Andropon hypogynus and Axonopus purpuii without grazing; Amazon - Mesic savanna with dominance of Trachypogon plumosus in Amapá state: (3a) rotational management with Santa Inês sheep breed, (3b) overgrazed grassland and (3c) cattle grazing; (4a) Lavraderos horses in a native grassland in Roraima state; Pampa – overgrazed grassland (5a) and grassland structure under low (5b) grazing intensity treatment and (5c) aerial photo of low-intensity grazing treatment with patchy vegetation structure in a LTER experiment at the experimental station of the Federal University of Rio Grande do Sul. Authors of images in Appendix A.
A common feature in almost all grassy ecosystems is the seasonality of primary productivity and, consequently, the quantity and quality of forage available to grazers. For the grasslands in the Pampa and in the south Brazilian highland region, the factors that limit primary productivity are low winter temperatures and, with considerable variability among years, droughts in summer (Overbeck et al., 2007). In the Pantanal, Cerrado, Caatinga and part of the grassy ecosystems of the Amazon biome, the rainfall regime is characterized by well-defined dry and wet seasons (Andrade et al., 2017; Ratter et al., 1997; Townsend et al., 2012). Additionally, in the Pantanal and on alluvial soils of the Amazon, grassland productivity is also impacted by seasonal flooding productivity (Aquino et al., 2017; Townsend et al., 2012). In any given situation, it will be necessary to establish management strategies that consider these specific conditions to reduce potential negative impacts, prevent overgrazing and maintain adequate conditions for the animals. Among the possible strategies to be adopted, we can mention some already recommended by EMBRAPA for intensive livestock in natural ecosystems such as: the use of stockpiling (such as in situ forage banks) combined with energy and protein supplementation, use of smaller areas of planted pastures with non-invasive exotic species in a separate area as complementary food source, the use of a variable stocking rate, or even withdrawal or sale of animals (Jaurena et al., 2021).
In many grazing systems, additional management activities may be used periodically, such as mowing and fire. Fire (see Box 1), together with grazing, has been important in the development of grassy ecosystems in the tropics and subtropics and is considered important in maintaining the ecosystem properties in many ecosystems (Koerner and Collins, 2014). Although the effects of each disturbance individually are well known, the interactive effects of fire and grazing regimes on plant communities are not (Kirkpatrick et al., 2016). In general, grazing reduces the flammable biomass and thus can reduce the frequency and intensity of fires (Hobbs, 1996). Vegetation recovering from fire is very attractive to large herbivores due to high palatability and high nutrient concentration, and this can result in changes in community structure and composition, so that these can become different from communities that are only burned or only grazed (Kirkpatrick et al., 2016; Donaldson et al., 2018). Grazing after burning is predominantly associated with an impeded recovery of woody plants (Capozzelli et al., 2020) but it is also associated with an increase in cover of unpalatable woody plants (Radloff et al., 2014).
Similar to grazing, fire is an important disturbance that can maintain structure and processes of grassy ecosystems (Hoobs, 1996; Podgaiski et al., 2013; Vasconcelos et al., 2016), either independently of grazing or in an integrated management scheme. Roughly, we can distinguish ecosystems into two groups: fire-influenced (fire-tolerant) and fire-sensitive systems, and grassy regions in general are within the first group (Pivello, 2011). The use of fire for conservation has been a taboo in the management of Brazilian grassy ecosystems, but acceptance by managers has recently increased. In addition, integrated fire management strategies have been developed and widely implemented for conservation in protected areas of grassy regions (for discussion see e.g. Durigan, 2020; Fidelis, 2020). However, burning native vegetation remains prohibited as a management tool in private areas in the Cerrado (Durigan and Ratter, 2016), but its use is regulated in other grassy regions, such as in the grazed highland grasslands in the southern part of the Atlantic Forest domain, where farmers use fire as a management tool. Overall, and as to be expected based on studies from around the world, Brazil’s grassy ecosystems appear to be resilient in response to variable frequencies of burning (Fidelis et al., 2010; Pivello and Coutinho, 1996).
We clearly are in need of more studies addressing grazing effects on natural grassy ecosystems in many regions of Brazil, and these can be integrated into the development of more sustainable land use practices, especially in those regions where grazing is the most common management practice, e.g. in the South Brazilian grasslands and in the Pantanal (Santos et al., 2011; Jaurena et al., 2021). For this, research and decision-making should bring together the knowledge of the ecological characteristics (biological features of the system and their environmental drivers) of each grassy ecosystem type, and involve stakeholders and environmental agencies, as to gather the existing knowledge about the ecosystems to be managed (Fig. 4). The adoption of adaptive management approaches can help in this context (Allen and Gunderson, 2011).
When discussing the potential role of grazing in Brazil’s grassy ecosystems, we should strive to answer a number of questions: what is the effect of different grazers on each ecosystem? What is the appropriate grazing intensity for different ecosystems, based on variation in primary productivity? And what are the effects of grazing to conservation goals? By creating partnerships with farmers for testing the most plausible management options in different types of grassy ecosystems, aiming at maintaining their biodiversity and multiple ecosystem services (including animal production), we can begin to reduce uncertainties about the effects of management practices. For each situation it is important to define clear management objectives in which both conservation and animal production goals can be balanced out (Fig. 4). Implementation of management should then be followed by monitoring of grazing effects on vegetation, as well as production-oriented aspects (e.g. weight gain by cattle). To build the necessary scientific support, a network of research sites and the promotion of knowledge exchange among scientists and practitioners will be essential.
ConclusionsGrassy ecosystems in tropical and subtropical South America developed under the influence of large herbivores until the Early Holocene, and a large body of evidence exists on the importance of disturbance regimes by grazing animals and/or fire for maintenance of the high biodiversity of these systems. Yet, knowledge about the effects of different grazing practices on biodiversity and ecosystem services is still lacking across the different types of grassy ecosystems. Grazing – in a similar way as fire – has been often considered a priori to be negative for biodiversity and ecosystem functioning due to supposedly negative effects in general. However, this negative perspective often was, and is, based on negative effects in specific systems only, such as adjacent systems that are grazing sensitive (even though these effects are manageable), or based on effects on specific species groups, but without any more detailed consideration of overall effects and reflection of overall conservation goals. Further, existing studies on grazing management have been mostly limited to single locations and often ignore cultural and socio-economic determinants, thus cannot be easily generalized or transferred to different situations.
We argue that grazing can be an important conservation tool when properly applied in terms of grazing strategy (stocking rate, grazing periods). As grazing can ally conservation with economically interesting activity, its use appears to be of special relevance in Legal Reserves in Brazil. A research agenda, developed in partnership with land users so that both conservation and economic feasible use can be evaluated, is necessary. This research agenda should include, across distinct grassy ecosystems, the study of the interaction between grazing and biodiversity and natural resources, as well as socioeconomic feasibility and determinants of grazing. In Brazil’s grassy ecosystems, grazing in the Legal Reserve can represent the rescue of a socio-cultural tradition which, if supported by science, will enlarge and strengthen the knowledge of the system’s dynamics by and for its users, researchers and policy makers, generating additional income and at the same time, expanding the conservation of grassy ecosystems.
RB received a CAPES PhD scholarship, VP, GEO and GD were supported by CNPq (respectively, grants 307689/2014-0, 310345/2018-9 and 303179/2016-3). An early version of this paper was part of the PhD thesis of the first author in the Graduate Program in Ecology of Universidade Federal do Rio Grande do Sul, and was later expanded with the support of a fellowship to RB in the framework of the INCT EECBio (National Institute of Science and Technology on Ecology, Evolution and Conservation of Biodiversity). GEO and GD are members of GrassSyn, conducted within SinBiose through CNPq/MCTI (grant 442348/2019-3).
Valério De Patta Pillar – Graphical abstract.
Giselda Durigan – Fig. 3, pics 1a–d.
Gerhard E. Overbeck – Fig. 3, pic 2a.
Sandra Aparecida Santos, Fig. 3, pics 2b–c.
Newton de Lucena Costa Fig. 3, pics 3a–c.
Ramayana Menezes Braga Fig. 3, pic 4a.
Olivier Bonnet Fig. 3, pics 5a–b.
Paulo Marsiaj Fig. 3, pic, 5c.