Understanding the impacts on the Brazilian semi-arid coast, which is a drought-prone area (>1000 km) in the tropical Atlantic, and how ecosystems survive and adapt to such extreme environments requires socioecological studies to create a theory for conservation. Here, we highlight five main ongoing changes in tropical semi-arid areas, namely (1) the decrease in rainfall rates due to climate change, which alters freshwater flows, alters water residence times, and promotes hypersalinity (>37) in low-inflow estuaries; (2) sea-level rise, groundwater hazards, increased erosion of beaches and nearshore mangroves, and landward mangrove forest expansion due to enhanced saline intrusion along river basins; (3) the decrease in land-ocean fluxes due to silting and closure of sandy bars in estuarine mouths; (4) warming and increased intensity and frequency of extreme events (e.g., heat waves, droughts, and sea swells); and (5) growing eutrophication and hypoxia, loss of vegetation cover and biodiversity due to urbanization, aquaculture (shrimp farming), agriculture, and land-use change, which includes building dams for water supply. The alteration of biogeochemical processes (“Arctic Paradox” hypothesis) and acidification that potentialize the impact of contaminants and nutrients is also highlighted. These impacts have effects on food and water security and multiple trophic levels, which should preferentially be studied through a long-term approach for advancing research. Based on these concerns, we propose key questions that should guide research in the context of the Decade of Ocean Science for Sustainable Development (2021–2030) to support science-based management actions in Brazil and other similar semi-arid areas worldwide.
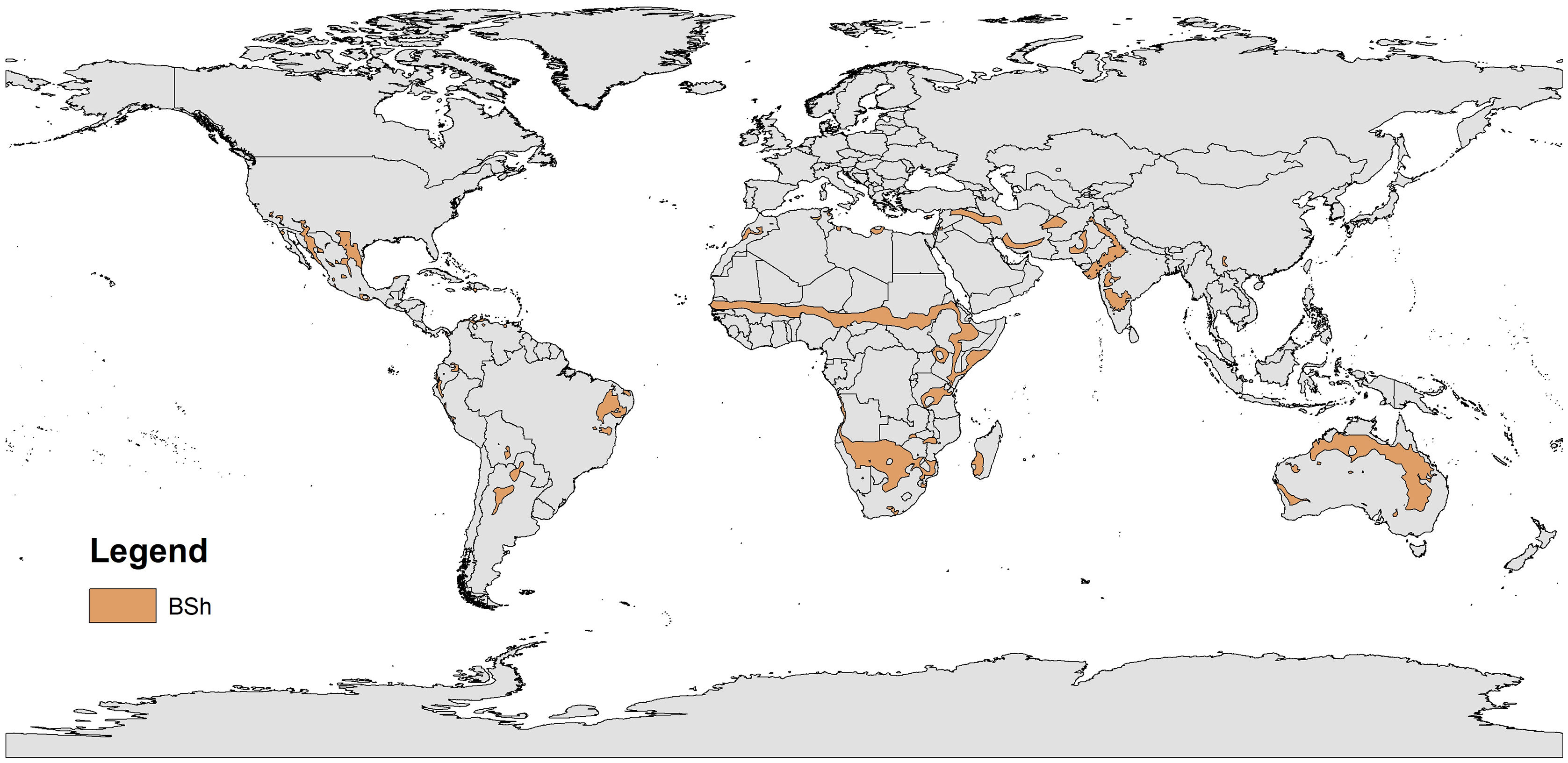
Coastal ecosystems are undergoing global environmental changes, which require urgent science-based actions to provide support for effective management and environmental planning, especially in ecologically and socially vulnerable areas worldwide (Bennett et al., 2016; Keys et al., 2019). Among the highly productive and biodiverse intertropical ecosystems (Pimm et al., 2014), estuaries and mangroves are essential for sustaining terrestrial and aquatic life and human communities. They provide carbon sequestration (blue carbon), pollutant filtration (Guannel et al., 2016), food security (e.g., protein provision) for traditional human communities (Costanza et al., 2014; Claudet et al., 2020), and coastal protection against climate change impacts, such as sea-level rise and sea swells (Saunders et al., 2014). Nevertheless, research in tropical environments of developing countries is still scarce compared with that in subtropical and warm-temperate coastal systems (Largier, 2010). Dry coasts (arid, semi-arid, and dry subhumid) are even more neglected, such as the coasts of Brazil (Barroso et al., 2018), Iran (Mafi-Gholami et al., 2019), Egypt (Eissa et al., 2018), the USA (Breaux et al., 2019; Lopez et al., 2020; Douglas et al., 2021), Mexico (Valderrama-Landeros et al., 2020), Spain (Sanz et al., 2002), Cape Verde (Larrue et al., 2020), and Australia (Adame et al., 2020). The socioecological dynamics of these dry coasts are not well known despite their increased population density, vulnerability, and unique biodiversity (Huxman et al., 2004; Poulter et al., 2014; Adame et al., 2020) (Fig. 1).
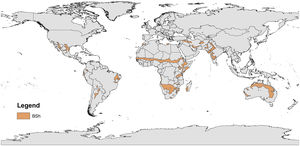
Hot (BSh, Köppen climate classification) semi-arid environments worldwide. Adapted from Beck et al. (2018).
Most of the knowledge of how environmental factors influence the structuring of populations and communities in semi-arid coastal zones is based on high-flow river systems (Canuel et al., 2012). Historically, we have based our understanding of many ecosystems with diverse functions, such as dry coastal systems (Largier, 2010; Tweedley et al., 2019), on perennial riverine ecosystems that cross dry lands, but are not limited by water deficits. Coastal ecosystems located in warm (>27 °C) semi-arid climates (Fig. 1) are under high-temperature and low-precipitation regimes with high solar radiation and evaporation rates (Huxman et al., 2004; Schwinning et al., 2004; Poulter et al., 2014). Thus, it is common for such environments to present stressful conditions, such as low humidity and hypersalinity (Largier, 2010; Douglas et al., 2021), for many species, which leads to physiological stress related to freshwater scarcity (e.g., high physiological costs of osmoregulation), changes in recruitment rates, and reduced productivity compared to ecosystems not limited by water availability (Weatherall et al., 2018; Breaux et al., 2019; Adame et al., 2020). Moreover, the increased salinity in dry regions may trigger large-scale mortality events of key tropical ecoengineering species (e.g., seagrasses) and/or stress-sensitive species across distinct taxa (e.g., fishes and mangroves) (Johnson et al., 2020). These processes also shape the supply of environmental goods and services, and the resistance and resilience of these ecosystems to local impacts and climate change (Waters et al., 2016; Cheng et al., 2019).
The Brazilian semi-arid coast is characterized by a hot semi-arid climate and is one of the most densely populated areas worldwide. The area has significant resource exploitation, high levels of social inequality, and is one of the least known regions in the tropics. This perspective article discusses the main ongoing impacts in this extensive and ecologically diverse drought-prone area (>1000 km) in the tropical South Atlantic, with a focus on the estuarine zone and related coastal ecosystems, such as mangrove forests, dunes, and sandy beaches.
Section “Brazilian semi-arid coast” presents a characterization of the Brazilian semi-arid coast and Section “Main environmental changes in the Brazilian semi-arid coast” presents the main ongoing impacts related to environmental changes. After each set of impacts, the main scientific question for advancing long-term research in the context of the United Nations (UN) Decade of Ocean Science for Sustainable Development (2021–2030) is identified. Using the questions as an agenda for future research will substantially enhance the understanding of those areas and similar semi-arid areas worldwide. This up-to-date knowledge will provide practical relevance for supporting science-based public policies.
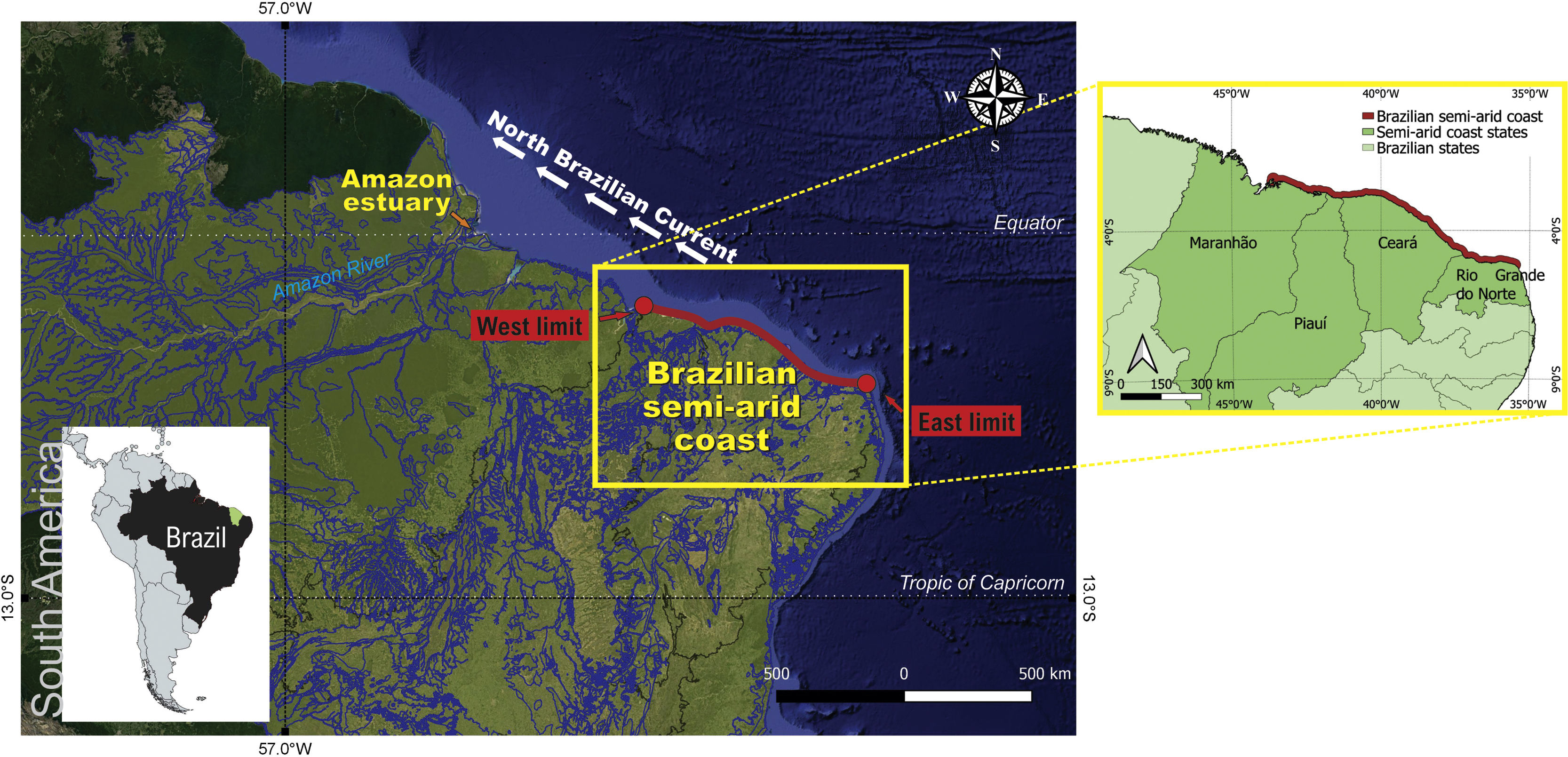
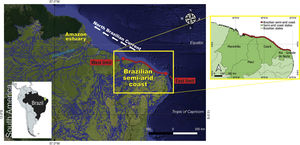
Brazilian semi-arid coastThe Brazilian semi-arid coast is located in the equatorial South Atlantic (Fig. 2). It is a macro geomorphological area on the northeast coast of Brazil between Ponta dos Mangues Secos in Maranhão State (west limit) and Cabo do Calcanhar in Rio Grande do Norte State (east limit), which is the only stretch of the Brazilian coast under the increasing and direct influence of the hot semi-arid climate. This dry coast borders the semi-arid Caatinga phytogeographical domain, namely the driest Brazilian ecosystem that is covered mostly by deciduous forests (Moro et al., 2016), and the seasonal savannas of the Cerrado domain. Owing to the climatic influence of the very dry Caatinga and the seasonal Cerrado, this coastal region differs from the rest of the Brazilian littoral zone. While most of the Brazilian coast harbors mainly humid forests in the Atlantic Forest domain or extensive mangrove systems in the Amazon, the semi-arid coast contains semi-deciduous forests, savannas, grasslands, and smaller mangrove systems associated with the shallow-water estuaries (Moro et al., 2015). With the exception of a few large rivers (São Francisco and Parnaíba), most of the river systems are temporary, with hypersaline mangroves and meadows (apicuns). Small estuaries are usually neglected despite being locally important because they support human populations and fishing resources.
The climate in this region is largely driven by the position of the Intertropical Convergence Zone (ITCZ). The sea surface temperature is high and has low intraannual and interannual variation (26–30 °C) (Teixeira and Machado, 2013). Seasonal, interannual, decadal, and multidecadal variations in the position of the ITCZ cause significant changes in the rainfall regime (interannual and intraannual variation), thereby resulting in hot semi-aridity (Utida et al., 2019). The region has a rainy season between the austral autumn and winter (March to June) when the ITCZ reaches its southernmost position. Indeed, a large part (70%) of the rainfall predicted for a year can fall in just a single month (e.g., May or June). However, a total annual reduction in precipitation has been observed in the past three decades (Marengo et al., 2017), and the area is now considered one of the most vulnerable to climate change in South America owing to the increased frequency of droughts (Utida et al., 2019). For example, Parnaíba (Piauí coast) showed a trend of reduction in total annual rainfall, with an estimated value of 118 mm for 30 years. Moreover, Fortaleza (Ceará coast) had a long-term trend of reduction for the total annual number of rainy days (19 days) (Carvalho et al., 2019), which represent a concentration of rainfall. Therefore, there is an increased negative risk to coastal ecosystems, possibly increasing flood events, erosion processes in shallow soils, and intense disturbances, such as drastic changes in estuarine salinity (Barroso et al., 2018; Carvalho et al., 2019).
In addition to rainfall, the meeting of the southeast and northeast trade winds in the ITCZ subject the semi-arid coast to a strong eolian regime (4 to 11 m s−1), particularly during the dry season (July to December with wind speed sometimes above 10 m s−1) (Funceme, 2009). This wind pattern affects the coastal and oceanic circulation in this area, which connects this semi-arid coast to the Amazon and Caribbean regions by the northwestward-flowing nutrient-poor coastal currents and the North Brazil Current (Fig. 2) (Santos and Silva, 2013).
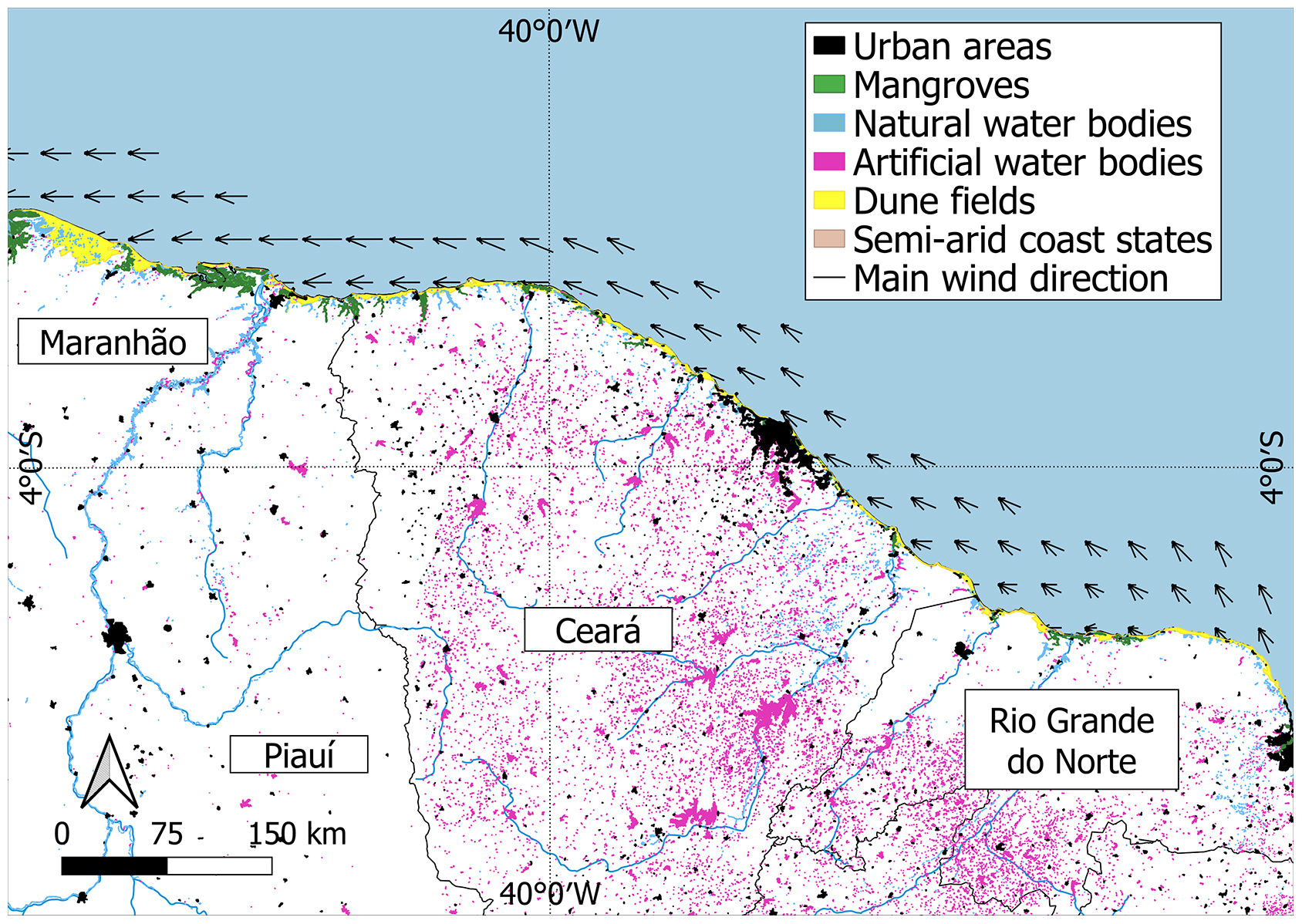
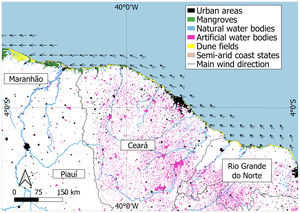
Owing to the lack of high river runoff (in contrast to the neighboring Amazon region) (Fig. 2), the Brazilian semi-arid coast is affected by low sedimentary processes, and a large proportion of the coast is currently facing erosion (Pinheiro et al., 2016). Most of the coastline is covered by sedimentary layers deposited during the Neogene and Quaternary periods (Morais et al., 2018). The combined action of swells, low river runoff, a mesotidal regime, and increased wind speed erodes these sediment deposits, thereby resulting in intense longshore drift and complex sediment exchanges between the shore and the mainland. These factors result in a unique set of oceanographic, ecological, geographic, and geomorphological features. For example, this semi-arid coastal area has the largest dune fields in South America (Santos et al., 2019), and most of the shallow rivers have bar-built estuaries that characterize semi-enclosed estuarine systems (Morais and Pinheiro, 2011) (Fig. 3).
Brazilian semi-arid coast (equatorial Southwestern Atlantic, northeastern Brazil) showing the states included (Maranhão, Piauí, Ceará, Rio Grande do Norte), land cover (urban areas, mangroves, natural and artificial water bodies, dunes fields) and main wind direction. Data sources - water bodies: ANA (2020); wind direction: Amarante et al. (2001); Urban areas: Farias et al. (2017); Mangrove areas: Freitas et al. (2018).
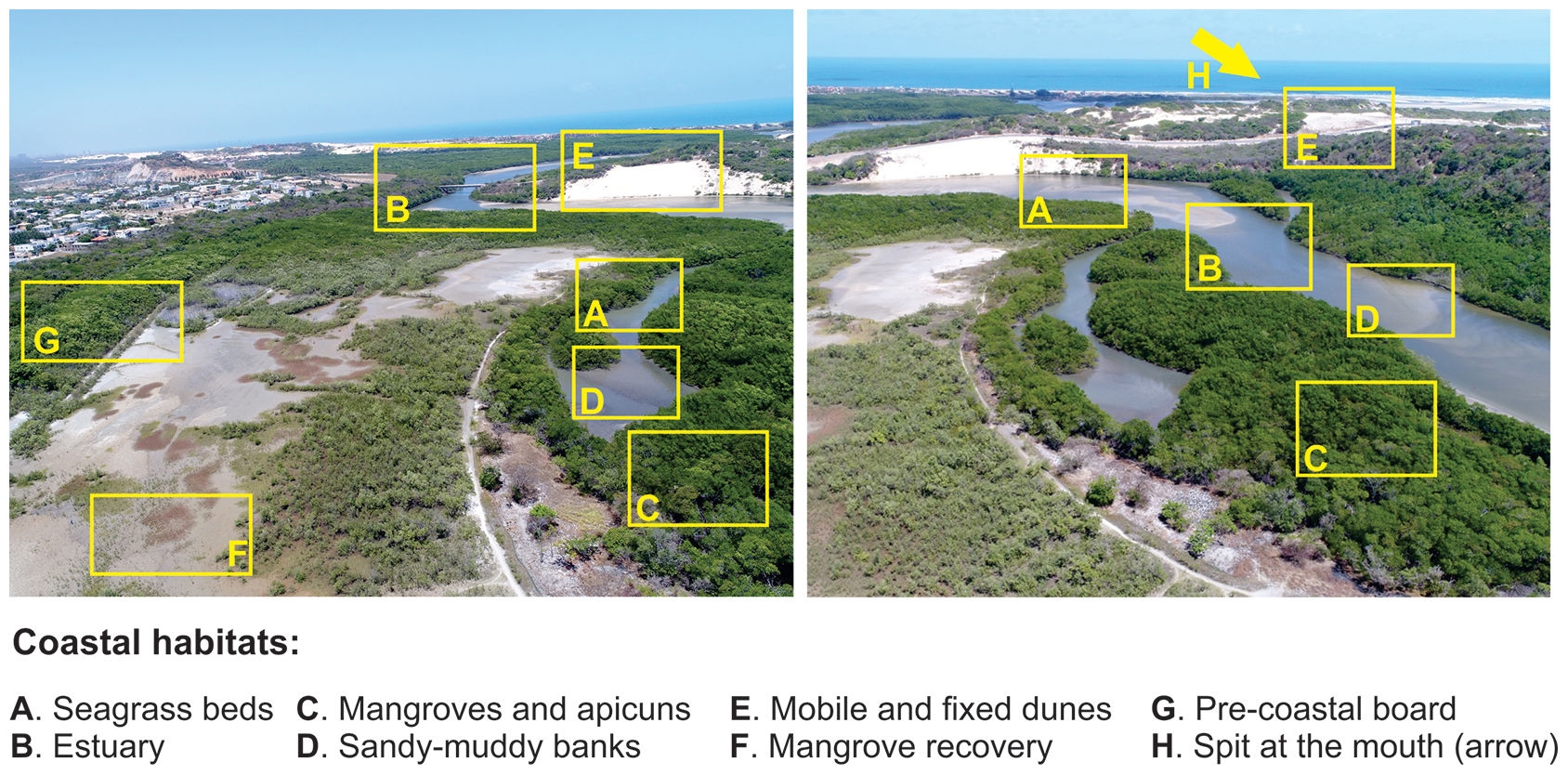
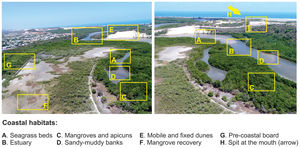
A complex set of tropical landscapes (Fig. 4), seascapes, and traditional human communities, such as fishers and shellfish gatherers, are found in this area. The area encompasses a variety of features, such as sandy beaches, mobile and fixed dunes (Fig. 3), seagrass and rhodolith beds, and mangroves (Fig. 3) in freshwater-deprived estuaries, which make up interconnected environments of high ecological complexity (Godoy and Lacerda, 2015; Barros et al., 2016; Pinheiro et al., 2016; Soares et al., 2017; Godoy et al., 2018; Costa et al., 2020) (Fig. 4). The key impacts on these ecosystems, which often persist at the edge of the physiological tolerances of their species, include extreme drought events, reductions in groundwater inputs and fluxes between continents and oceans (e.g., presence of dams) (Fig. 3) (Dias et al., 2016), sea-level fluctuations, and increases in nutrient loading (e.g., shrimp farms, discharge of raw sewage, and urban runoff) (Soares et al., 2017; Barroso et al., 2018; Lacerda et al., 2020; Marins et al., 2020). These impacts also affect the coast via transport reduction to the ocean and the formation of estuarine plumes on the inner continental shelf in exceptionally rainy years (Lacerda et al., 2017).
Main coastal ecosystems located on the Brazilian semi-arid coast. Both aerial drone-photos are from the Pacoti River estuary, Ceará coast, northeastern Brazil. This shallow estuary is seasonally hypersaline (during the dry season; August to December) like most of the short and low-inflow estuaries on the Brazilian semi-arid coast.
The small size of the rivers also results in low-inflow, hypersaline, and shallow estuaries (sensuLargier, 2010). Hypersalinity occurs seasonally and at different intensities in these systems (Schettini et al., 2017; Valentim et al., 2018). The phenomenon of hypersalinity is recurrent in several estuaries worldwide, but in Brazil, it occurs seasonally in this semi-arid coastal zone (Fig. 2), in the north of the state of Rio de Janeiro (Kjerfve et al., 1996), and during drier years in Todos os Santos Bay (Bahia State) (Lessa et al., 2019). Therefore, these extreme environments can be used as models for predicting future scenarios by providing answers to how the socioecological systems may behave in response to climate change and for assessing the resilience of biological and human communities (e.g., artisanal fishermen) to such impacts. Hypersalinization and high water residence time (Verissimo et al., 2017; Barroso et al., 2018; Garcia et al., 2020) are related to several local and global factors, including the location of the river basin in a semi-arid region, short and bar-built estuaries (Pelage et al., 2021), multiple dams along the upstream basin (Fig. 3), high evaporation rates, global warming, higher insolation rates (Lacerda et al., 2012, 2020), low runoff, and low river depth (Schettini et al., 2017).
In these freshwater-deprived estuaries, among the key factors that influence the structuring of communities, we highlight the salinity, strong seasonality of rainfall/fluvial discharge, high concentrations of inorganic nutrients, important geochemical carriers and carbon fluxes (Maia et al., 2018; Mounier et al., 2018; Santos et al., 2019), and organic matter characteristics (Cavalcante, 2019). A mixture of freshwater and seawater can rapidly consume freshwater CO2 by the carbonate buffering capacity of seawater (Cotovicz et al., 2020a). These processes are maximized by the high temperatures and distinct hydrological conditions (e.g., high residence time) of the estuarine water body (Dias et al., 2011, 2013). The reduction in the sediment and nutrient loads from the estuary not only has consequences on beach erosion, river basin silting, and the export of nutrients from the inner continental shelf (Morais and Pinheiro, 2011; Dias et al., 2013, 2016; Ximenes Neto et al., 2018), but also affects the adjacent marine ecosystems depending on the energy and matter flows that may be sensitive to reductions in available organic matter (Rossi et al., 2019). The changing sedimentation rates and quality of the sediments also alter the substrate available to benthic organisms and may be related to contamination (Nilin et al., 2013; Rios et al., 2016), as presented in Section “Main environmental changes in the Brazilian semi-arid coast”.
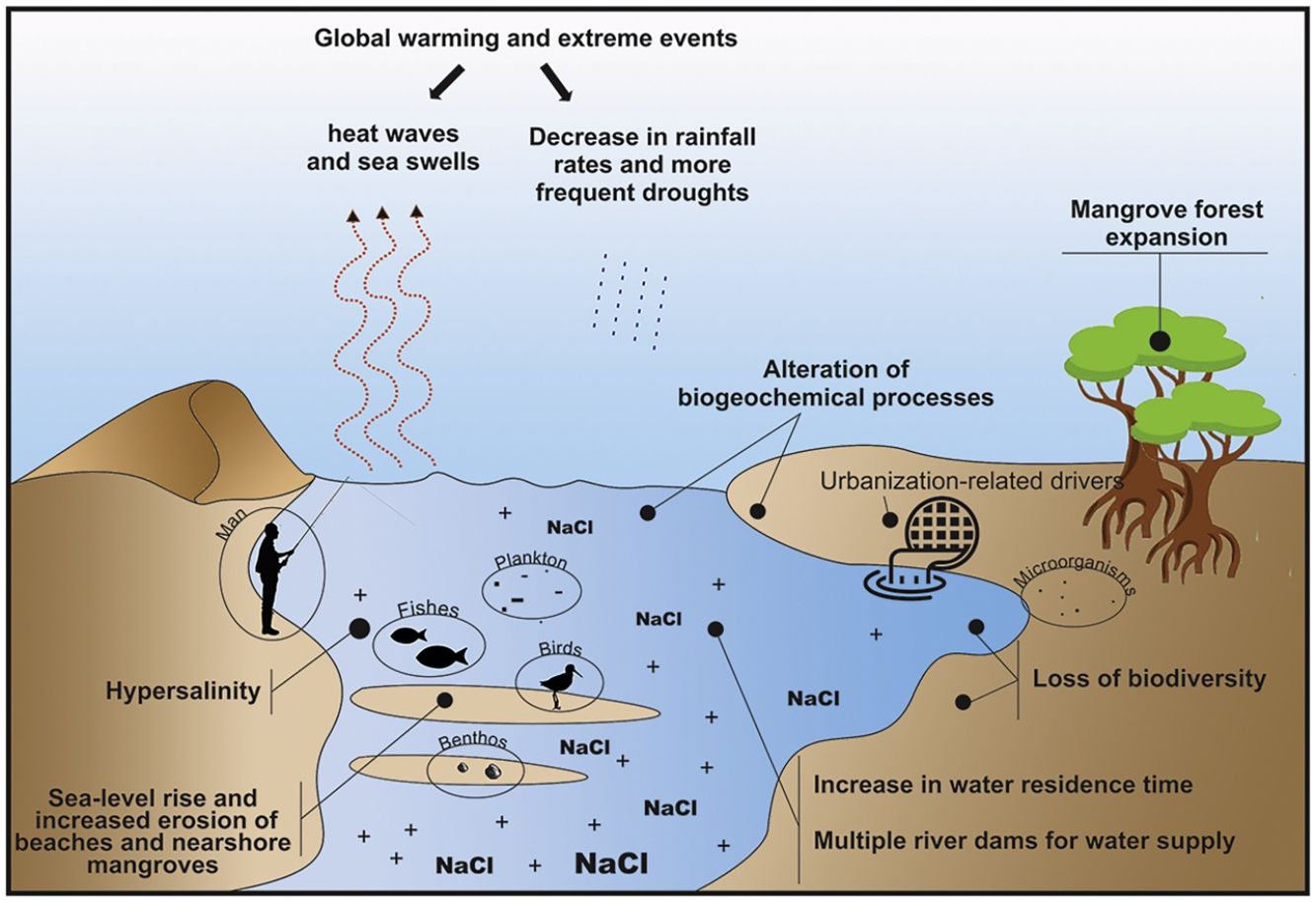
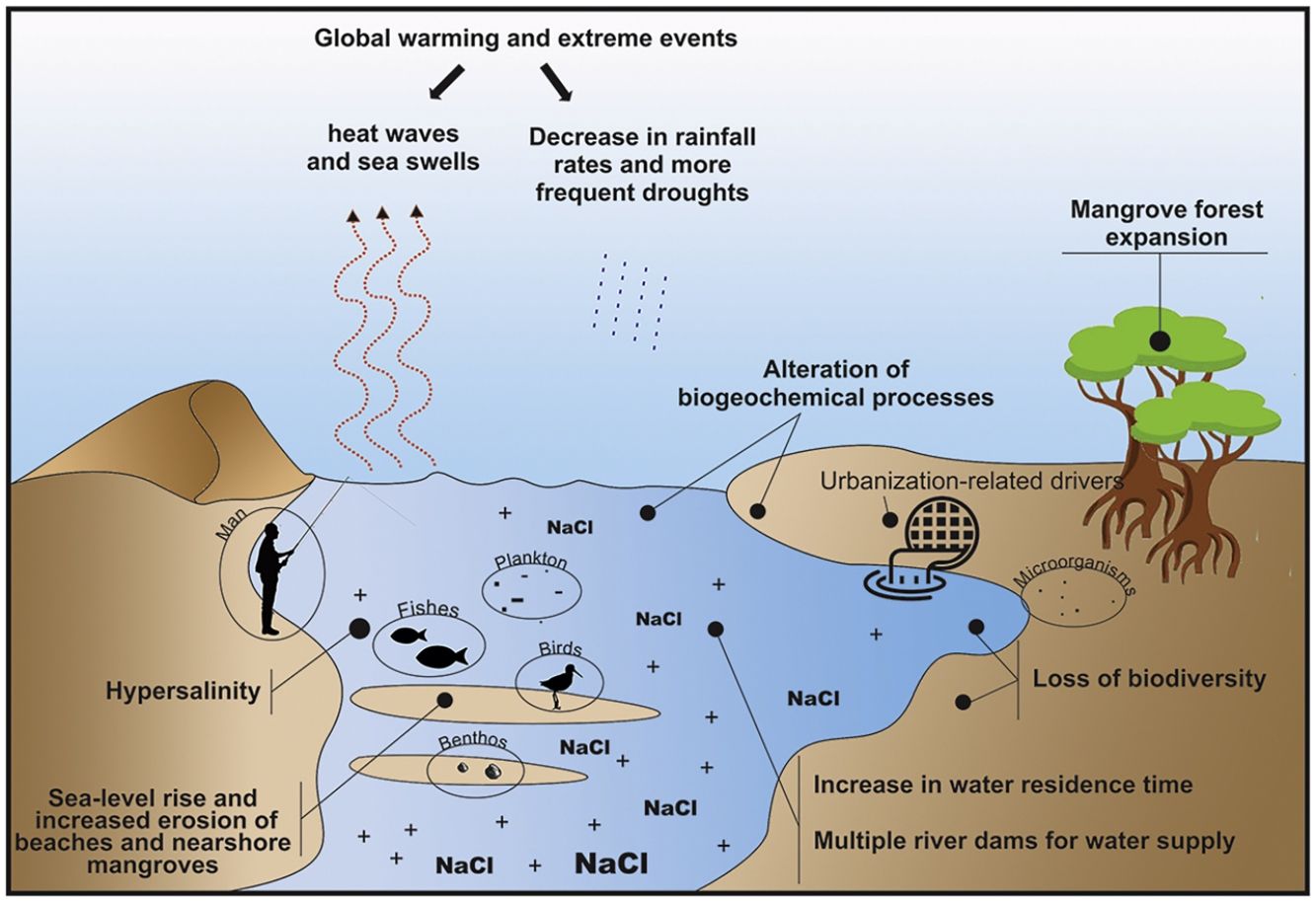
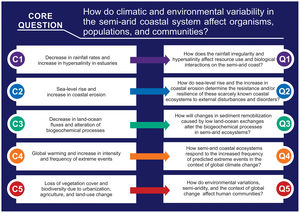
Main environmental changes in the Brazilian semi-arid coastAmong the main impacts that drive changes in semi-arid coastal ecosystems, the following five are highlighted here: (1) the expected decrease in rainfall rates due to climate change, which alters water flows and the water residence time and promotes hypersalinity (>37) in low-inflow and shallow estuaries; (2) the sea-level rise, increased erosion of beaches and nearshore mangroves, and inland mangrove forest expansion due to enhanced saline intrusion along the river basins; (3) the decrease in land-ocean fluxes due to silting and closure of sandy bars in the estuarine mouth; (4) global warming, acidification, and the increased intensity and frequency of extreme events (e.g., heat waves, droughts, and sea swells); and (5) growing eutrophication and hypoxia, the loss of vegetation cover and biodiversity due to urbanization, aquaculture (shrimp farming), agriculture, and land use change, which includes building dams for water supply along the semi-arid river basin (Fig. 3). Moreover, the alteration of biogeochemical processes (“Arctic Paradox” hypothesis) can leverage the impact of contaminants, such as trace metals, and nutrients, which can increase the trophic state of oligotrophic waters.
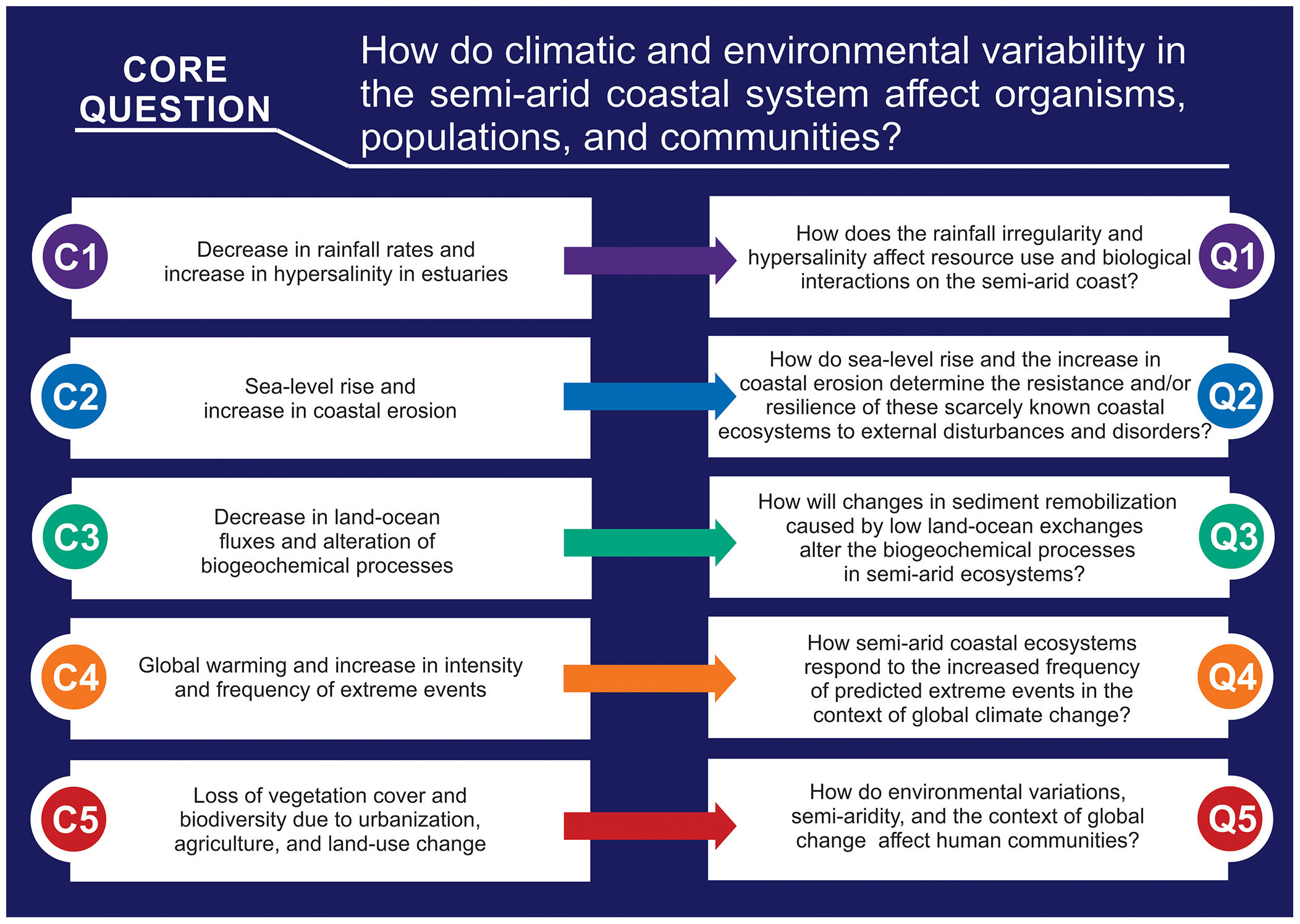
Considering these five main ongoing impacts, which will be presented in detail in this section, there is an urgent need for interdisciplinary approaches and the generation of long-term socioecological databases. These data could be used to understand the structure and dynamics of communities and their relationships with environmental variability. To pursue this task, we raise the following core question: how do climatic and environmental variability in the semi-arid coastal system affect organisms, populations, and communities? This central question was divided into five sub-questions aligned with the five main environmental changes identified in the Brazilian semi-arid coast (Fig. 5).
Droughts and their socioeconomic and ecological consequences have coexisted for centuries in the Brazilian semi-arid region. However, the frequency and intensity of this phenomenon have intensified due to climate change (Marengo et al., 2020; Wu et al., 2020). This region experienced the worst drought ever recorded in Brazilian history between 2011 and 2017 (Marengo et al., 2020). A deficit of approximately 60% in the average accumulated precipitation was observed during this period compared with that in other years. Moreover, this region might experience greater increases in temperature, rainfall anomalies, and more frequent/intense dry spells and droughts in the next few decades (IPCC, 2014; Marengo et al., 2017). Regarding precipitation, decreases in the current rainfall rate of 10%–20% by 2040 and 25%–35% by 2070 (PBMC, 2014) are predicted to impact these coastal ecosystems. Such effects will have knockout effects on several small, low-inflow, and short estuaries due to the increased salinity, which can result in long-term hypersalinity.
Some reported alterations due to hypersaline waters are a reduction in species richness, with the survival of the most salt-tolerant species (Pillay and Perissinotto, 2008, 2009; Nche-Fambo et al., 2015). A previous study in a Brazilian semi-arid estuary suggested the presence of some groups that are well-adapted to deal with hypersalinity, such as phytoplankton (Barroso et al., 2018). Other detected patterns include changes in zooplankton composition (Campos, 2018; Garcia et al., 2020) and in the diversity and biomass of benthic animals in the dry period (Freitas et al., 2006; Maia et al., 2018), which are probably due to the flourishing of stress-tolerant species. Many estuaries worldwide are hypersaline and have elevated water residence times (Lancelot and Muylaert, 2011; Tirok and Scharler, 2013; Barroso et al., 2018). Under these conditions combined with nutrient enrichment, the phytoplankton biomass can increase (du Plooy et al., 2014; Hemraj et al., 2017; Barroso et al., 2018). However, this primary productivity enhancement in hypersaline conditions is not always beneficial for the general estuarine functioning, since harmful algae blooms can occur (Sahraoui et al., 2013; Wetz et al., 2017). In fact, in estuarine environments the synergistic effect of nutrient enrichment, long water residence times, and hypersaline conditions have been associated with several symptoms of eutrophication, such as high concentrations of chlorophyll, harmful algae blooms, episodic hypoxia, and fish kills (Wetz et al., 2017; Hallett et al., 2018).
Osmotic stress increases the risk of mortality of individual species and may result in a reduction in interspecific competition (Vilas et al., 2009). Some fish species experience physical stress due to changes in salinity. For example, the reactive oxygen species activity significantly increases with changing salinity (Hossain et al., 2016). Species that are poor osmoregulators or are not able to rest during periodical salinity stresses will be substituted by salt-tolerant species that are more adapted to or able to rest during unfavorable periods. This will lead to a shift in the species composition (e.g., dominance by salt-tolerant species) and community structure, thereby affecting the human economy and food security (e.g., fishing) (Feyrer et al., 2015). Hypersalinity can also cause the trophic systems to become truncated in terms of carbon flow to higher trophic levels because zooplankton predators in coastal food webs are more vulnerable to hypersalinity (Campos, 2018).
Furthermore, the longer water residence times in these Brazilian semi-arid estuaries have caused landward saline intrusion (Maia and Lima, 2004), thereby leading to the expansion of mangroves dominated by a few tolerant plant species over new areas (Godoy and Lacerda, 2015; Godoy et al., 2018). This can also lead to an increase in hypersalinity in the landward direction, thereby significantly increasing the associated marine fauna of the systems (e.g., jellyfishes and sea stars) that can be potentialized by basin drainage systems, such as multiple dams (Dias et al., 2011; Dias et al., 2013, 2018; Barroso et al., 2018; Medeiros et al., 2018; Valentim et al., 2018).
In this scenario, the irregularity of rainfall exerts a key control over the life histories (Feitosa and Rezende, 2020) and physiological characteristics of biota (Abrantes et al., 2020) because the short rainy period concentrates freshwater and riverine nutrient inputs in 3–4 months (Funceme, 2009). Thus, during most of the year, the biota face critical survival conditions related mainly to extremes of salinity, evapotranspiration, and water residence times. Therefore, the following two situations are possible: (a) rain could provide relief from suboptimal conditions for species that survived the long dry season, or (b) rain could be a disturbance because the rainfall is usually concentrated in a few months of the year. However, the limits of this adaptability, resilience, and diversification among members of the estuarine community and how semi-aridness affects the supply of resources to traditional communities are unclear.
Therefore, a major scientific challenge is to understand the responses of ecological communities to the ongoing intensification of the water deficit and increased saline intrusion. Thus, we raise the following question: how do rainfall irregularity and hypersalinity affect resource use and biological interactions on the semi-arid coast? To answer this question, long-term monitoring and research are strongly advised because changes in the stability and connectivity of communities can have key consequences for the ecosystem’s ability to resist external environmental disturbances. Also, predicting the effects of dryer scenarios and higher sea levels on saline estuarine intrusion as well as better river-dam management are essential to address adaptability and mitigation under climate change scenarios.
Sea-level rise and increase in coastal erosionGlobal mean sea-level rise, which is caused by meltwater from glaciers and ice sheets and water expansion due to heat accumulation in the ocean, threatens different areas worldwide, including the Brazilian semi-arid coast. Along this coastline, at least three areas are at high or very high risk of flooding, and thus are more susceptible to sea-level rise (Tessler, 2008; Muehe, 2010; Nicolodi and Petermann, 2010). These areas also sustain large urban concentrations of approximately 180 K to 3000 K inhabitants. Therefore, despite the lack of precise sea-level scenarios under climate change for the region, at least 3.5 million people face a potential risk of being permanently or temporarily impacted by coastline regression by the end of this century. Regional models dealing with this sea-level rise impact are urgently needed to assess the magnitude and prevalence of potential risks, such as the destabilization of the extensive semi-arid coastline and many threats to water security, such as flooding and saltwater intrusion on coastal groundwater (Befus et al., 2020).
Coastal erosion is another consequence of sea-level rise and inappropriate land use (Section “Loss of vegetation cover and biodiversity due urbanization, aquaculture, agriculture, eutrophication, and land use change”). Current trends in coastal dynamics, combined with nearshore recession driven by sea-level rise, could result in the near extinction of almost half of the beaches worldwide by the end of the century (Vousdoukas et al., 2020). Approximately 42.4% of the Brazilian semi-arid coast is facing erosion (Morais et al., 2018; Paula et al., 2018; Vital et al., 2018). The vulnerability of this region and the continuation of this process will lead to considerable losses of coastal land in the coming decades, thereby affecting economies and low-lying human communities, increased groundwater hazards, and livelihoods (Franco et al., 2012; Befus et al., 2020). However, a non-linear increase in the erosion intensity is also expected due to the decrease in precipitation along this coast, which will increase the eolian transport of sediment from the shore to the mainland (Muehe, 2010). This lack of sediments amplifies the vulnerability of semi-arid coastal areas to erosion (Muehe, 2010), while possibly increasing the size of dune fields. This in turn may lead to siltation and burial of continental water bodies (Pinheiro et al., 2006; Tsoar et al., 2009), which will have severe consequences for estuarine and lagoon habitats. Coupled with local factors, such as river damming, these processes have already decreased the input of sediments to shallow marine areas, including sandy beaches (Morais and Pinheiro, 2011).
These ongoing processes may result in serious modifications of the coastal dynamics, thereby impacting the structure and function of adjacent marine ecosystems (e.g., seagrass beds and coral reefs) and compromising the production of vital environmental goods and services, such as coastline stabilization and freshwater provision. In such a scenario, management actions such as mapping erosion-susceptible regions, protecting coastal vegetation and regulating coastal occupation are primordial. Thus, to understand the alterations caused by environmental changes in these ecosystems, we pose the following question: how do sea-level rise and the increase in coastal erosion determine the resistance and/or resilience of these semi-arid coastal ecosystems to external disturbances and disorders?
Decrease in land-ocean fluxes and alteration of biogeochemical processesMore than half of the rivers worldwide are intermittent or ephemeral, meaning that they can naturally cease their flow or remain completely dry for a long time (Datry et al., 2016). These rivers are commonly present in hot semi-arid regions (Pusey et al., 2010). Most rivers along the Brazilian semi-arid coast are small and intermittent (Maltchik and Medeiros, 2006). During the dry season, sediments accumulate in the river channels and the freshwater flow is almost nonexistent. The connection between the land and the ocean is commonly maintained by the intrusion of saltwater caused by high tides. Therefore, transitional systems have an estuarine-lagoon morphology and are frequently subjected to hypersalinity. However, during the wet season, intense but short-lived rainfall carries sediments, nutrients, and contaminants to the ocean and establishes typical estuarine circulation. This results in intense exchanges of matter and energy between the continent-ocean interface, which is a fundamental process for local coastal dynamics in the inner continental shelf. Moreover, acidic ocean waters can alter heavy metal’s dynamics and increase their bioavailability and toxicity. These pollutants can decrease CO2 sequestration by the blue carbon ecosystems accelerating climate changes (Zeng et al., 2015). Finally, coastal regions, such as the Brazilian semiarid coast, are more vulnerable to the impact of acidification due to large influxes of pollutants from land-based sources and hypoxia levels (Melzner et al., 2013). In this sense, environmental protection from coastal pollution has a large potential for mitigating ocean acidification risk (Zeng et al., 2015; Cotovicz et al., 2020b).
However, changes in the drainage of the river basins in the past three decades (especially after the construction of multiple dams) (Molisani et al., 2006) combined with climate-change-driven reductions in rainfall (Cunha et al., 2019) have reduced the freshwater inputs during the rainy season, thereby compromising the pivotal land-ocean fluxes. This reduction increases the water residence time (Dias et al., 2011) and the sediment accumulation in the inner river areas (Morais and Pinheiro, 2011; Godoy and Lacerda, 2013), thereby perennializing the tide-dominated status of such estuaries (Pinheiro and Morais, 2010; Medeiros et al., 2018).
As the residence time of estuarine water increases, the reactivity of many substances is enhanced. Trace metals of environmental significance, such as mercury, become more reactive. Their bioavailability can also increase by complexing with dissolved organic carbon. Paradoxically, this is the opposite of the phenomenon observed in the Arctic Ocean, in which increasing flows of mercury into the ocean and bioaccumulation in biota are associated with increased river discharge due to the melting of glaciers and continental ice (Dai et al., 2009). The phenomenon of increasing pollutant mobilization due to global changes is independent of the variations in emissions and is stronger in extreme ecosystems worldwide; it has been described as an “Arctic Paradox” by Lacerda et al. (2020). Thus, the levels of a contaminant in the local biota are increasing even when the total load of the pollutant is generally decreasing.
This process is claimed to cause increasing mercury exposure in local consumers of fishery products on the semi-arid coast. Moreover, it is hypothesized that the accumulation of mercury could be an indirect consequence of the increase in semi-arid mangrove areas because it expands the metabolism-based dissimilatory SO42- reduction to a larger portion of the estuary (Lacerda et al., 2020). The longer water residence time in the dry period leads to a longer flooding time in the upper estuary, thereby leading to the production of large amounts of mercury-organic complexes due to the incomplete respiration of organic matter by anaerobes. The complexes are then transported to the lower estuarine areas where they bioaccumulate (Vaisman et al., 2005; Rios et al., 2016). Landward mangrove expansion has been reported in the Brazilian semi-arid coast as saline intrusion increases toward upstream areas (Godoy and Lacerda, 2015; Godoy et al., 2018). This increase, along with mangrove erosion next to beaches and river mouths, contributes to the remobilization of sediments and to the release of sulfur-metallic compounds, thereby dissociating them and releasing the metals to the water column, where they are much more bioavailable (Marins et al., 2020). For this reason, efficient dam management to avoid sediment retention could help to minimize such effects. Thus, to better understand the responses of these ecosystems to this changing scenario, we pose the following question: how will changes in sediment remobilization caused by low land-ocean exchanges alter biogeochemical processes in semi-arid ecosystems?
Global warming, intensity, and frequency of extreme eventsThe occurrence of extreme events, such as heatwaves, temperature increases, intense and frequent droughts, and annual rainfall reduction, have been predicted and reported in the Brazilian semi-arid coast (Kundzewicz et al., 2014; Marengo et al., 2019; Soares et al., 2019). The average marine heatwave (MHW) frequency was close to 3 events per year from 1982 to 2020 in some Brazilian coastal regions, but was more than 10 events per year in this low-latitude coast. The frequency of MHW events has increased in the last 40 years, and a clear trend of a 0.2 °C temperature increase per decade was detected in the Brazilian semi-arid coast (Soares et al., 2021). These recent results indicate that the Brazilian semi-arid coast is one of the most-affected regions by climate change impacts, such as global warming, acidification, and extreme events such as heatwaves (Marengo et al., 2016, 2017, 2018; Soares et al., 2021).
A gradual increase in the temperature of 0.5 °C and 1.0 °C is expected by 2040 and 2070, respectively (PBMC, 2014). This increased warming trend and MHWs are currently impacting distinct semi-arid ecosystems, such as marginal reefs subjected to coral bleaching (Soares et al., 2019) and short low-inflow estuaries under hypersaline conditions (Barroso et al., 2018). The temperature increase is also expected to impair the trophic transfer efficiency from the individual level via changes in growth costs and carbon-use efficiency to the ecosystem level (Barneche et al., 2021).
A global-scale analysis of coastal species has shown abundant changes linked to warming, with richness declines expected in equatorward areas in the next five decades because of failures to adapt to rapid climate change (Hastings et al., 2020). Given that the ranges of marine species are closely related to their physiological thermal tolerances, global warming may lead to increases in abundance poleward and decreases in abundance toward the equator (Hastings et al., 2020), which is where the Brazilian semi-arid coast is located (Fig. 2). Acidification is another global stressor (Cotovicz et al., 2020b) that represents an additional impair mainly to calcifier organisms, some of them with key habitat-forming importance (e.g., corals and coralline algae) (Crook et al., 2012; Soares et al., 2019) and socioeconomic relevance (e.g., bivalves in brackish waters) (Waldbusser et al., 2015). Thus, determining how semi-arid coastal ecosystems respond to the increased frequency of predicted extreme events in the context of global climate change is pivotal for improving the predictions of community dynamics under climate change. Moreover, management actions, such as improving prediction models of regions susceptible to extreme events (e.g., storm surges and floods), better control of coastal occupations, and protection of coastal vegetation are key to deal with these global changes.
Loss of vegetation cover and biodiversity due urbanization, aquaculture, agriculture, eutrophication, and land use changeThe Brazilian semi-arid coastal urbanization process accelerated during the 20th century owing to intense human migration from inland areas to coastal areas related to prolonged droughts and the desire to seek refuge and better living conditions. This growth became more pronounced when development activities, such as the installation of ports, shrimp farms, industries, and services, were initiated (Cerqueira et al., 2020; Lacerda et al., 2021). The intense land use and uneven distribution of governmental and private investments resulted in segregated and socially unequal access to the city, which resulted in several areas with deficient sanitation and sewage systems and high demands for housing, employment, and income. These areas put pressure on coastal ecosystems (e.g., dunes, coral reefs, estuaries, and beaches) through water and soil contamination, eutrophication, siltation, and the removal of vegetation cover.
The increase in urbanization caused strong deleterious changes. The reduction and fragmentation of vegetation cover in northeastern Brazil are prominent owing to the link between vegetation (e.g., mangrove forests) and other ecosystem components. Vegetation is a main attribute of conservation of terrestrial biodiversity and environmental services, and its loss will result in poorer ecosystems (Moro et al., 2015). Among other changes, urbanization causes soil compaction and waterproofing (caused by buildings and pavement), hydrological destabilization (resulting from hydric works or the imbalance between the withdrawal and return of water in water bodies), and water quality deterioration with eutrophication (either due to source pollution from sanitary and industrial sewage or diffuse pollution from surface runoff carrying livestock residues, mineral fertilizers, or pesticides) (Adams et al., 2001), thereby directly impacting the coastal ecosystem (Martins et al., 2012).
The main impacts on these ecosystems have been known in the Brazilian scientific literature since 2004, in which Gorayeb et al. (2004) identified mangrove deforestation as a major damage to the Pacoti estuary (Figs. 3 and 4), mainly due to the use of firewood to produce charcoal for domestic consumption and inadequate garbage disposal. Moreover, tourism and leisure activities without proper planning and mismanagement near the mouth of the rivers are other significant threats.
Some parameters are strong indicators of human influence on water quality and determinants of negative ecological impacts in coastal environments, such as biodegradable organic matter, which promotes the depletion of dissolved oxygen and hypoxia in shallow-water urbanized estuaries (MacPherson et al., 2007); lower dissolved oxygen in hypereutrophic estuaries (Dai et al., 2006); increased inorganic nutrients (mainly in the forms of NO3, NO2, NH4/NH3, PO4, and SiO4), which cause eutrophication, harmful algae blooms, and imbalances in primary productivity and the food chain (Cerqueira et al., 2020; Malone and Newton, 2020); turbidity, which causes obstruction of light and decreased photosynthetic activity (MacPherson et al., 2007); and pathogenic microorganisms, which pose risks to public health through bathing or through consumption of contaminated seafood in beaches, mangroves, and estuaries (Lipp et al., 2001). Considering this complex scenario, we raise the following multicomponent question: how do environmental variations, anthropogenic changes, and the context of global change affect ecosystem services and human communities? Answering this question is crucial to guiding stakeholders in creating accessible public policies aimed at preserving both traditional human populations and natural ecosystems.
Conclusions and final remarksWe raised five main environmental issues concerning the Brazilian semi-arid coast and proposed fundamental socioecological questions to guide future research in the field (Fig. 5). Understanding the structure and functions of these dry coastal ecosystems will help to clarify the intricate relationships and connectivity between the different communities in the land-ocean continuum. This, together with participatory data collection, will generate information to understand how biodiversity is related to stability under global environmental changes. Understanding the ongoing impacts on traditional communities living in this semi-arid coastal zone is pivotal to building science-based actions to support their livelihoods and decrease social inequality.
Regional/local (e.g., shrimp farming and organic pollution) and global-scale pressures (e.g., marine heatwaves and extreme droughts) act concomitantly (e.g., cumulative or synergistically), driving species-specific effects and, consequently, deep changes in the current and future state of semiarid coastal ecosystems such as mangroves (Marins et al., 2020; Lacerda et al., 2021) and coral reefs (Soares et al., 2017). Climate change could modify the resistance or resilience of coastal ecosystems, potentially making them more vulnerable to human impacts (i.e. overfishing and deforestation), and direct impacts may amplify climate change constraints in coastal ecosystems. Such drastic changes will have severe and negative effects on the energy budgets of key organisms as well as on their phenology and population viability (Rossi et al., 2019).
These historically neglected human populations have been heavily impacted by decreases in both environmental and human health status. They were recently affected by the largest environmental disaster in Brazilian history (an oil spill that reached the northeastern Brazilian coast in 2019/2020) and by the COVID-19 pandemic, which was intensified by the high levels of poverty and social inequality (Magalhães et al., 2020; Soares et al., 2020). Between 2011 and 2017, the Brazilian northeast faced recurrent drought events, which were considered the most intense in terms of duration, severity, and recurrence (Cunha et al., 2019). Droughts are associated with serious global economic and social losses and affect more people than any other natural disaster (Keyantash and Dracup, 2002).
In this context, interdisciplinary ecological and socioeconomic indicators that can monitor the effects of global climate change are essential to define, characterize, and quantify the consequences of this phenomenon on the drought-prone coast and society, especially vulnerable populations (Andrade and Soares, 2017; Queiroz et al., 2017). An effective strategy is to identify and monitor processes that not only confirm that the climate is changing, but also indicate how and to what extent this will affect ecosystem goods and services (Lacerda et al., 2010, 2012, 2020). Such ecosystem services include blue carbon stocks and sequestration (Macreadie et al., 2019), of which Brazil is an important global provider through mangroves, macroalgae, saltmarshes, and seagrass beds (Turra and Denadai, 2016; Gouvêa et al., 2020). For example, mangroves on the semi-arid coast of Brazil capture, on average, more carbon (above- and belowground) per hectare (413 Mg C ha-1) than the Amazon rainforest (approximately 280 Mg C ha-1), with values close to that of the Amazonian mangrove forest (511 Mg C ha-1), which has the highest average carbon capture per hectare in Brazil (Kauffman et al., 2018a, 2018b). However, we do not know how the climate regulation service provided by these estuaries will be affected by mangrove expansion and retraction (Sections “Decrease in rainfall rates and increase in hypersalinity in estuaries” and “Sea-level rise and increase in coastal erosion”) and the complex impacts of climate change (Kauffman et al., 2018a; Marengo et al., 2020).
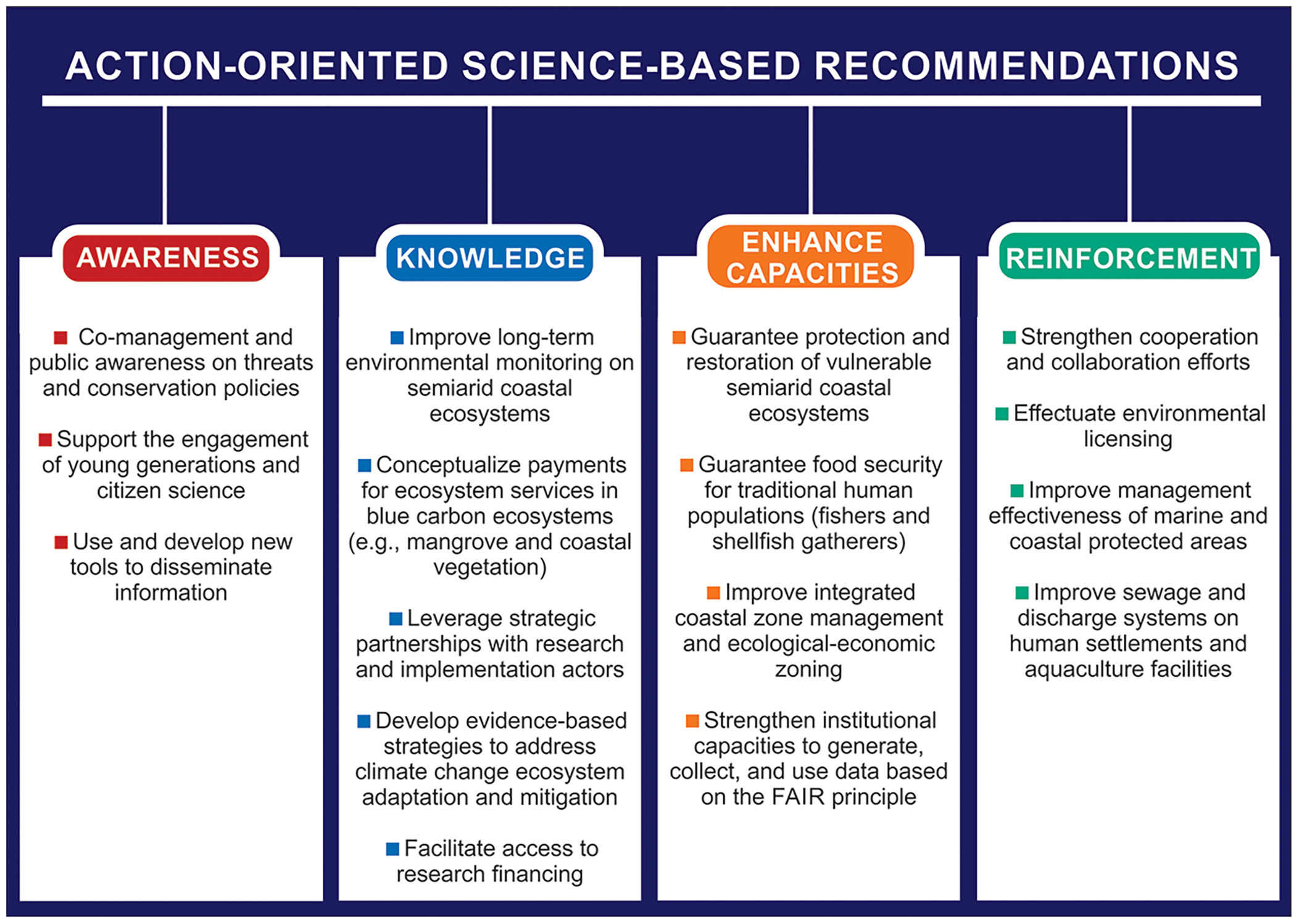
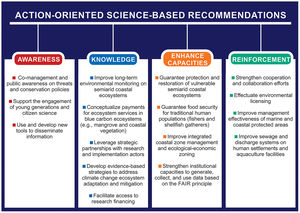
Such scientific information will help to develop important science-based tools for use in drought-prone regions by water resource managers, scientists, social movements, community associations, NGOs, society, and local governments (Fig. 6). Furthermore, studying coastal ecosystems under extreme environmental conditions (e.g., inverse and hypersaline estuaries) in the long term can provide important information for other regions of Brazil and the world that are undergoing increasing aridity. Many other ecosystems will be impacted by similar stressors in the short, mid, or long term.
Using the changes, impacts, and five questions presented here (Fig. 5) to guide the structure of coastal research strategies and to provide access to long-term scientific data can benefit scarcely known semi-arid systems and unknown adjacent submarine areas. This can help to predict the effects of environmental changes on the sustainability of ecosystem goods and services. Moreover, the five questions can guide science-based proposals of public policies (Fig. 6) aimed at achieving the Sustainable Development Goals and the actions/goals in the context of the ongoing UN Decade of Oceanic Science for Sustainable Development (2021–2030) in Brazil and similar semi-arid coasts worldwide.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This perspective article outlines the Brazilian Semi-Arid Coast Long Term Ecological Research Project (PELD-CSB) and the first research site on this coastline focusing on the following main scientific question: how do climatic and environmental variability in the semi-arid coast affect organisms, populations, and communities? We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Research Productivity Fellowship Nos. 307061/2017, 309140/2018-8, 310165/2020-2, and 313518/2020-3), PELD Costa Semiárida do Brasil-CSB (No. 442337/2020-5), CAPES-PRINT, CAPES-PNPD (HSB Fellowship), MCTIC/CNPq 28/2018 – Universal 423628/2018-6, Pronex PR2-0101-00052.01.00/15, INCT AmbTropic, and Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (Chief Scientist Program) for their financial support. This is PELD-CSB contribution #2.