Birds are important bioindicators of environmental change and perform essential processes for ecosystem functioning. Afforestation of grasslands may lead to the reduction or loss of specific functions performed by bird species. We investigated the effects of afforestation of grasslands in southeastern South America on taxonomic and functional diversity of birds using multidimensional functional diversity indices. We collected data from bird communities in mosaics of native habitat and in eucalyptus plantations. We recorded taxonomic impoverishment in plantations, but no reduction in functional diversity was found in planted areas. The results demonstrate greater functional redundancy in riparian forest, which may confer greater stability for these communities, while higher values of functional diversity in plantations may indicate quite dynamic and less stable communities from a functional point of view. None of the diet categories were strongly associated with the sampled habitats. Nonetheless, omnivorous and insectivorous bird species were among the first to colonize plantations after cutting. Since the response of species to habitat changes are mediated by their traits, we can infer that, for a group of species, forest plantations are not totally unsuitable. However, to conserve different dimensions of diversity, it is essential to conserve mosaics of native vegetation to maintain bird diversity.
The conversion of native habitats to other land uses is expected to increase in the coming years as human population growth and demand for consumer products increase (Newbold et al., 2013). Grassland ecosystems are under strong anthropogenic pressure from changes in land use caused mainly by the expansion of monocultures such as soybeans and eucalyptus (Azpiroz et al., 2012). Until a few years ago the main economic activity in grasslands of southeastern South America was raising livestock, a traditional activity that, when managed properly, is compatible with the conservation of native diversity (Azpiroz et al., 2012; Overbeck et al., 2015). Such grasslands comprise a heterogeneous landscape featuring a natural mosaic of native grasses, shrubs and forest patches restricted to riparian zones (Hasenack et al., 2010). The most representative portion of this ecosystem in Brazil is concentrated in the southern region of the state of Rio Grande do Sul (Pampa), comprising the so-called southeastern South American grasslands (SESA grasslands) (Azpiroz et al., 2012). Recent years have seen rapid expansion of eucalyptus plantations over these grasslands, replacing native habitats that harbor a high concentration of species of conservation interest.
Afforestation of grassland ecosystems leads to changes in vegetation structure, resulting in simplification of the vegetation. Such changes impact local bird assemblages, in this sense, the major impacts on bird communities are changes in abundance, diversity and composition of species, resulting in the loss and homogenization of biodiversity (Dias et al., 2013; Jacoboski et al., 2019). Typical grassland bird communities that depend exclusively of grassland resources are very sensitive to the afforestation of grasslands and become locally extinct (Jacoboski et al., 2019; Phifer et al., 2016). For birds of the riparian forest of the Pampa, eucalyptus plantations may serve as a complementary habitat for some species with less specialized diets and habitat requirements (Pezda, 2015). With increasing intensity and change in land use, and the consequent homogenization of the habitat, bird communities become less diverse and dominated by particular types of species, mainly generalist species with regard to habitat and diet (De Coster et al., 2015). The characteristics and width of the ecological niche of each species can influence their ability to exploit different habitat types, whether degraded habitats or new habitats created by human activities (Zurita et al., 2017).
Thus, changes in habitat may not only alter species richness of a particular location but may also cause changes in the occupation of functional space by removing species with traits that are not adapted to the habitat (Mouillot et al., 2013). Understanding the impact of the conversion of native ecosystems on bird biodiversity is of critical importance because birds contribute a wide range of ecosystem services. More and more studies are focusing on different components of diversity that explain different dimensions of a given ecosystem and describes a unique aspect of a given community (López-Ricaurte et al., 2017). Diversity metrics focused on functional aspects constitute an additional tool beyond the traditional taxonomic approach, since these metrics represent the variety of functional roles played by species within a community (Petchey and Gaston, 2006). Species traits represent how species influence ecological processes in a given assemblage and may correspond to ecological processes such as pollination and seed dispersal (Luck et al., 2013), determining ecosystem functioning as well as services for humans. Several studies have shown that the ecological traits of species, including different taxa (plants, mammals, birds, amphibians, ants), help explain species responses to changes in land use (Corbelli et al., 2015; Ernst et al., 2006; Flynn et al., 2009; Jacoboski et al., 2016). Previous studies with birds have shown that structurally and biologically less diverse anthropogenic habitats, such as eucalyptus plantations, lead to reductions in the taxonomic and functional diversity of their communities compared to native forest ecosystems (Corbelli et al., 2015; Jacoboski et al., 2016; Phifer et al., 2016). For grassland ecosystems, afforestation has also been found to lead to reductions in birds species richness and abundance in planted areas in an Argentinian savanna (Phifer et al., 2016) and changes in bird species composition in grasslands of southern Brazil (Dias et al., 2013). Similarly, López-Ricaurte et al. (2017) found that grassland specialist birds can be very sensitive to palm oil plantations in Colombian savannas. Critically, however, no study has examined the relative functional vulnerability of grasslands bird communities in grassland ecosystems in relation to the expansion of eucalyptus plantations.
In this sense, the aim of the present study was to evaluate whether afforestation of grassland ecosystems leads to a reduction in taxonomic and functional diversity of bird communities by comparing native vegetation mosaics (grassland and forest) to eucalyptus plantations at different stages of development. Considering that plantations can lead to a homogenization of the local community diet, with the predominance of insectivores and omnivores, a reduction in the performance of diet-related functions can be expected. Similarly, the reduction in the quality and quantity of foraging and nesting substrates in plantations, may lead to local extinction of species and specific traits. We can also expect a reduction in the mass of the bird community in plantations due to the absence of large frugivores, which directly affects seed dispersal. Thus, we hypothesized that: (1) as changes in land use can lead to reductions in the taxonomic and functional diversity of bird communities (Edwards et al., 2013; Jacoboski et al., 2016), grassland afforestation will decrease taxonomic and functional diversity of birds, while grazing will not; and (2) Since ecosystem services, such as pest control, can be maintained in highly modified landscapes (Luck et al., 2013; De Coster et al., 2015), afforestation conversion of grasslands will cause changes in the functional composition of diet traits of bird species, with traits related to pest control (insectivory) being predominant in areas of adult eucalyptus plantings and in areas after eucalyptus cutting.
Material and methodsStudy areaThe study was conducted in the grasslands of extreme southern Brazil (Pampa). These grasslands are part of the so-called southeastern South American grasslands (SESA grasslands), the region with the largest expanse of grassland ecosystems in the Neotropics (Azpiroz et al., 2012). Forests within this ecoregion are mostly restricted to riparian zones. Sampling was performed at 50 sites in four municipalities located in the central-western portion of the state of Rio Grande do Sul: São Gabriel (30°20′11″ S, 54°19′12″ W), Rosário do Sul (30°15′30″ S, 54°54′51″ W), Santa Margarida do Sul (30°20′19″ S, 54°4′15″ W) and Vila Nova do Sul (30°20′17″ S, 53°52′33″ W). Eight different sites in each of six different habitat types, based on vegetation, were sampled (except the riparian forest, with 10 sites): (1) Riparian forest (FOR): arboreal vegetation ranging in height from six to eight meters; undeveloped understory in some areas due to the presence of cattle. (2) Ungrazed native grassland (UNG): herbaceous-grassland vegetation with some isolated shrubs; average height of vegetation ranging from 60cm to one meter; without any management (cattle, fire) for at least six years; refers to Áreas de Preservação Permanente (APPs, permanent preservation areas) located within eucalyptus plantations. (3) Grazed native grassland (GRA): herbaceous-grassland vegetation with a predominance of grasses; intermediate level of grazing; average height of vegetation ranging from five to 40cm. (4) Eucalyptus plantations in the initial stage of development (IEP): eucalyptus plantations less than one year old and at most two meters high; undergrowth dominated by herbaceous-grassland vegetation. (5) Adult eucalyptus plantations (AEP): eucalyptus plantations of over four years old and over 15m in height; understory absent. (6) Plantings after cutting (PCE): no vegetation with a predominance of residues such as branches and bark.
Bird samplingWe sampled birds during the austral spring–summer 2014–2017, encompassing three breeding seasons of the bird species. We performed a visit at each of the 50 sites and sampled the birds using the point count method (Bibby et al., 1992). We distributed sampling so that all different habitat types were sampled in each breeding season. The counting points were distributed according to the size of each site, ranging from three to nine points per site. Points were allocated at random and were separated from each other by a distance of at least 200m (Bibby et al., 1992), maintaining a minimum distance of 50m from the edge of the habitat. According to Bibby et al. (1992), this distance is ideal to guarantee statistical independence among sampling points. All species of birds seen or heard within a fixed radius of 50m at each point during 10min were recorded. The observation radius was limited to 50m to maximize detectability and decrease potential for observer error when identifying cryptic species over long distances (Howick et al., 2015) and to maximize the detectability of species that are heard at shorter distances in forest environments. Of the six habitat types sampled had a total of 50 count points for a grand total of 300 count points. Sampling started 10min after sunrise and extended for up to 3h. All sampling was performed on days without wind or rain. Birds in flight were not considered.
Functional traitsTraits data were collected for the recorded bird species. The traits were taken by species and, therefore, without intraspecific variation. Four traits were selected for analysis: body mass, predominant diet (omnivore, insectivore, granivore, frugivore, nectarivore, carnivore, saprophage), foraging substrate (soil, vegetation, air, water) and nesting substrate (soil, vegetation). These traits were chosen because they are more likely to respond to habitat changes caused by eucalyptus plantations. Moreover, this choice was based on other studies that used the same traits and showed that changes in land use lead to changes in functional diversity (Luck et al., 2013; Jacoboski et al., 2016). The loss or reduction of these traits may result in the loss of key ecosystem processes, such as decreased predation rates, seed dispersal and pollination (Luck et al., 2013; Newbold et al., 2013). Different sources were used to characterize bird species in relation to their traits (Del Hoyo et al., 1992–2002, 2003–2006; Wilman et al., 2014).
Statistical analysesInitially, to assess spatial correlation among the sampled points a Mantel correlation analysis was performed between a distance matrix from the taxonomic composition matrix and a matrix made form the geographic coordinates of each counting point. For other analyses, each site was considered a sample unit. The taxonomic diversity index of recorded bird species was subsequently calculated using the Simpson diversity index. The functional diversity index (FD) was also calculated. Since FD is known to be dependent on species richness, sesFD was calculated, which standardizes functional diversity values by removing the effects of species richness (Swenson, 2014). The null model taxa.labels, which maintains species richness in each community, was used to calculate sesFD (Swenson, 2014).
We also calculated four multidimensional indices of functional diversity calculated: FRic, FEve, FDiv and FDis. These indices measure the distribution of species traits in a multidimensional space and are independent of species richness and of one another (Villéger et al., 2008). Functional richness (FRic) represents the amount of functional space occupied in a community (Villéger et al., 2008). The index FEve measures the regularity of species distribution and abundance across functional space, a gradient of traits and equitability in the distribution of abundance across species (Villéger et al., 2008). FDiv demonstrates how abundance is distributed within the volume of space of a functional trait occupied by a species. The index FDis is the average distance of the trait in multidimensional space of a single species to the centroid of all species, which can account for species abundance shifting the position of the centroid toward the most abundant species and weighing distances from each species by its relative abundance (Laliberté and Legendre, 2010). Univariate analysis of variance (ANOVA) followed by Tukey's test were performed with the values for each of the calculated metrics for each of the sampled sites to compare the contrasts between habitats in order to evaluate possible differences in taxonomic and function structure of the bird communities among sampled habitats. All analyses were performed using the packages picante and FD of R software (R Core Team, 2017).
In addition to these analyses, functional traits that were positively or negatively related to each of the sampled habitats were determined and quantified using RLQ analysis (Dolédec et al., 1996). This multivariate approach links information on species abundance, species traits and environmental characteristics. This metric uses three different matrices: a matrix of species×traits (matrix Q), a matrix of sample units×species (matrix L) and a matrix of sample units×environmental variables (matrix R), with each of the sampled habitats being considered as environmental variables (Dray and Legendre, 2008). RLQ is an ordering method that joins the data of the three matrices and generates a matrix fourth of traits×environmental variables. The null model used was modeltype=6, the only one that does not inflate type I error (Dray and Legendre, 2008). This model is a combined approach that permutes sites in rows and species in columns. After a permutation test (9999 permutations), the strength of the association between species traits and each habitat can be identified. The analysis was performed used the ade4 package of R software (R Core Team, 2017).
ResultsA total of 109 bird species were recorded in the sampled habitats (Table 1, Supplementary Material). Only one species, Zonotrichia capensis, was recorded in all habitats. Three of the recorded species are in some category of global threat, according to IUCN (2019): Culicivora caudacuta, Sporophila cinnamomea and Xanthopsar flavus. Two other species have a record of regional threat: Sporophila pileata and Mackenziaena severa (DOE, 2014). All of the species, except M. severa, were recorded in areas of native grassland (grazed or ungrazed). The Mantel test indicated that there was no significant spatial correlation among sampled points (r=0.02, P=0.23).
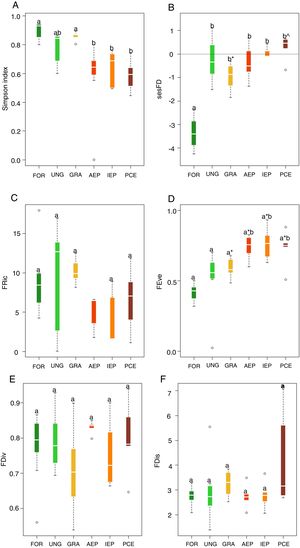
The highest taxonomic diversity was recorded in the riparian forest and grasslands while the lowest were in plantation areas, considering all stages of development (Fig. 1A). The majority of the species recorded in adult plantations were also recorded in riparian forest. Riparian forest had the lowest level of sesFD (lower FD than expected given species richness) (Fig. 1B), while FEve was higher in the eucalyptus plantations at all stages of development (Fig. 1D). Indices FRic, FDiv and FDis did not differ significantly among treatments (Fig. 1C,E, F) (Table 2, Supplementary Material).
Boxplots for values of: (A) taxonomic diversity, (B) functional diversity (sesFD), (C) functional richness (FRic), D) functional equitability (FEve), (E) functional divergence (FDiv) and (F) functional dispersion (FDis). Different letters indicate significant differences, and same letters with different symbols also indicate significant differences. Same letters unaccompanied by any symbol indicate no significant difference. Legend: FOR (riparian forest), UNG (ungrazed grassland), GRA (grazed grassland), AEP (adult eucalyptus plantation), IEP (initial eucalyptus plantation), PCE (post-cut eucalyptus).
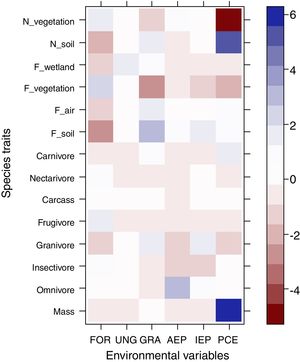
The most evident relationships between traits and habitat were related to post-cut eucalyptus. Species that nest on the soil and those with higher body mass were positively associated with plantings soon after cutting, while those that nest in vegetation were negatively associated with this plantation stage (Fig. 2). None of the diet categories were associated with habitats.
Relationship between functional traits and habitat. Intense colors represent the strongest associations between traits and habitat. Blue color represents positive associations between traits and habitat, while red color represents negative associations. Legend: N_vegetation (nesting in vegetation), N_soil (nesting in soil), F_wetland (foraging in water), F_vegetation (foraging in vegetation), F_air (foraging in air), F_soil (foraging in soil), FOR (riparian forest), UNG (ungrazed grassland), GRA (grazed grassland), AEP (adult eucalyptus plantation), IEP (initial eucalyptus plantation), PCE (post-cut eucalyptus).
Our results demonstrate that afforestation of grasslands does not alter taxonomic diversity and functional diversity in a similar manner. Afforestation of grassland ecosystems reduces taxonomic diversity mainly due to habitat similarity and reduced availability of resources for birds (Jacoboski et al., 2016; Phifer et al., 2016). This result demonstrates the importance of mosaics of native vegetation for maintaining greater bird diversity. In forest plantations, species with higher degrees of specialization are replaced by habitat generalists, a mechanism that promotes functional homogenization (Almeida et al., 2018). Despite the absence of plantation specialist species, reduced levels of functional diversity were not recorded for the different plantation stages. That is, species substitution occurs between the different habitats, but no turnover in traits takes place. The negative values for functional diversity (sesFD) in riparian forest indicate short functional distances between species that coexist in these areas; in other words, there is evident functional grouping. In this case the random loss of any of these species may not result in the loss of functional diversity (Luck et al., 2013). In accordance with our results, some studies have also shown that species-rich forest bird assemblages have functional grouping and high levels of functional redundancy (Almeida et al., 2018; Prescott et al., 2016). It is probable that these communities, and the ecosystem services they provide, are more stable over time, conferring greater resilience to riparian forest (Sayer et al., 2017).
On the other hand, bird communities in native grassland areas (grazed and ungrazed) and eucalyptus plantations in different stages are more functionally dispersed, which may indicate the occurrence of species with unique traits, as evidenced by Almeida et al. (2018). When many of the species of a community are functionally unique the loss of one only species can have negative consequences for the ecosystem (Luck et al., 2013). Thus, the grassland bird communities of the present study appear to be more vulnerable since they are restricted to grasslands, while plantation species can be found in riparian forest. The life history traits of species were correlated with habitat structure, indicating that functional traits depend on specific habitat characteristics (Smith et al., 2018; Chapman et al., 2018). In this sense, grassland-dependent species do not find the specific habitat structure they need in plantations. On the other hand, most species present in plantations are not dependent on a specific habitat.
Among the analyzed multidimensional indices of functional diversity, only functional equitability (FEve) had significant differences among the sampled habitats. Communities with the highest FEve values were those of plantations in all their stages of development. Higher FEve values can indicate under exploitation of space, suggesting that the habitat is not very structurally complex (Mason et al., 2005). In other words, there is less abundance of species, which means that there are fewer uniformly-occupied niches and fewer interactions among bird species (Schleuter et al., 2010). Thus, functional equitability may increase with the intensification of habitat disturbances (Villéger et al., 2008), probably due to simplification of habitat structure, such as in eucalyptus plantations. Although habitat complexity was not quantified in the present study, we can infer that there is less complexity within plantations (Jacoboski et al., 2016), due to homogenization and lower vertical stratification of vegetation, leading to high levels of functional equitability.
The other multidimensional indices analyzed had no significant differences among habitats. The results for FRic demonstrate that, in both native and plantation habitats, effectiveness of resource use is similar across communities, as niche space is similarly occupied (Mason et al., 2005; Schleuter et al., 2010). Similar levels of FDiv indicate that both native habitat and plantation bird communities maintained a similar diversity of resource use characteristics, as there is a similar distribution of species in function trait space (Prescott et al., 2016; Edwards et al., 2013). Regarding functional dispersal, the conversion of native habitats to plantations did not lead to changes in FDis levels among habitats and, therefore, there was no reduction in ecosystem functioning (Prescott et al., 2016). FDis is associated with landscape diversity, which filters species via their ecological traits (Barbaro et al., 2014). However, in the present study we did not verify the filtering effect of eucalyptus plantations since the communities, both of native and plantation habitats, appear to be quite similar in functional terms, as shown by the results presented here.
Regarding our second hypothesis, which predicted the maintenance of ecosystem services, such as pest control, in areas after eucalyptus cutting, strong positive or negative associations between these traits and habitats were not recorded. However, body mass and species that nest on the soil were found to be positively associated with plantings after cutting. Species that nest in vegetation, on the other hand, were negatively associated with this plantation stage. The occurrence of Rhea americana in plantations after cutting certainly contributed to the association between large body size and this habitat. The association of some traits with this plantation stage demonstrates the plasticity of many typical open-area species that return to areas shortly after cutting. Since not all species respond negatively to human disturbance, many species of the pool return when the area approaches its original aspect (i.e., no tree vegetation). As birds have high mobility capacity, species respond quickly to changes in the landscape. This is a very interesting result, especially for studies involving restoration of grassland areas. Although without any vegetation in these areas, omnivorous and insectivorous species, such as R. americana and Xolmis cinereus, eventually find additional resources within these areas and are among the main colonizers. Luck et al. (2013) and De Coster et al. (2015) suggest that ecosystem services, such as pollination and biological control, can be maintained even in highly modified landscapes.
The present study demonstrates that, despite the reduction in species taxonomic diversity, afforestation of southern grasslands does not result in reduced functional diversity, suggesting that the combination of species traits and use of functional space are relatively similar across habitats. In other words, bird species in each habitat appear to be functionally equivalent but differ in their responses to disturbance. While habitat and diet specialists become locally extinct from plantations, generalist species tend to expand their populations to plantations, so some ecosystem services, such as insect pest control, can still be maintained on forest plantations. In this sense, eucalyptus plantations in the studied region may not be totally unsuitable for a portion of the species found in the local pool. On the other hand, our results demonstrate the importance of vegetation mosaics in the studied region for the maintenance of a greater diversity of birds. Thus, if we want to maximize the conservation of different dimensions of diversity, the ideal is to ensure the conservation of mosaics of native vegetation, including original grassland vegetation and forest vegetation restricted to riparian zones.
Conflict of interestNone.
We thank CMPC Brasil for financial and logistical support. We are grateful to E.F. de Araújo for all the logistics for the realization of the fields and for the information on the study area. We also thank the owners who authorized bird sampling on their properties. Thanks also go to R.K. Paulsen, B.A. Maslak, A.M. Pezda, and J.K.F. Mähler Jr. for help in the field. We thank L.S. Duarte, L. dos Anjos and R.A. Dias for their valuable suggestions for the final version of the manuscript. Thanks to two anonymous reviewers for all sugestions that improved our manuscript. L.I.J received a scholarship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), and S.M.H has a research grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), process 304820/2014-8.