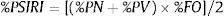Vertebrates introduced in non-native habitats have contributed to several extinctions in the modern era, with direct effects mainly over birds, mammals and reptiles on islands. Fernando de Noronha Archipelago, in tropical Atlantic Ocean, is a World Heritage natural site, holding the most diverse breeding seabird community off Brazil, in addition to endemic landbirds and reptiles. It also holds invasive black rats (Rattus rattus), tegu lizards (Salvator merianae) and feral cats (Felis catus), among the 26 exotic species reported in the archipelago, all of which are species with potentially high impact upon native fauna. Aiming to assess the role of exotic vertebrates on the fauna, we investigated their diets through stomach content and scat analysis, and stable isotope analysis (SIA) followed by isotopic mixing models. The main food items for tegu lizards were fruits, with relative importance (Prey-specific Index of Relative Importance – %PSIRI) of 41.3%, and the endemic Noronha skink (Trachylepis atlantica; 19.6%) and for black rats was Noronha skink (30.3%). The diet of feral cats was composed by rodents (31.6%), birds (28.6%) and Noronha skink (18.8%). SIA and Bayesian mixing models provided evidences that invasive species use marine matter to synthesize tissues, probably derived directly or indirectly from seabird colonies. This study demonstrated that exotic species feed on other exotic, as well as endemic species. The most heavily impacted species was the endemic Noronha skink. We demonstrated the predation pressure of exotic fauna upon endemic vertebrates, and strongly recommend the implementation of an invasive species control and eradication plan.
Invasive predators have contributed to several extinctions in the modern era, the vast majority being birds, mammals and reptiles (Doherty et al., 2016). Invasive species can affect the population structure of native species, modify the community composition and ecological processes through competition, predation or emergence of diseases (Lockwood et al., 2007). Introduced rats, for instance, are the main cause of the high mortality observed in several bird species, including seabirds (Caut et al., 2008a; Leal et al., 2016), especially at the chick phase (Jones et al., 2008; Tabak et al., 2016). Cats and lizards are also known to prey native birds at nests, small mammals, reptiles and amphibians (Loss et al., 2013; Mazzotti et al., 2015; Woinarski et al., 2020), accounting for extinction or decline of species (Bovendorp et al., 2008).
Islands have high endemism rates due to limited distribution ranges and dispersion of terrestrial species (Kier et al., 2009). Whereas endemic species could lack defensive traits due to their evolution in the absence of natural predators (Banks and Dickman, 2007), island ecosystems become particularly vulnerable to the effects of introduced species (Medina et al., 2011). Insular endemic species threatened by invasive species added up to 81% of the “Endangered” and “Extinct” categories (Doherty et al., 2016), and most well-documented bird species extinctions of modern era (78.7%) occurred on oceanic islands (Szabo et al., 2012).
Fernando de Noronha Archipelago, 360km off the Brazilian coast, is an Important Bird Area (BirdLife International http://datazone.birdlife.org/site/factsheet/arquip%C3%A9lago-de-fernando-de-noronha-iba-brazil), particularly for breeding seabirds (11 species; Mancini et al., 2016), and also hosts endemic and threatened species of skinks and landbirds. Among seabirds that breed on the archipelago, the red-footed booby (Sula sula), white-tailed tropicbird (Phaethon lepturus) and red-billed tropicbird (Phaethon aethereus) are nationally listed as “Endangered”, while the Audubon's shearwater (Puffinus lherminieri) is listed as “Critically Endangered” (MMA, 2018). The endemic landbirds Noronha vireo (Vireo gracilirostris) and Noronha elaenia (Elaenia ridleyana) are globally listed as “Near Threatened” and “Vulnerable”, respectively (IUCN, 2020). Two endemic reptiles, Noronha skink (Trachylepis atlantica) and Ridley's worm lizard (Amphisbaena ridleyi) are “Critically Endangered” in the Pernambuco state red list (CPRH, 2017). There was also an undescribed flightless rail (Olson, 1981) and an endemic rodent (Noronhomys vespuccii), both now extinct possibly due to the arrival of humans and introduction of the exotic black rat (Rattus rattus) brought on vessels (Carleton and Olson, 1999). Additionally, there are 26 exotic species reported to the Fernando de Noronha Archipelago (IABIN, 2020), including black rats, feral cats (Felis catus) and tegu lizards (Salvator merianae) (Dias et al., 2017; Abrahão et al., 2019; Gatto-Almeida et al., 2020). Cats and rats prey upon the majority of the animal species available in the environment, affecting nesting of seabirds and endemic species where they occur (Bonnaud et al., 2007; Dickman, 2009; Dias et al., 2017). Tegu lizards have their diet based on fruits and leaves, but also feed opportunistically on invertebrates, carcasses and animal tissues in general, including other lizards and birds (Hines, 2011; Silva et al., 2014; Abrahão et al., 2019).
Determination of diet is important to establish consequences of invasive species over native species. This assessment can be made through direct diet examination, using stomach contents or scat samples (Bonnaud et al., 2007), and complemented with intrinsic elemental markers, such as stable isotopes (Galetti et al., 2016). Stable Isotope Analysis (SIA) assesses food resources effectively assimilated into animal tissues during a variable period (i.e. days, weeks or months), whereas the traditional diet analysis can only show food items consumed recently (i.e. hours or days), despite providing identification to higher taxonomic resolution (Rounick and Winterbourn, 1986). The combination of both techniques can enhance inferences on the trophic role of consumers and their food sources (Barrett et al., 2007).
Quantifying consumption of endemic species and determining the most important dietary items of an invasive species allow the prioritization of threatened species and management actions towards conservation. The aim of the current study was to determine the main food items of invasive cats, rats and tegu lizards of Fernando de Noronha Archipelago. We also assessed the importance of endemic and threatened vertebrate species on the diet of these invasive species and its potential effects on conservation of the island biodiversity. To our knowledge, our study is the first to investigate the feeding patterns of the large-sized tegu lizard using the conventional diet assessment methodology associated with SIA. It is also the first study to analyze and quantify the diet of feral cats and black rats of Fernando de Noronha, where control and eradication plans are currently under intense debate and black rats were recently eradicated from Meio Island, within the archipelago. Our results could ultimately support the development of management plans for control or eradication of exotic species in Fernando de Noronha, thus contributing to the conservation of threatened species.
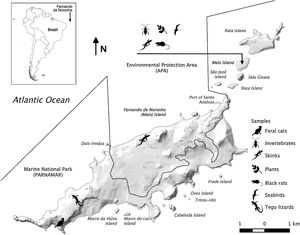
Material and methodsStudy areaStudy occurred on two islands located at Fernando de Noronha Archipelago (3°52′S; 32°26′W), the Meio Island and the Main Island. The archipelago is in the Equatorial South Atlantic Ocean, 360km from the mainland (Fig. 1) and has 21 volcanic islands with total area of 18.2km2 (Dias et al., 2017). Climate is tropical with well-defined seasons: dry, from August to January, and wet, from February to July. The archipelago presents a great habitat variety (Russel et al., 2018), and the only island with permanent human settlement is the Main Island (Ilha Principal), with population estimated over 3000 inhabitants, plus up to 3000 tourists each month during the peak season (IBGE, 2019).
As a semi-arid ecosystem with marked dry season, Fernando de Noronha holds low vertebrate diversity but high endemism, resulting from the long isolation of terrestrial vertebrates (Serafini et al., 2010). Meio Island is a small island hosting Noronha skinks, the nationally “Endangered” yellow-crabs (Johngarthia lagostoma), breeding seabirds and a recently eradicated abundant population of black rats. These species are also found in other islands and islets of the archipelago. Feral cats inhabit the Main Island only and the tegu lizards are found in the Main and Rata Islands (Abrahão et al., 2019). The archipelago is inside a National Marine Park in uninhabited areas, while urban areas are within an Environmental Protection Area (EPA).
Sampling and sample processingFeral catsA total of 78 scat samples of feral cats were collected throughout 2015, along tracks in uninhabited areas of the Main Island, thus targeting feral cats only (Fig. 1). These samples were stored frozen until analysis. Scat samples were used, instead of stomachs, to perform conventional diet analysis and to identify prey ingestion of the same day (Rounick and Winterbourn, 1986). Scats are easily found and usually provide good taxonomic resolution due to remains of bones, wings and scales, for instance. In addition, it would be hard to sample cat stomach contents without a running program for control or eradication. We were unable to obtain blood or tissue samples to evaluate the diet of feral cats through SIA.
Scat samples of feral cats were washed over a sieve (0.5mm) under a shower of warm water (Nogales et al., 1988). All food items found e.g. feathers, bones, fur and seeds, were separated, compared to reference material for identification, and quantified by number (N) and volume (V). Item counting was performed using single, diagnosable food item remains (e.g. mandible, jaw, head) or pairs (e.g. forepaws, hind paws, wings). We used graded glass beaker and glass dish over a graded paper to obtain the total volume of food items (Hellawell and Abel, 1971). The length of structures was measured in cubic millimeters on the dish, and converted to ml (i.e. 1mm3=0.001mL). This procedure was also applied to stomach contents of tegu lizards and black rats, described below. Moreover, plant samples found on feral cat scats (e.g. grass and seeds) were not included in analysis, as their occurrence is related to accidental intake and do not represent food contribution value.
Tegu lizardsSamples of tegu lizards were previously collected in uninhabited areas on Main Island in four expeditions between 2014–2016 (Fig. 1). However, tegu lizards have a home range that extends over 10ha (Abrahão et al., 2019) and could have access to both inhabited and natural areas. Samples used in the current study were gathered in other studies where culling had been planned. Tegu lizards were trapped and euthanized using an association of ketamine (20–30mg/kg) and midazolam (1–2mg/kg) followed by cerebral perforation, according to Brazilian Council of Animal Experimentation and the American Veterinary Medical Association (Leary et al., 2013). Animals were necropsied, whole blood samples of 29 individuals were collected for SIA and 22 stomach contents for diet analysis. Blood samples were collected for SIA in order to assess the recent diet (weeks) of tegu lizards, due to high rates of isotopic turnover (Peterson and Fry, 1987; MacAvoy et al., 2006). Whole blood samples were collected before euthanasia in order to avoid potential chemical contamination. Samples were then lyophilized, homogenized, weighed (1mg) and stored in tin capsules for SIA analysis. Stomach contents were analyzed in order to obtain high taxonomic resolution of the ingested prey. Under a stereoscopic magnifying microscope (10×40), food items were quantified and identified at the lowest taxonomic level possible.
Black ratsSamples of black rats were collected on Meio Island during two expeditions (Fig. 1), in October 2016 and April 2017. Rats were trapped at night with handnet and Tomahawk traps (50×21.5×20cm) and euthanized through cervical dislocation according to Brazilian guidelines of Ethics, Bioethics and Animal Welfare Commission (CFMV, 2013) also to avoid risk of chemical contamination on tissues for SIA. Liver, muscle and stomach contents were collected from 10 black rats. Samples were stored in 70% ethanol, due to impossibility to freeze samples in field conditions at Meio Island. This conservation method seems to be adequate to SIA analysis, as it does not result in changes of δ15N and δ13C values (Hobson et al., 1997).
Stomach samples were also analyzed under a stereoscopic magnifying microscope and food items identified and quantified as described above. Liver samples were used complementary to stomach analysis to determine, through SIA, the previous diet of black rats over a medium (months) period (Tieszen et al., 1983). Muscle samples of black rats were also used as a potential food item to tegu lizards in SIA analysis. Because SIA aims tracing protein from source to consumers, lipids from muscle and liver samples were extracted using Soxhlet apparatus with solvent petroleum ether in 6h cycle (Mancini and Bugoni, 2014). Then, muscle and liver samples were lyophilized, homogenized, weighed (1mg) and stored in tin capsules.
Potential prey samplingIn order to perform a Bayesian isotopic mixing model, a range of potential food items were sampled based on those available in the archipelago, found in stomach contents, and identified in previous diet studies on tegu lizards from Fernando de Noronha (e.g. Kiefer and Sazima, 2002; Castro and Galetti, 2004; Abrahão et al., 2019) and rats (Stapp, 2002; Caut et al., 2008a).
Potential prey samples were collected in October 2016 and April 2017. Sampling of muscle of Noronha skinks were obtained on Main (n=5) and Meio (n=6) islands, and muscle of yellow-crabs (n=18) were collected on Meio Island only. Both were obtained through a non-lethal collection, by tail autotomy and by removing a pereiopod, respectively. Blood samples of masked booby (Sula dactylatra) on Meio Island (n=12) were collected using syringe and sterile hypodermic needle. Ants (Solenopsis and Camponotus genus) and wolf spiders (Lycosidae) were collected through pitfall traps and manually, respectively, both on Meio Islands. All animal samples were stored in 70% ethanol. Plant leaves were collected manually on Meio Island in a 100m transect previous performed as a sample of the entire island, representing the two possible photosynthetic pathways, i.e. with C3 (Ipomoea piurensis and I. alba) and C4 (Paspalum pleostachyum and Cyperus atlanticus). Samples were sun dried and stored in ziplock plastic bags. Fruits found in stomach contents of tegu lizards such as cashew (Anacardium occidentale), in addition to fruits collected on surroundings of Main Island, e.g. jocote (Spondias purpurea) were used in SIA to verify the contribution of fruits to tegu lizard diet.
Sugars were removed from fruit pulp after centrifugation for 10min at 4000rpm and washed with acetone in 50mL vials (Rossmann et al., 1997). Plants C3 and C4 were oven-dried for 48h at 60°C. After, plant samples were macerated, weighed (3mg) and encapsulated. All SIA samples were sent to the Center for Stable Isotopes at the University of New Mexico (UNM-CSI) and analyzed in Isotope Ratio Mass Spectrometer (IRMS). Standards used were Vienna Pee Dee belemnite and atmospheric air, for carbon and nitrogen, respectively. Standard deviation for δ13C and δ15N were 0.08‰ and 0.03‰, respectively, obtained from the internal laboratory standards, soy, casein, whey protein and tuna.
Data analysisThe prey-specific index of relative importance was determined through relative measures to quantification of prey using %PSIRI index (Brown et al., 2011), using:
Measurements used were obtained from the frequency of occurrence (FO), abundance or numerical contribution (N) and volume (V). We obtained the FO from the number of samples containing the selected food item, and the relative frequency of occurrence (%FO) is the percentage of FO regarding to the total number of samples analyzed of each consumer species. The N value is obtained counting the number of consumed food item, assessed through each food item that appears on the sample, i.e. number of predated individuals or consumed fruits. The numeric proportion (%PN) is the average of equivalent percentages to N of each sample, excluding those samples in which the food item was absent. Likewise, the total volume (V) of a food item is the sum of volume values obtained in all samples for this item, and the relative volume ratio (%PV) is the percentage of a given item in relation to the total volume of samples in which that item was present.
Bayesian isotopic mixing models were used to assess the relative contribution of food items for the diet of each invasive consumer, through SIAR package (Parnell et al., 2010) on R environment (R Core Team, 2014). Trophic discrimination factors (TDF), denoted as ΔN and ΔC for the δ15N and δ13C, respectively, were chosen according to consumer tissues, i.e. whole blood for tegu lizard samples, and liver for rat. For liver samples of black rats, we used diet-dependent discrimination factors, where values are applied for each potential food item in rat diet instead of a single TDF value for the whole diet. We applied values and standard deviation of 0.1‰ previously calculated (Caut et al., 2008a) based on experimental analysis with black rats fed basically with fish meal, corn flour, alfalfa meal and casein (Caut et al., 2008b). Values to each potential items were, seabirds (ΔC = −0.84‰, ΔN=1.49‰), skinks (ΔC=−1.77‰, ΔN=−0.03‰), insects (ΔC=1.07‰, ΔN=0.8‰), fruits (C3) and C4 plants (ΔC=1.21‰, ΔN=2.73‰; ΔC=−2.78‰, ΔN=2.09‰, respectively). The determination of adequate TDF values for modeling of reptiles usually result in large variations according to taxa, size, metabolism or breeding period (Steinitz et al., 2016; Durso et al., 2020) that ideally should be considered in a mixing model application. In addition, SIDER package, used to generate TDF values to mammals and birds (Healy et al., 2018) does not holds suitable values to Bayesian mixing models using reptiles, due to limited phylogenetic and isotope data for the taxa. All these factors associated with the lack of TDF values specific to tegu lizards in literature, prompted a necessity to evaluate potential TDF's in ecologically similar groups. Therefore, the most suitable value found related to diet, metabolism, behavior and body size for whole blood of tegu lizards was that measured in rock iguanas (Cyclura spp.) (Steinitz et al., 2016). In this study, overall TDF across the three iguana species was suggested as suitable for studies with other reptile species, thus, we used TDF values of ΔC=2.5±0.6‰ and ΔN=4.1±0.4‰.
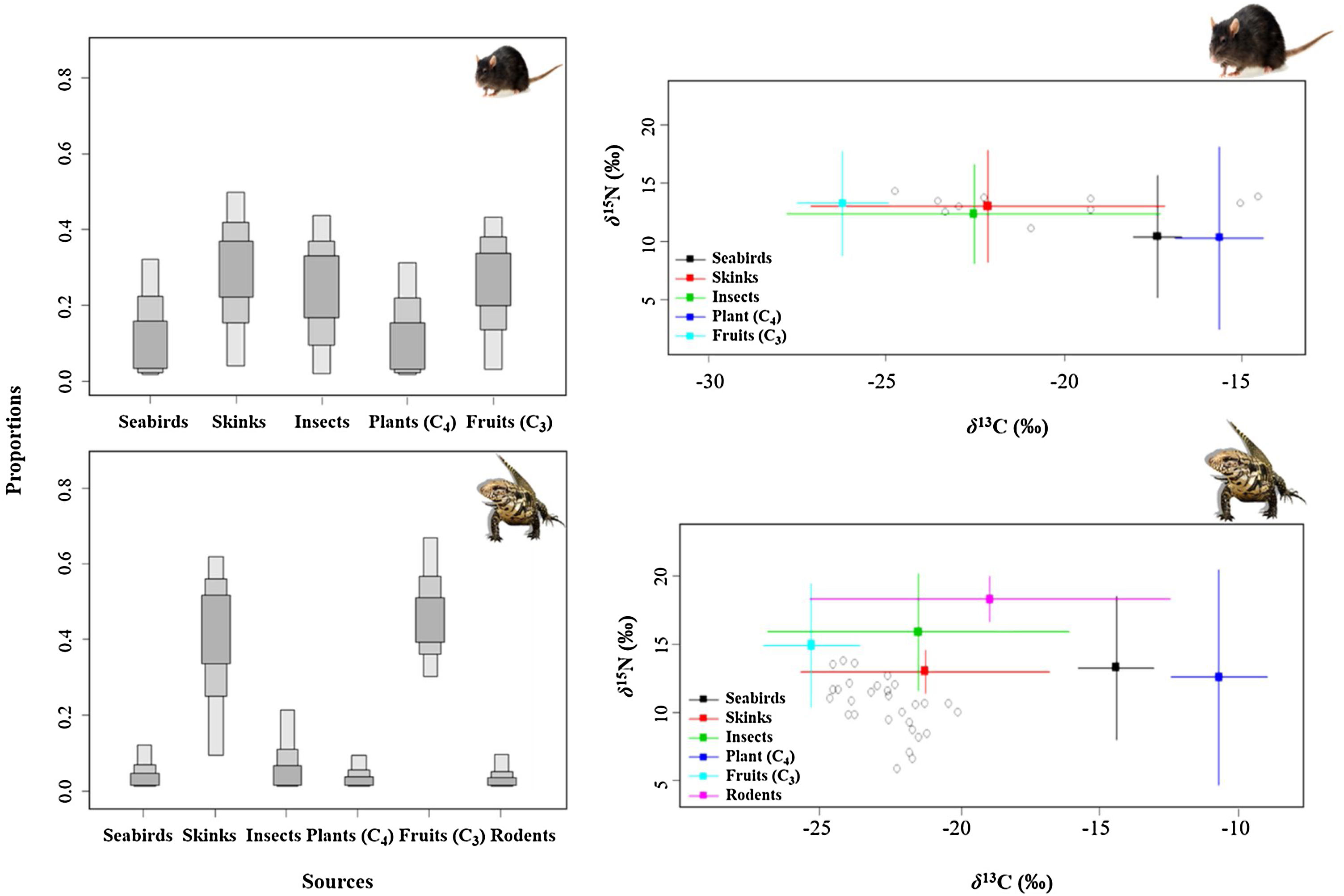
The range of isotopic values and their standard deviation to consumers and prey were included in the modeling procedure (Table 1). We considered a vast range of potential food items available at Meio and Main Islands, including those indicated by stomach content analysis. Potential food items with similar isotopic values and ecology were pooled (Phillips et al., 2005). Thus, two genera of ants (Solenopsis and Camponotus) represented the “Insects” category, while two different grasses (P. pleostachyum and C. atlanticus) represented “Plants (C4)” group. Plants with C3 photosynthetic pathway (Ipomoea spp.) were pooled with fruits – group “Fruits (C3)” –, as they have similar isotopic values. The taxa “Skinks” and “Seabirds” were represented by the endemic Noronha skink and the masked booby, respectively. We used masked booby samples as a primary marker of marine matter, as seabirds are a key carriers of marine nutrients, through laying eggs, their chicks, carcasses, and through guano and food remains (Sánchez-Piñero and Polis, 2000; Caut et al., 2012).
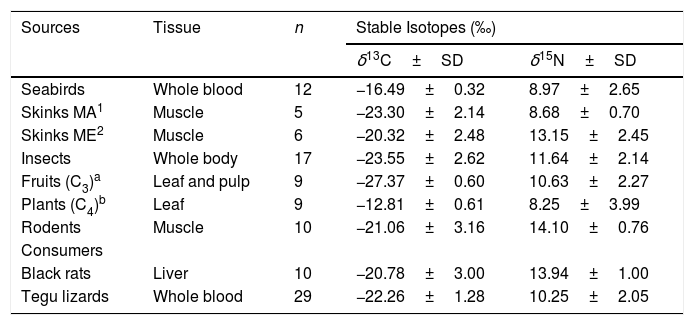
Range of isotopic values (mean ± 1 standard deviation) in tissues of consumers and food sources used in the Bayesian Mixing Models, and estimated food items contribution to the diet of exotic species in Fernando de Noronha Archipelago, Brazil.
| Sources | Tissue | n | Stable Isotopes (‰) | |
|---|---|---|---|---|
| δ13C±SD | δ15N±SD | |||
| Seabirds | Whole blood | 12 | −16.49±0.32 | 8.97±2.65 |
| Skinks MA1 | Muscle | 5 | −23.30±2.14 | 8.68±0.70 |
| Skinks ME2 | Muscle | 6 | −20.32±2.48 | 13.15±2.45 |
| Insects | Whole body | 17 | −23.55±2.62 | 11.64±2.14 |
| Fruits (C3)a | Leaf and pulp | 9 | −27.37±0.60 | 10.63±2.27 |
| Plants (C4)b | Leaf | 9 | −12.81±0.61 | 8.25±3.99 |
| Rodents | Muscle | 10 | −21.06±3.16 | 14.10±0.76 |
| Consumers | ||||
| Black rats | Liver | 10 | −20.78±3.00 | 13.94±1.00 |
| Tegu lizards | Whole blood | 29 | −22.26±1.28 | 10.25±2.05 |
| Food items contribution (CI95%) | ||||||
|---|---|---|---|---|---|---|
| Seabirds | Lizards | Insects | Fruits (C3) | Plants (C4) | Rodents | |
| Black rats diet | 0–33 | 4–50 | 2–46 | 0–32 | 3–43 | – |
| Tegu lizards diet | 0–12 | 7–59 | 0–24 | 0–10 | 29–66 | 0–10 |
After initial mixing model runs with a range of food items as potential sources, food items for mixing models were selected according to lowest correlations among them, as indicated in SIAR manual (Inger et al., 2010). Previous models performed poorly due to highly negative correlations between food items, i.e. yellow-crabs present high negative correlation with “Fruits (C3)”, while wolf spiders also negatively correlated with Noronha skink. Thus, we excluded yellow-crab and wolf spiders in order to maintain “Fruits (C3)” and “Skinks” due to high proportion of these resources in the diet, in detriment of crabs and spiders. Final models developed were similar for both black rats and tegu lizards, due to potential prey available on study area and food habit. However, we used Noronha skinks collected on Main Island as “Skinks MA” on tegu lizard diet model, whereas for black rats we used this food item from Meio Island, “Skinks ME” (Table 1). It decreased negative correlations and resulted in models performing better. Moreover, black rat muscle previously collected composed the “Rodents” group to tegu lizard diet model. Final models were built as below:
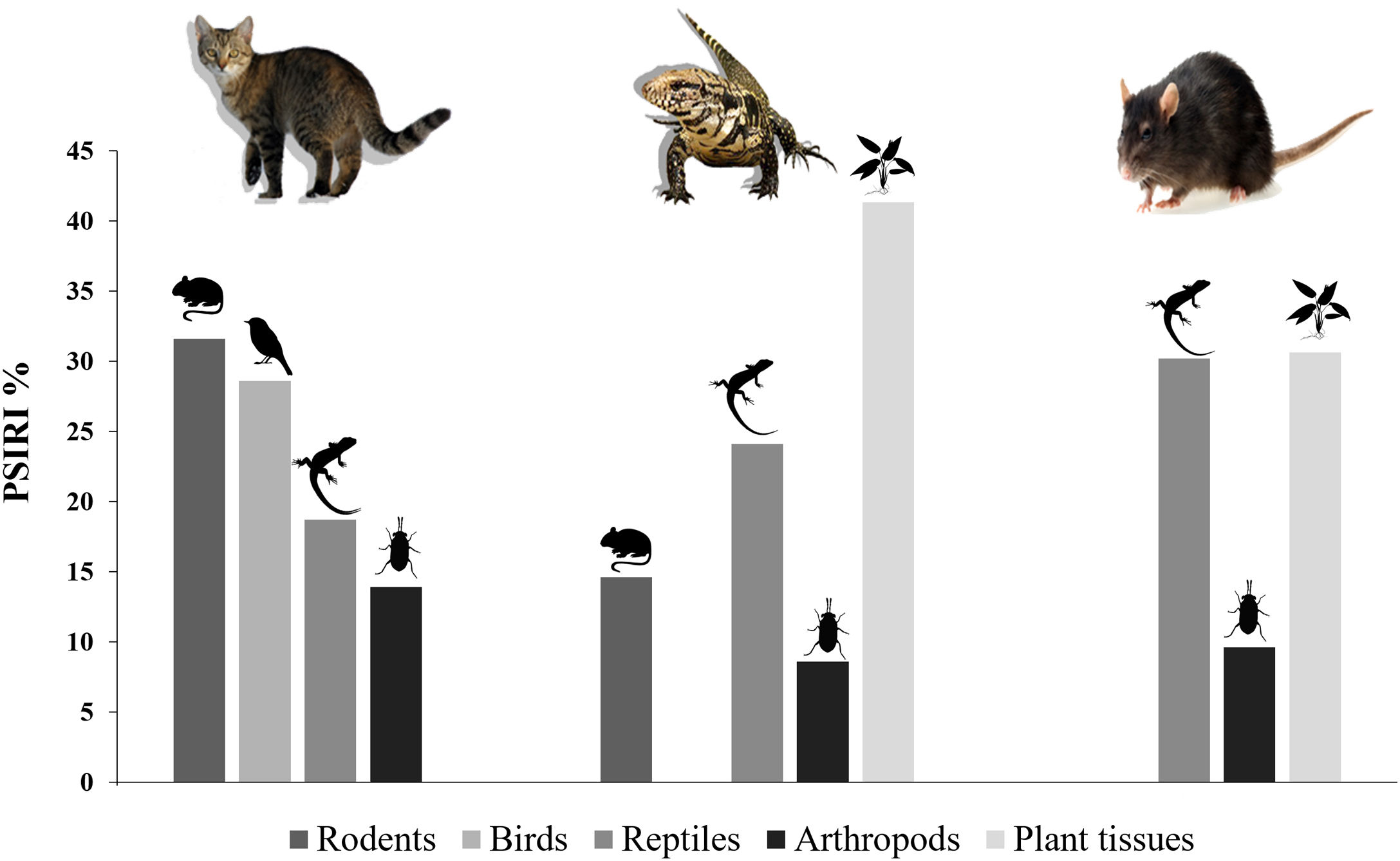
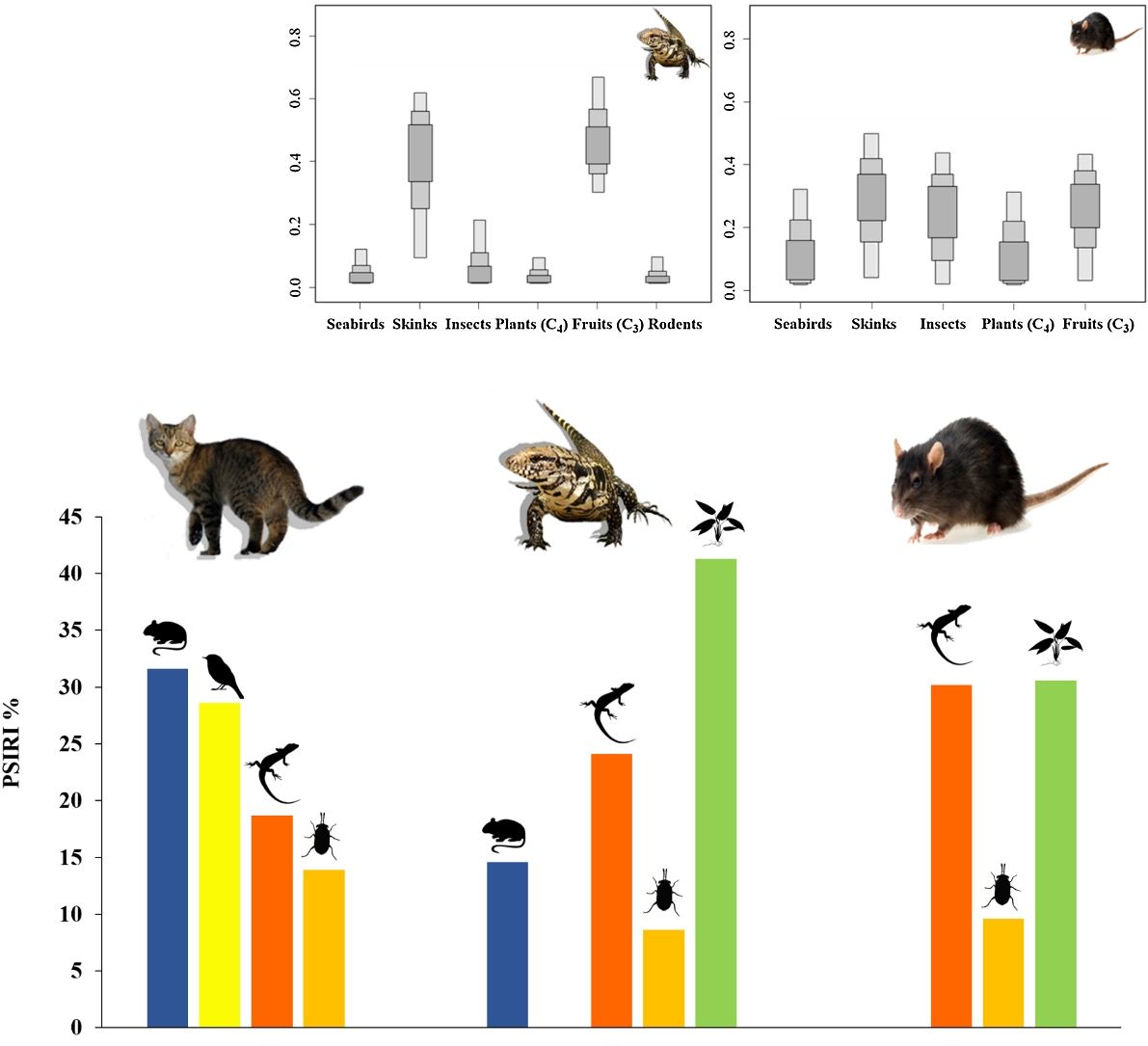
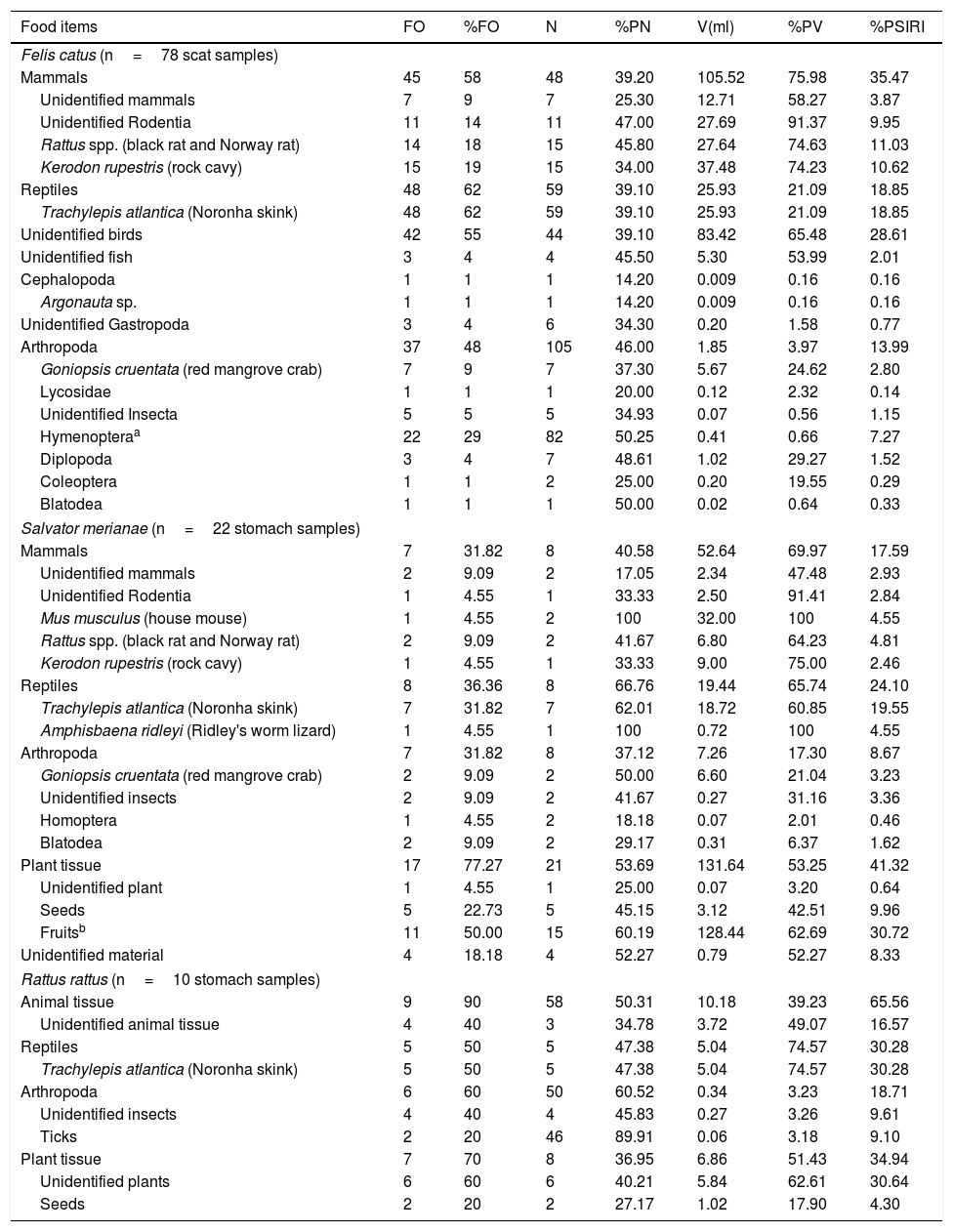
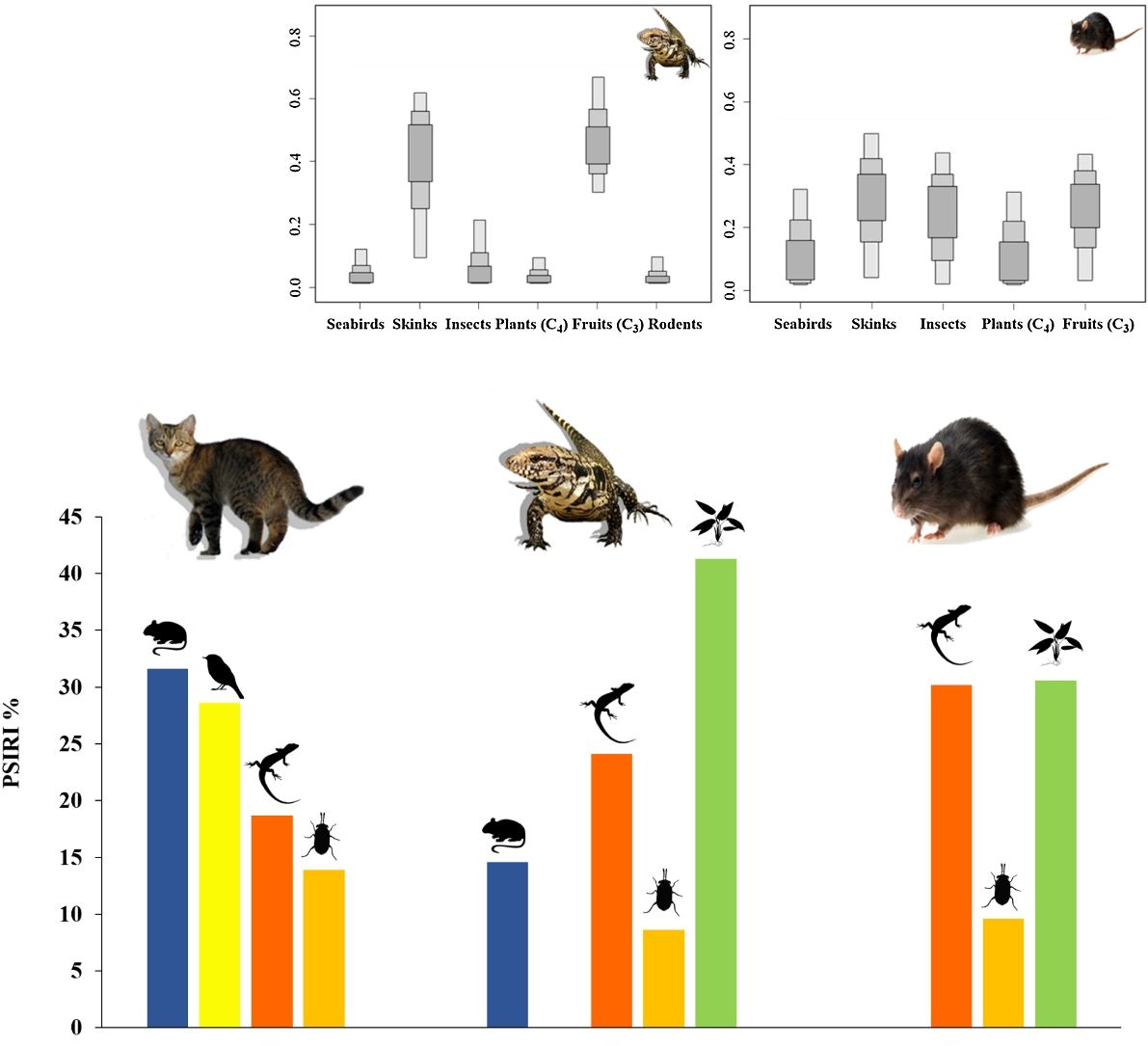
ResultsDiet of feral catsFrom the analysis of 78 feral cat scats, 13 food items were identified (Table 2). The main food group identified was Reptiles, which summed 59 individuals of a single species, the Noronha skink (FO=62.0%). Birds were represented by 44 individuals with FO=55.0%, followed by rodents (41 individuals), with Rattus spp. and the rock cavy (Kerodon rupestris) showing highest occurrence (FO=18.0% and 19.0%, respectively). %PSIRI demonstrated that rodents contributed with 31.6% in the diet of feral cats, with birds and reptiles contributing 28.6% and 18.8%, respectively (Fig. 2). Arthropods, including crabs and insects, contributed 13.9%. Other groups had %PSIRI values equal or lower than 2.0%, and included fish, gastropods and cephalopods (Table 2).
Diet composition of feral cats Felis catus, tegu lizard Salvator merianae and black rats Rattus rattus in Fernando de Noronha Archipelago, Brazil, determined through scats and stomach content analysis.
| Food items | FO | %FO | N | %PN | V(ml) | %PV | %PSIRI |
|---|---|---|---|---|---|---|---|
| Felis catus (n=78 scat samples) | |||||||
| Mammals | 45 | 58 | 48 | 39.20 | 105.52 | 75.98 | 35.47 |
| Unidentified mammals | 7 | 9 | 7 | 25.30 | 12.71 | 58.27 | 3.87 |
| Unidentified Rodentia | 11 | 14 | 11 | 47.00 | 27.69 | 91.37 | 9.95 |
| Rattus spp. (black rat and Norway rat) | 14 | 18 | 15 | 45.80 | 27.64 | 74.63 | 11.03 |
| Kerodon rupestris (rock cavy) | 15 | 19 | 15 | 34.00 | 37.48 | 74.23 | 10.62 |
| Reptiles | 48 | 62 | 59 | 39.10 | 25.93 | 21.09 | 18.85 |
| Trachylepis atlantica (Noronha skink) | 48 | 62 | 59 | 39.10 | 25.93 | 21.09 | 18.85 |
| Unidentified birds | 42 | 55 | 44 | 39.10 | 83.42 | 65.48 | 28.61 |
| Unidentified fish | 3 | 4 | 4 | 45.50 | 5.30 | 53.99 | 2.01 |
| Cephalopoda | 1 | 1 | 1 | 14.20 | 0.009 | 0.16 | 0.16 |
| Argonauta sp. | 1 | 1 | 1 | 14.20 | 0.009 | 0.16 | 0.16 |
| Unidentified Gastropoda | 3 | 4 | 6 | 34.30 | 0.20 | 1.58 | 0.77 |
| Arthropoda | 37 | 48 | 105 | 46.00 | 1.85 | 3.97 | 13.99 |
| Goniopsis cruentata (red mangrove crab) | 7 | 9 | 7 | 37.30 | 5.67 | 24.62 | 2.80 |
| Lycosidae | 1 | 1 | 1 | 20.00 | 0.12 | 2.32 | 0.14 |
| Unidentified Insecta | 5 | 5 | 5 | 34.93 | 0.07 | 0.56 | 1.15 |
| Hymenopteraa | 22 | 29 | 82 | 50.25 | 0.41 | 0.66 | 7.27 |
| Diplopoda | 3 | 4 | 7 | 48.61 | 1.02 | 29.27 | 1.52 |
| Coleoptera | 1 | 1 | 2 | 25.00 | 0.20 | 19.55 | 0.29 |
| Blatodea | 1 | 1 | 1 | 50.00 | 0.02 | 0.64 | 0.33 |
| Salvator merianae (n=22 stomach samples) | |||||||
| Mammals | 7 | 31.82 | 8 | 40.58 | 52.64 | 69.97 | 17.59 |
| Unidentified mammals | 2 | 9.09 | 2 | 17.05 | 2.34 | 47.48 | 2.93 |
| Unidentified Rodentia | 1 | 4.55 | 1 | 33.33 | 2.50 | 91.41 | 2.84 |
| Mus musculus (house mouse) | 1 | 4.55 | 2 | 100 | 32.00 | 100 | 4.55 |
| Rattus spp. (black rat and Norway rat) | 2 | 9.09 | 2 | 41.67 | 6.80 | 64.23 | 4.81 |
| Kerodon rupestris (rock cavy) | 1 | 4.55 | 1 | 33.33 | 9.00 | 75.00 | 2.46 |
| Reptiles | 8 | 36.36 | 8 | 66.76 | 19.44 | 65.74 | 24.10 |
| Trachylepis atlantica (Noronha skink) | 7 | 31.82 | 7 | 62.01 | 18.72 | 60.85 | 19.55 |
| Amphisbaena ridleyi (Ridley's worm lizard) | 1 | 4.55 | 1 | 100 | 0.72 | 100 | 4.55 |
| Arthropoda | 7 | 31.82 | 8 | 37.12 | 7.26 | 17.30 | 8.67 |
| Goniopsis cruentata (red mangrove crab) | 2 | 9.09 | 2 | 50.00 | 6.60 | 21.04 | 3.23 |
| Unidentified insects | 2 | 9.09 | 2 | 41.67 | 0.27 | 31.16 | 3.36 |
| Homoptera | 1 | 4.55 | 2 | 18.18 | 0.07 | 2.01 | 0.46 |
| Blatodea | 2 | 9.09 | 2 | 29.17 | 0.31 | 6.37 | 1.62 |
| Plant tissue | 17 | 77.27 | 21 | 53.69 | 131.64 | 53.25 | 41.32 |
| Unidentified plant | 1 | 4.55 | 1 | 25.00 | 0.07 | 3.20 | 0.64 |
| Seeds | 5 | 22.73 | 5 | 45.15 | 3.12 | 42.51 | 9.96 |
| Fruitsb | 11 | 50.00 | 15 | 60.19 | 128.44 | 62.69 | 30.72 |
| Unidentified material | 4 | 18.18 | 4 | 52.27 | 0.79 | 52.27 | 8.33 |
| Rattus rattus (n=10 stomach samples) | |||||||
| Animal tissue | 9 | 90 | 58 | 50.31 | 10.18 | 39.23 | 65.56 |
| Unidentified animal tissue | 4 | 40 | 3 | 34.78 | 3.72 | 49.07 | 16.57 |
| Reptiles | 5 | 50 | 5 | 47.38 | 5.04 | 74.57 | 30.28 |
| Trachylepis atlantica (Noronha skink) | 5 | 50 | 5 | 47.38 | 5.04 | 74.57 | 30.28 |
| Arthropoda | 6 | 60 | 50 | 60.52 | 0.34 | 3.23 | 18.71 |
| Unidentified insects | 4 | 40 | 4 | 45.83 | 0.27 | 3.26 | 9.61 |
| Ticks | 2 | 20 | 46 | 89.91 | 0.06 | 3.18 | 9.10 |
| Plant tissue | 7 | 70 | 8 | 36.95 | 6.86 | 51.43 | 34.94 |
| Unidentified plants | 6 | 60 | 6 | 40.21 | 5.84 | 62.61 | 30.64 |
| Seeds | 2 | 20 | 2 | 27.17 | 1.02 | 17.90 | 4.30 |
Ten food items or categories were identified in the diet of tegu lizards, with fruits as the main food item (%PSIRI=41.3), which were present in 77.3% of samples (Table 2), followed by reptiles (%PSIRI=24.1). In the reptile group, at the species level, the Noronha skink had the largest contribution (%PSIRI=19.6), with the remaining food items represented by the endemic Ridley's worm lizard (%PSIRI=4.6). The Rodents group was composed by at least three species of exotic vertebrates: rats – black and Norway rats (Rattus norvegicus) –, house mouse (Mus musculus) and the rock cavy, which contributed together with 14.7% for the %PSIRI and had FO=22.7%. Lastly, arthropods, including crabs and insects, contributed with 8.7% for %PSIRI to tegu lizards' diet (Fig. 2).
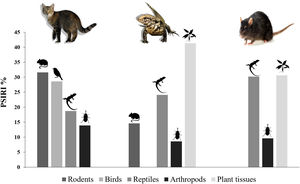
Mean stable isotopic values of whole blood of tegu lizards applied in the mixing model in SIAR indicated that tegu lizards fed mainly on fruits (95% credibility interval - CI). Noronha skink appears on second position, followed by insects. Seabirds and rodents were less important (Table 1; Fig. 3).
Diet of black ratsAnimal and plant tissues were identified in the black rat diet in a very fragmented state (%PSIRI=65.6 and 34.9, respectively). Main food items were Noronha skink (%PSIRI=30.3 and FO=50.0%), insects and seeds, contributing 9.6% and 4.3% for the %PSIRI, respectively (Table 2; Fig. 2). Ticks, typical from seabirds, had %PSIRI=9.1.
Mean SI values in liver samples of black rats applied on model reveals a homogeneous prey contribution to the diet. Results demonstrated that all food items had high contribution, but the main food items of black rats were skinks, followed by fruits (C3). Insects were important food items and then, seabirds and plants (C4) (Table 1; Fig. 3).
DiscussionBased on the combination of classic dietary methods and intrinsic markers as the stable isotopic analysis we demonstrate that the three exotic vertebrates – rats, cats and tegu lizards – introduced on Fernando de Noronha have diets widely composed by native, endemic and threatened birds and reptiles. Also, SIA provided evidences that marine matter, probably derived from seabirds and other marine food items, contributed directly or indirectly to the synthesis of consumer tissues.
Feral catsFeral cats are strictly carnivores and scats found in the study site had proven that they prey mainly upon rodents, birds and the Noronha skink. The primary prey was black rats, an abundant prey that contribute largely to the persistence and high abundance of feral cats on the archipelago, as also demonstrated at Port-Cros Island, Mediterranean Sea, where both species co-occur (Bonnaud et al., 2007). On the other hand, the high contribution of birds on feral cat diet can be due to seabirds nesting on the ground, in burrows or rock crevices (Leal et al., 2016), which makes them vulnerable to predation. In addition, the endemic passerines, Noronha elaenia and Noronha vireo, are abundant in the National Marine Park on the Main Island (Mestre et al., 2016; Luna, 2017), which overlap with an area of high density of free roaming cats (Dias et al., 2017), making endemic landbirds easily accessible to cats, which are known to be hunters able to capture a wide variety of birds (Bonnaud et al., 2011). Also, some scat samples analyzed on this study contained remiges feathers of the Noronha elaenia and Noronha vireo.
According to dietary results, feral cats consumed the greatest proportion of birds among invasive species analyzed, thus a major threat to land birds and seabirds. Noronha skink also contributed on feral cat as well as tegu lizard and black rat diets, showing the vulnerability of this endemic species.
Tegu lizardsThe main food items in tegu lizard diet were fruits and endemic skink, which had high contribution in both conventional diet analysis and estimated by SIA. The endemic Ridley's worm lizard appeared only once in stomach contents of tegu lizards. Tegu lizard has an omnivore diet that also includes vertebrates and invertebrates, but is mainly composed by plants and fruits (Kiefer and Sazima, 2002; Castro and Galetti, 2004). The substantial contribution of Noronha skink on tegu lizard diet was expected due to similarity on their niches and predation facility, as both have diurnal habits and live in open habitats with anthropic influence (Bovendorp et al., 2008; Rocha et al., 2009; Abrahão et al., 2019).
Similarly, high δ15N values detected in the SIA analysis demonstrated that tegu lizard tissues have signatures of indirect consumption of marine matter. High contribution of plants and fruits added to the less important contribution of seabird, no negative correlation and a lack of food items of marine origin on conventional diet analysis shows that tegu lizards use marine matter indirectly, through the ingestion of 15N-enriched plants. This finding highlights that despite tegu lizards have access to inhabited areas, they continue using natural and enriched food to tissue synthesis as key resources. Furthermore, Noronha skink also presented high contribution on tissue synthesis, demonstrating the food and energy importance of this food item on tegu lizard diet.
Black ratsBlack rat diet analysis has demonstrated Noronha skink as an important food item in both conventional diet analysis and SIA, which reveals high frequency of the endemic skink in its diet. Noronha skinks are an easy prey to the rats, as other small lizard species living in other oceanic islands (Monks et al., 2014), because native predators were currently absent. Plants (C3) and insects were also important to the black rats diet as expected, as rats have an omnivorous diet (Caut et al., 2008a). However, seabird contribution was detected only by SIA, not in conventional diet analysis. Frequently, conventional diet could also not provide results with high taxonomic resolution, due to morphology and food habits of consumers, as occurs in rodents, which grind their food. However, persistent remains such as fish scales were frequently found, while soft tissue as muscle and egg content were unnoticed. It is worth noting that marine signature is present on a wide range of resources in oceanic islands (Russel et al., 2020), but at a small islet such as Meio Island, with restrict resources, no negative correlation between food items was found in Bayesian mixing models, and thus we consider reliable that black rats use seabirds as a resource as well as skinks, insects and 15N-enriched plants. The SIA highlighted that black rats could be using seabirds directly, or seabird-related sources as food resources to tissue synthesis. Thus, seabirds are part of black rat diet and contribute in their marine matter signatures, despite it is not clear whether it occurs by predation of chicks, eggs, scavenging, or indirectly, e.g. through ingestion of seabird ticks, which were found in the dietary direct analysis. Nevertheless, 15N-enriched plants and fruits seem to contribute widely to the high δ15N values on black rat tissue, derived from marine matter input on the island.
Consequences and applicationsThe importance of invasive rodents – rats, rock cavy and house mouse – in the diet of tegu lizards and feral cats, suggest that invasive vertebrates could contribute to the persistence and high numbers of feral cats and tegu lizards, estimated to hold populations in Fernando de Noronha of 1287 and 12,270 individuals, respectively (Dias et al., 2017; Abrahão et al., 2019). Such prey could be particularly important during food scarcity as occurs in the dry season and the non-breeding periods of seabirds. Moreover, humans feed a large proportion of the non-feral cat population on the archipelago (Dias et al., 2017). Because exotic rodents are also key food resources to other invasive vertebrates, a management plan for control or eradication of invasive species should focus over the species simultaneously, avoiding eventual subsequent increase of predation pressure over native species, due to “mesopredator release effect” (Russell et al., 2009; Molsher et al., 2017).
Environmental effects previously reported on Fernando de Noronha Archipelago agree with current dietary findings, with predation negatively affecting the community dynamics on islands (Thoresen et al., 2017). For instance, the population of Noronha skink in the Main Island is at least 50.7% below the expected abundance, when compared to the density in secondary islands (Gasparotto et al., 2020). Tegu lizards and feral cats could account for such discrepancies, even among other causes such as human direct impact. Furthermore, the distribution of seabird nesting sites at Fernando de Noronha is currently restricted to peripheral islands and islets – also inhabited by rats – or over trees and steep cliffs when nesting on the Main Island (Mancini et al., 2016). This finding can also be probably related to predation pressure mediated by feral cats and tegu lizards, as seen on islands elsewhere (Pontier et al., 2008; Hohnen et al., 2016). Predation pressure over natural populations culminates in the current status of seabirds at Fernando de Noronha Archipelago, such as the red-billed tropicbird (Mancini et al., 2016), or the Audubon's shearwater, “Critically Endangered” in the Brazilian Red List and with population size estimated as fewer than 50 mature individuals (Lopes et al., 2014). Native landbirds are also affected, as the small populations of Noronha elaenia and Noronha vireo (Mestre et al., 2016; Luna, 2017), listed, respectively, as “Vulnerable” and “Near Threatened” globally (IUCN, 2020).
In addition to direct threats derived by exotic species over populations of native species, invasive species also affect island communities and ecosystem functioning. On nutrient and energy poor insular terrestrial environments, such as tropical oceanic islands, marine nutrients can be the main source of energy available and make terrestrial communities dependent on marine sources (Stapp et al., 1999). Seabirds, as well as sea turtles, are great carriers of marine matter and largely responsible to input nutrients that fertilize the soil and plants and provide energy to different trophic levels (McLoughlin et al., 2016). The presence of marine signatures on consumer tissues highlights the importance of marine nutrients to the insular trophic web studied. The marine input increases the stability of systems and allows species coexistence by sharing abundant food (Mellbrand and Hambäck, 2010). Thus, threats to marine-terrestrial transboundary carriers such as seabirds, caused by exotic species, can disrupt energy flows to the whole terrestrial food web, cause variations in resource availability and modify the dynamics between consumers and resources (Bauer and Hoye, 2014).
Similar to the scenario demonstrated here for Fernando de Noronha Archipelago, invasive species affects several native and endemic species in other oceanic islands around the world (Harper and Bunbury, 2015; Thibault et al., 2017; Bicknell et al., 2020). The eradication has been considered a powerful tool to protect native threatened wildlife (Jones et al., 2016; Bicknell et al., 2020) benefiting targeted species and the entire insular ecosystem. Defining the main food items in the diet of invasive species is one of the first and key steps for the development of a management plan. Usually, programs for eradication of exotic species are established only assuming predation upon native and endemic species, but with no quantitative data on their impacts (Howald et al., 2010; Capizzi et al., 2016; Algar et al., 2020). For effective conservation, the identification of threatened species and the impact caused over those species is crucial to act reducing risks of extinction (Burger, 2018), as well as convincing stakeholders on the need to control exotic species.
Overall, our findings determined the role of invasive vertebrates as predators of endemic and threatened species at Fernando de Noronha Archipelago, and proved predation occurring among invasive species. This predation represents an important and cumulative pressure upon native species (Thibault et al., 2017), which may lead to a long-term decline, specially to the Noronha skink population at the Main Island, that is already impacted and face densities much lower than expected in comparison to human uninhabited islands of the archipelago. Similarly, the predation pressure over breeding seabirds could explain colonies located in marginal areas and largely reduced in the Main Island. This study highlights the importance to assess and control the impacts of invasive species in order to protect the endemic and threatened species of Fernando de Noronha Archipelago. We strongly recommend the implementation of the rat eradication plan to other islands of the archipelago, and the elaboration and implementation of an action plan to control and, if feasible, eradicate other invasive species on the archipelago, with main focus on native species, predators as feral cats and tegu lizards.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
FundingThis work was supported by the Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico, CNPq [PQ 310550/2015-7, 422759/2016-3]; Programa de Excelência Acadêmica (PROEX-CAPES) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior do Ministério da Educação, CAPES [PVE 88881.065000/2014-1].
We are grateful to the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) of Fernando de Noronha for granting permission and providing logistical support for this study. Centro Nacional de Pesquisa e Conservação das Aves Silvestres (ICMBio/CEMAVE) also provided metal bands for birds, and Centro Nacional de Pesquisa e Conservação de Répteis e Anfíbios (ICMBio/RAN) provided support to C.R.A. Authors are also grateful to all who supported data and sample collection during fieldwork, in particular Guilherme Tavares Nunes and Sophie Bertrand team. We also thank Vinícius P. O. Gasparotto for providing Noronha Skink samples for this research. Procedures were approved by Ethics Committee of the School of Veterinary Medicine of São Paulo University (licenses 2724150515, 677813114 and 1827250515) and by the Brazilian Ministry of Environment (SISBio 41682-6, 43589-2 and 22697-7).