Dogs are considered an invasive species, whose presence in natural habitats adversely affects wildlife. We investigate the effects of household and road proximity, and of dog population size in the surroundings on the invasion of cacao agroforest by dogs, and evaluate if dogs raised in the vicinity are more likely to invade agroforests than dogs of unknown origin. The study was conducted in a landscape dominated by agroforests, within the cacao growing region of Southern Bahia, Brazil. Dogs were recorded by camera-traps in 15 agroforest sites, and we identified dogs inhabiting the vicinity of each sampled agroforest site by visiting all households up to 800m from sampling sites. We obtained 115 photographic records of 47 individuals, and identified 213 dogs inhabiting the site surroundings. The number of individuals and frequency of visit of dogs tend to be higher in agroforests located nearer a household, but were not associated with the distance to the nearest road or the dog population size in the surroundings. The frequency of visits in agroforests did not differ between dogs residing in the surroundings and dogs of unknown origin. Our results indicate that the surroundings are not the main source of dogs invading agroforests, most likely because dogs perform long-distance movements in association to humans. Strategies to reduce the impacts of dogs on wildlife will gain from studies on movement ecology and should include practices to restrict dogs’ home range.
After more than 15,000 years interacting with humans (Vila et al., 1997), dogs (Canis familiaris) stand as the most abundant and widely distributed Canidae in the world (Ferreira et al., 2011; Wandeler et al., 1993). Current global dog population exceeds 700 million individuals (Hughes and Macdonald, 2013). These animals can use different types of environment and a large territory, especially in rural areas, where they enter agricultural systems and pastures, as well as remnants of native vegetation, and interact with wildlife (Vanak and Gompper, 2010). In rural areas the dog/human rate is generally higher than in urban areas and the number of dogs exceeds the number of humans (Wandeler et al., 1993).
Biological invasions are increasing around the world and are currently considered the second largest threat to biodiversity, after habitat loss (Baillie et al., 2004; Hulme, 2009). Introduced in all environments where man has settled, dogs have been considered an invasive species whose presence in natural habitats can negatively affect wildlife. Dogs may function as competitors, predators and/or pathogen reservoirs (Young et al., 2011; Hughes and Macdonald, 2013; Doherty et al., 2017). Through these interactions, they affect several levels of biological organization. For instance, behavioral changes have been reported in pudu (Pudu puda) and chilla foxes (Lycalopex griseus), with individuals of these species avoiding areas used by dogs (Silva-Rodríguez et al., 2010; Silva-Rodríguez and Sieving, 2012); Manor and Saltz (2004) report a negative relationship between gazelle (Gazella gazella gazella) recruitment and dog records; and Randall et al. (2006) indicate the high suscetibility of Ethiopian wolves (Canis simensis) to generalist pathogens transmitted from domestic dogs, specially rabies and canine distemper virus. Dog invasion of natural environments are also likely to change ecosystem processes through negative effects on key species, such as medium-sized mammals responsible for seed dispersal (Galetti and Sazima, 2006).
Agroforestry landscapes are rural areas with great potential for species conservation. Agroforestry systems might provide habitat or act as corridors between habitat patches, increasing the chances of (meta)population persistence (Cassano et al., 2009; Schroth and Harvey, 2007). As other human-modified landscapes, agroforestry mosaics are subjected to dog invasion (Cassano et al., 2014; Frigeri et al., 2014). In cacao agroforests in northeastern Brazil, the occurrence and detectability of some native species are negatively related to the frequency of dogs (Cassano et al., 2014) and invasion by dogs occur predominantly during days and times of greatest human activity (Frigeri et al., 2014). In other words, rural workers may be mediating the presence and the use of space by dogs in agroforestry mosaics.
Several factors are likely to influence the frequency of free-ranging dog (sensuVanak and Gompper, 2009) visits in agricultural or natural environments, the proximity to human settlement figuring among the most important. Soto and Palomares (2014) detected a higher abundance of dog signs near the edges of a protected area (i.e. edges close to human settlements) compared to sites far away from these edges. Through camera trapping, Srbek-Araujo and Chiarello (2008) also found a high number of dog records in forest edges next to human residences. In a rural landscape, Odell and Knight (2001) reported that dogs were detected more frequently closer to human residences than farther away (considering the distance classes of 30, 180, and 330m), while wild canids (Vulpes vulpes and Canis latrans) showed the opposite pattern. Roads and trails may also influence invasion of agricultural and natural environments by facilitating dog displacement in rural landscapes (Sepúlveda et al., 2015).
Here we investigate the invasion of an agroforestry mosaic by dogs, addressing two inter-related hypotheses: (1) whether invasion of agroforests by dogs is negatively associated with the distance from the nearest household and distance from the nearest road, and positively associated with dog population size in the surroundings; and (2) if dogs residing in the vicinity are more prone to invade agroforests than dogs whose origin is unknown.
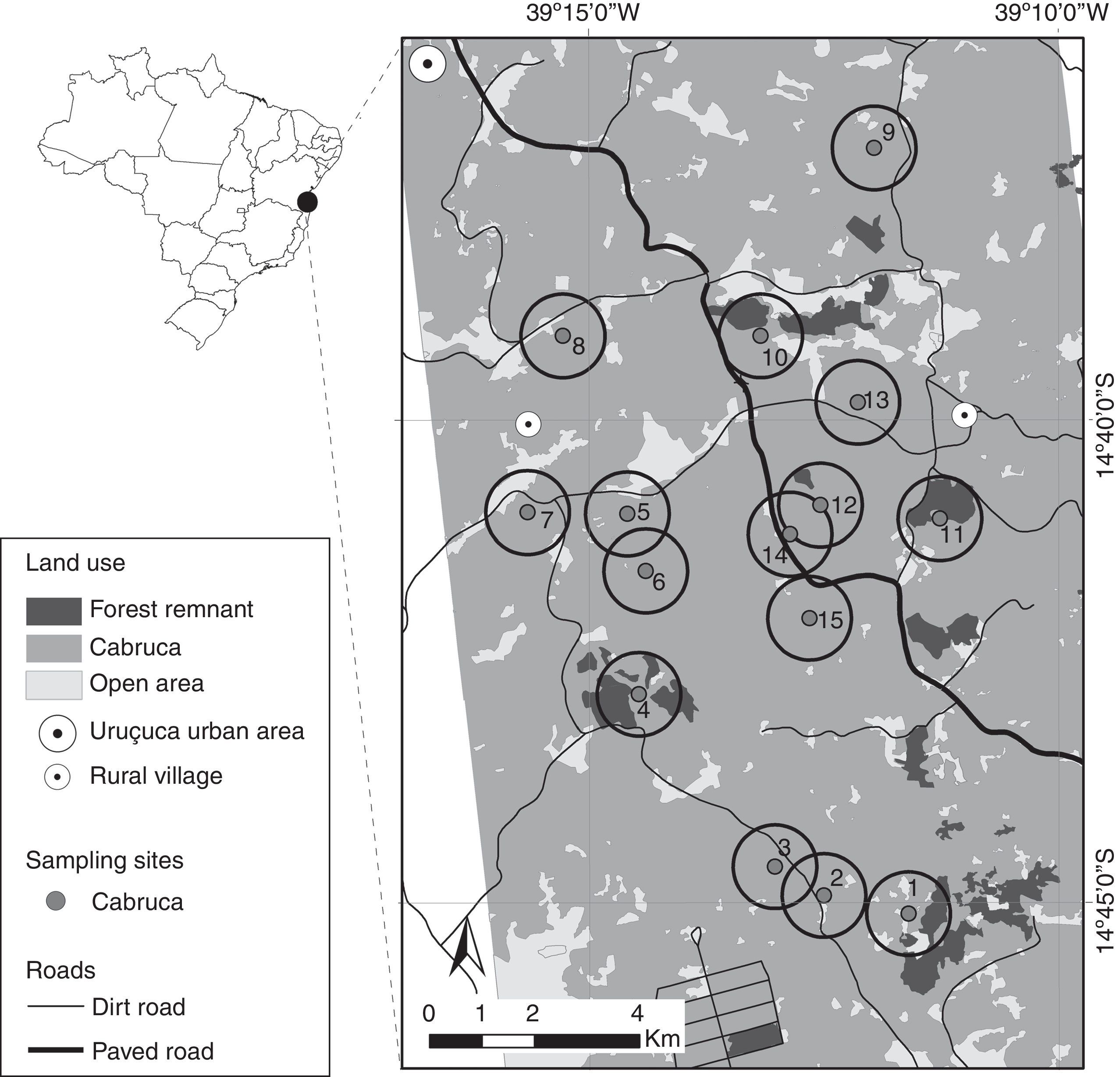
Material and methodsStudy areaThe study was conducted in the municipalities of Ilhéus and Uruçuca, Southern Bahia, Brazil, in a landscape of ∼400km2 (between 14°26′–14°50′ S and 39°03′–39°44′ W; Fig. 1). The study area is a typical landscape of the cacao growing region of southern Bahia, with a few small native forest fragments (10–200ha) immersed in a matrix predominantly composed of cacao plantations (Theobroma cacao), mainly under the agroforestry system locally known as cabruca. Cabrucas were historically set through native forest thinning and the removal of understory for cacao tree cultivation (Johns, 1999). The studied landscape is largely covered by cacao plantations (nearly 59% of landscape), with approximately 3% of area covered by native rain forest, the remaining land being kept as open areas or covered by water bodies or marshes.
Sampling designThe study was developed in 15 cabruca sampling sites (Fig. 1). Each sampling sites had a minimum distance of 800m from each other, varying distance from forest remnants (ranging from 250 to 2750m), roads (ranging from 150 to 1450m) and households (ranging from 200 to 1930m), and the number of households around each site (considering a 800-m radius buffer) varied from zero to 25 (Table S1).
Data collectionDogs in cabrucasPhotographic records of dogs were obtained during four surveys of one month each, covering the winter period (Jun–Jul) in 2013 and 2014, and summer (Jan–Feb) in 2014 and 2015. We installed one camera-trap (Trapa Camera® or Bushnell®) in each sampling site, at a height of 30cm from the ground, baited with sardines and banana. The cameras remained active during day and night, and were checked weekly for maintenance and bait refill. To avoid data to be inflated by sequential pictures of the same individual at the same camera, we computed the frequency of visits of dogs at each site discarding consecutive shots of the same individual in the same camera at intervals shorter than 1h. This procedure follows previous studies reporting large mammal records taken by camera-trap (e.g. Rovero and Marshall, 2009; Soares et al., 2013; Tobler et al., 2008). We individualized and counted the number of dogs recorded per sampling site per survey based on morphological characteristics such as race, sex, coat color, size and presence of scars. Number of dogs recorded per sampling site per survey was used to estimate the number of invading dogs per sampling site (see Data analysis).
Households, roads and dog population in the surroundingsWe located all households up to 800m from each cabruca sampling site through satellite image analysis in ArcGIS 10.1. The 800-m radius correspond to a reasonable range considering common distances traveled from household by free-ranging dogs (Vaniscotte et al., 2011; Ruiz-Izaguirre et al., 2015; Sepúlveda et al., 2015; Ribeiro, 2016), and allowed little overlap between cabruca surroundings (Fig. 1).
Households were visited twice in Nov-Dec in 2013 and 2014, when all resident dogs were cataloged and photographed. We then quantified: (1) the distance between each cabruca site and the nearest household, and (2) the number of dogs (dog population) residing in the vicinity of each cabruca. We also quantified: (3) the distance between each cabruca site and the nearest road (either dirt or paved), using a road map. The photos obtained in the households were compared to those taken by camera-traps (based on morphological characteristics) in order to identify whether the dogs registered in cabrucas reside in the vicinity of the sampling site (Fig. 2a and b).
Data analysisWe used two analyses to test the association between dog invasion across the 15 cabrucas and the independent variables distance to nearest household, distance to nearest road and size of dog population in the vicinity. First, we compared generalized linear models with the frequency of visits of dogs (negative binomial errors and log as the link function) constant, as function of the distance to the nearest household, as function of the distance to the nearest road, as a function of the size of dog population in the vicinity, or as a function of two of these variables. The second analysis compared the estimated number of invading dogs in the sampling sites using open N-mixture models (Dail and Madsen, 2011). Open N-mixture models yield estimates of the number of individuals using a site (parameter abundance λ) from point count data that account for imperfect detectability and do not require the closure assumption (Dail and Madsen, 2011). We built open N-mixture models with the parameter abundance λ constant, as function of the distance to the nearest household, as function of the distance to the nearest road, as a function of the size of dog population in the vicinity, or as a function of two of these variables; all models had the parameters recruitment (γ), survival (ω) and detection (p) constant. Each one-month survey was considered a sampling occasion in open N-mixture models.
Finally, we used a Mann–Whitney test to assess whether the frequency of visits of dogs in cabrucas differ among dogs residing in the vicinity and those of unknown origin. All analyses were performed with R environment (R Development Core Team, 2015), using packages “MASS”, “coin” or “unmarked”. Non-parametric tests were considered significant at p<0.05. We performed the model selections using the Akaike Information Criterion modified for small samples (AICc), and considered equally plausible those models with ΔAICc<2 (Burnhan and Anderson, 2002).
ResultsWe recorded 115 visits of 47 dogs in the 15 cabrucas, with a total sampling effort of 5120trap*day. Eighty-five households were located in the vicinity of the cabruca sites, where 213 dogs live (2.5±2.4 dogs per household; mean±SD), 19 (9%) of which were recorded in cabruca sites. The size of dog populations in the vicinity of cabruca sites varied from zero to 42 (14.2±12.1 dogs per cabruca vicinity).
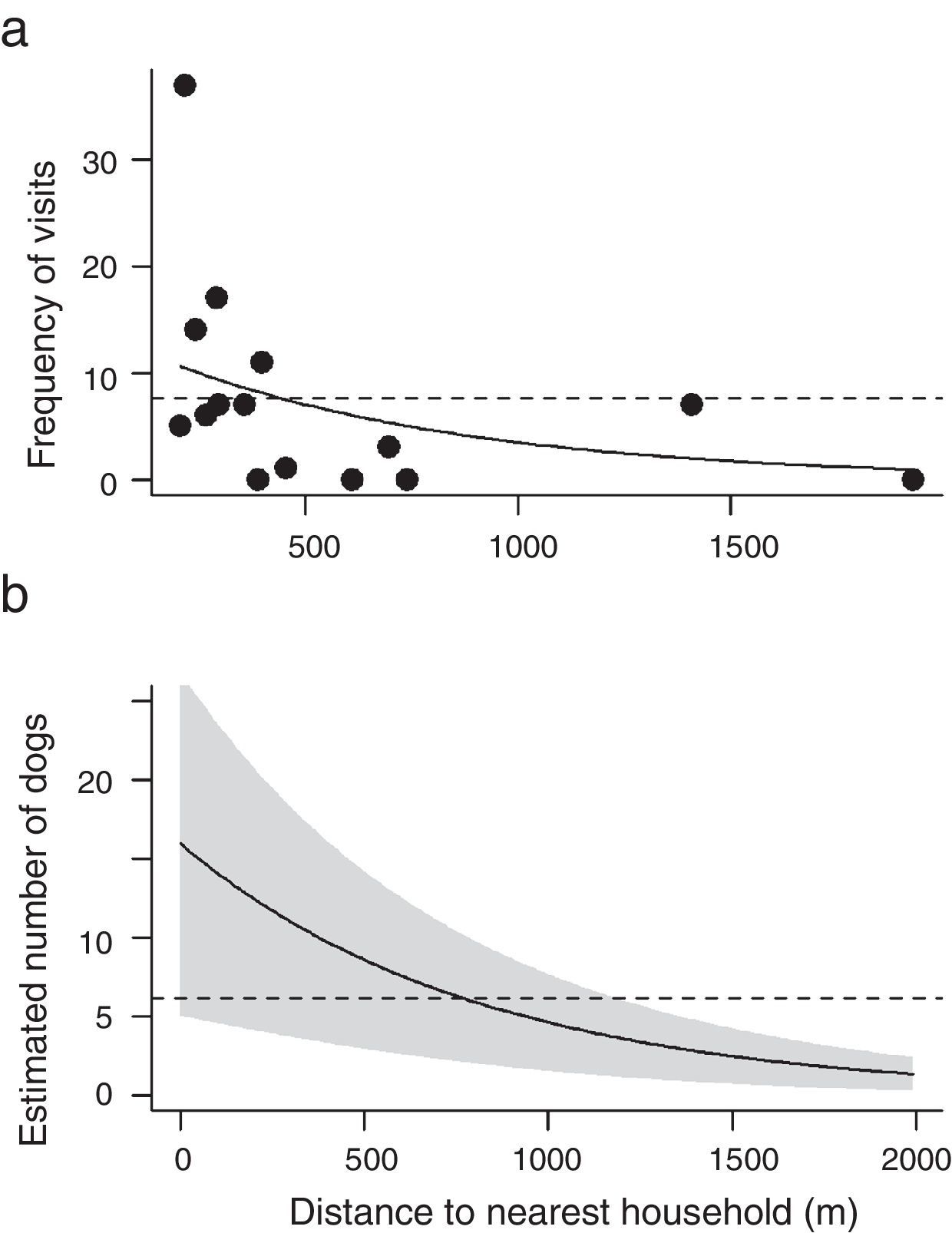
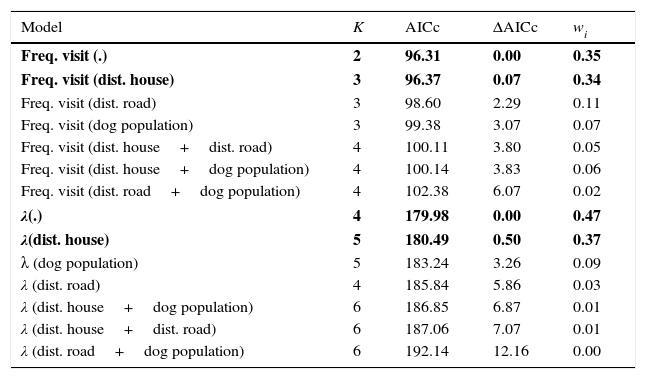
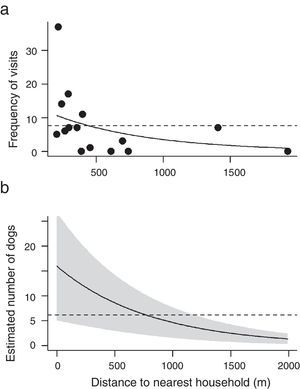
For both the frequency of visits of dogs and the estimated number of individuals across the 15 cabrucas, two models were selected: the reference model and the model containing the distance to the nearest household (Table 1). Thus, while the distance to nearest road and the size of dog population did not influence neither the frequency of visits nor the estimated number of dogs using cabrucas, the distance to the nearest household tended to decrease both aspects of dog invasion (Fig. 3a and b).
Generalized linear models for the frequency of visit of dogs, and open N-mixture model for estimating the number of dogs, across 15 cabrucas. Independent variables: dist. house: distance to the nearest household; dist. road: distance to the nearest road; dog population: dog population size in the vicinity. K – number of parameters; AICc – Akaike Information Criterion modified for small samples; ΔAICc – difference to the AICc of the best model; wi – model weight. Selected models are in bold.
| Model | K | AICc | ΔAICc | wi |
|---|---|---|---|---|
| Freq. visit (.) | 2 | 96.31 | 0.00 | 0.35 |
| Freq. visit (dist. house) | 3 | 96.37 | 0.07 | 0.34 |
| Freq. visit (dist. road) | 3 | 98.60 | 2.29 | 0.11 |
| Freq. visit (dog population) | 3 | 99.38 | 3.07 | 0.07 |
| Freq. visit (dist. house+dist. road) | 4 | 100.11 | 3.80 | 0.05 |
| Freq. visit (dist. house+dog population) | 4 | 100.14 | 3.83 | 0.06 |
| Freq. visit (dist. road+dog population) | 4 | 102.38 | 6.07 | 0.02 |
| λ(.) | 4 | 179.98 | 0.00 | 0.47 |
| λ(dist. house) | 5 | 180.49 | 0.50 | 0.37 |
| λ (dog population) | 5 | 183.24 | 3.26 | 0.09 |
| λ (dist. road) | 4 | 185.84 | 5.86 | 0.03 |
| λ (dist. house+dog population) | 6 | 186.85 | 6.87 | 0.01 |
| λ (dist. house+dist. road) | 6 | 187.06 | 7.07 | 0.01 |
| λ (dist. road+dog population) | 6 | 192.14 | 12.16 | 0.00 |
Frequency of visit (a) and estimated number of dogs in cabrucas (b) as a function of distance to nearest household. Solid lines indicate models adjusted for the independent variable “distance to nearest household” and dashed lines indicate the constant models. In panel b, shaded area indicates the standard error.
From the 47 dogs photographed in cabrucas, only 19 were found in the surrounding households. Although the highest frequency of visits to cabrucas was observed for three dogs that were raised in the surroundings, frequency of visits did not vary significantly among dogs living in the surroundings and those of unknown origin (Mann–Whitney: W=228, p=0.34).
DiscussionThe use of camera-traps has been a successful non-invasive sampling technique applied in the study of medium and large-sized vertebrates, allowing for information on occupancy, frequency of use, abundance and behavior (Burton et al., 2015). In the current study, we take advantage of camera-trap records not only to estimate frequency of use and the number of individuals, but also to associate camera-trap data and photographic records of dogs living in the surroundings, allowing for the identification of the origin of animals detected in a specific sampling site.
Most dogs recorded in cabrucas do not live in the immediate vicinity of the sampling sites. This explains the lack of relationship between the frequency of visits and number of invading dogs in a cabruca and the size of dog population in cabruca vicinity. This may also explain the weak responses to distance from the nearest household. Dogs of unknown origin that invade cabruca sites (>50% of recorded individuals) might inhabit residences located at distances greater than 800m from sampling sites or have no direct link to households (feral dogs, according to classification proposed by Vanak and Gompper, 2009). A behavioral study of free-ranging dogs in our study region shows that daily displacements exceeding 800m from household were rare, but may occur when dogs follow rural workers inside the cabrucas (C.L.A. dos Santos, unp. data). In fact, fine-scale movements of free-ranging dogs have reveled dog locations farther than 4km from household (Parsons et al., 2014; Ruiz-Izaguirre et al., 2015; Sepúlveda et al., 2015). These long displacements of owned dogs seems to be rare and accomplished by the minority of individuals – e.g. directional movement at least 200m from the household occupied only 5.3% of the total monitoring time and 76% of forays were displayed by 30% of dogs (Sepúlveda et al., 2015) – but they may greatly increase dog impact on native species as these displacements allow dogs to explore wildlife habitats (Vaniscotte et al., 2011; Ruiz-Izaguirre et al., 2015; Sepúlveda et al., 2015).
Some characteristics of our agroforestry landscape may explain the displacements of dogs away from human settlements. The number and extension of roads and well-marked trails as well as the open environments are expected to facilitate dog movements (May and Norton, 1996; Torres and Prado, 2010; Sepúlveda et al., 2015), and thus agroforest invasion. In our study, neither frequency of visits or number of invading dogs in a cabruca was associated to distance to nearest road. This lack of association suggests that other features might facilitate dog displacement in cabrucas, such as non-mapped trails or the relatively open vegetation (especially on ground level due to periodical removal of herbaceous vegetation) in this agroforestry system. Reports of rural workers indicate the use of dogs for company during work activities in cacao plantations and eventual hunting excursions in cabrucas and forest remnants (A.P. Silva, pers. observation). This worker–dog relationship may also contribute to cabruca invasion when dogs start displacing with their owners and further explore the area alone or in the company of other dogs. This human induced invasion of cabrucas is supported by Frigeri et al., (2014), who found that invasions occur predominantly in the days and hours of greatest human activity in this agroecosystem and that management intensification in agroforests led to a higher number dog records. Furthermore, 10 free-ranging dogs tracked in our study region only entered the cabrucas in day time when their owners were working this agroflorestry system (C.L.A. dos Santos, unp. data).
Several processes may mediate the negative effect of dogs on wildlife, and these processes are likely to be influenced by dog movement. Longer displacements may increase the probability of encounters, favoring processes that depend on direct contact such as hybridization, predation, and direct competition; all of which have been previously reported between dogs and wildlife (e.g.Campos et al., 2007; Khosravi et al., 2013). Longer displacements also imply larger areas with “marks” left in the environment, such as urine and feces deposition. Besides changing habitat use by others species (Silva-Rodríguez et al., 2010; Silva-Rodríguez and Sieving, 2012), these excreta can transmit pathogens if infected (Corrêa and Corrêa, 1992; Vaniscotte et al., 2011).
Dogs represent an important treat to biodiversity conservation worldwide, mainly due to wildlife predation, but also through environmental disturbance, disease transmission, competition and hybridization (Doherty et al., 2017). Processes driving the negative effect of dogs on wildlife in agroforestry systems are unknown. Nevertheless, Soares et al. (2013) highlight the presence of domestic animals (including dogs) as one factor threatening the diversity of wild mammals in agroforestry mosaics; and Cassano et al. (2014) report that dog record rates played a stronger negative effect on wildlife than the reduction of forest cover or local environmental simplification due to agroforestry management. The conservation value of agroforests and agroforestry landscapes depends, among other things, on the management of invasive species, such as the dog. An increasing number of studies have been reporting the invasion of wildlife habitat by free-ranging dogs and dog-wildlife interactions, taking advantage of common methods applied on large mammal studies such as camera-trapping and radio-tracking (e.g.Silva-Rodríguez et al., 2010; Vaniscotte et al., 2011; Ruiz-Izaguirre et al., 2015). Free-ranging dogs have been shown to display long distance movements in human-modified landscapes (Parsons et al., 2014; Ruiz-Izaguirre et al., 2015; Sepúlveda et al., 2015; this study). Such movements might be rare and displayed by the minority of dogs, nevertheless they can increase the negative impact of dogs on native species, by increasing the invasion of wildlife habitats and the probability of dog-wildlife interactions. The invasion of cabrucas is likely to increase dog-wildlife interactions as this system is home to several native species (Cassano et al., 2009), and might facilitate the invasion of native forest fragments surrounded by this agroecosystem. Knowledge on the origin of invading dogs, on movement ecology, and on current management of local dog population are valuable to guide conservation strategies.
Concluding remarksCabrucas near household tend to be more prone to invasion by free-ranging dogs, but the origin of the dogs that invade a cabruca is divided between the immediate vicinity (i.e. up to 800m) and more distant areas. The relatively long displacements of dogs through the landscape have implications for the impact on native fauna. Predation and competition are likely to be more widespread and pathogen circulation (among dogs and between this and other species) are likely to be higher when animals move freely. Biodiversity conservation actions in agroforestry landscapes are important because these environments are home to a significant diversity of fauna and flora. Limiting dog displacements through these areas is likely to increase landscape conservation value. Studies on movement ecology can provide useful information to the design of strategies aiming at reducing the impact of free-ranging dogs on wildlife.
Data accessibilityData and R script are available upon reasonable request. Please contact corresponding author.
We thank the owners of cacao farms for logistical support and permission to carry out the research on their farms, and Universidade Estadual de Santa Cruz [grant number 00220.1100.1481], FAPESB/PPP [grant number 008/2011] and CNPq/Universal [grant number 456759/2014-0] for financial support. CLAS acknowledges her scholarship from CNPq [131326/2014-7], SBS and APS acknowledge their scholarship from FAPESB [5356/2014 and 5659/2014, respectively].