Biological invasion is one of the main drivers of biodiversity loss and ecosystem damage. Invasive species are difficult to eradicate and prevention is considered the best approach. The pied crow (Corvus albus) was recently recorded in eastern Brazil (South America). This African bird species is being considered as a “native invader” in South Africa, and has the potential of causing serious ecological impacts. Therefore, it is important to identify the potential suitable areas and entry points of this species in Brazil. This sort of information allows for a better assessment of where surveillance is needed and if eradication procedures are required. We used ecological niche models to assess the potential distribution of the pied crow in Brazil. Models predicted high suitability for the Southeast, Central and Northeast regions of Brazil, mainly in the Atlantic Forest region. Pied crow occurrence was associated with human infrastructure. Binary models failed to include published records for C. albus in Brazil. However, suitable areas are found 46km away from known occurrence records. We argue that ports are non-intentional points of entry and that surveillance measures should be put into place to prevent novel propagules from arriving and establishing in Brazil.
Human activities are responsible for the introduction of species into localities outside species’ natural ranges (Richardson et al., 2011). Species introduction can occur with and without human intention and is usually favored in human altered landscapes (Mack, 2000). Invasive species are detrimental to biodiversity and human welfare due to its negative impacts on native species, community and ecosystem processes, human health and economy (Blackburn et al., 2014). However, for an invasive species to be successful in a new environment, it needs to overcome the following barriers (Blackburn et al., 2011: i) have access to a geographic region that was inaccessible prior to human assistance; ii) establish a viable population in the new geographic region, thus overcoming potential restrictions imposed by the new environment; iii) spread to novel localities within the new environment and; iv) cause deleterious impacts (sensu Blackburn et al., 2014) in the new environment. Climate matching has been pointed out as an important predictor of successful invasions (Hayes and Barry, 2008). The reason is that introduced areas that have similar climate to the invasive species’ native range are more propitious to the establishment of viable populations, because these areas will have the appropriate climatic conditions for positive population growth (Holt et al., 2005). Because the most efficient way to minimize ecological and economical damage is to prevent the establishment of invasive species (Sakai et al., 2001), species distribution models and ecological niche models can be used as predictive tools for determining the invasive potential of a species (Peterson, 2003). With these models in hand, monitoring procedures can be put into place to deter the establishment or advancement of invasive species (Venette et al., 2010).
In a recent meta-analysis review, Madden et al. (2015) pointed out that crows and ravens have an overall small negative effect on bird abundance and productivity (e.g., nesting success, brood size). However, two crow species – Corvus splendens and Corvus albus – are considered problematic. The first species has managed to establish breeding populations outside their native range in several countries, where they are responsible for ecological impacts (Ryall, 1992), economic loss (Kamel, 2014) and human health problems (Yap and Sodhi, 2004). The second species has yet to establish breeding populations outside of its Pan-African native range. However, in South Africa, C. albus has experienced a disproportionate increase in abundance and range (Cunningham et al., 2016). Scientific studies on the ecological impacts for this species are lacking, but there is a report of heavy predation pressure on a native tortoise (Fincham and Lambrechts, 2014) and of potential negative interactions with raptors (Simmons and Barnard, 2011). Indeed, it is possible that a process of “native invasion” by C. albus is currently happening in South Africa (Cunningham et al., 2016). This can be problematic because this species is a behavioral generalist that feeds on a wide range of organisms as well as carrion (Anjos et al., 2009). Moreover, human infrastructure can facilitate the spread of C. albus. For example, powerline and road infrastructure are associated with an increase in the numbers of C. albus in South Africa. The first probably provides the species with nesting and perching sites (Cunningham et al., 2016), while the latter provides alternative food sources via roadkills (Joseph et al., 2017).
The presence of C. albus in Brazil was first reported in 2004 (Silva and Olmos, 2007), and there are currently eight published records for this species in the coast of São Paulo State (Table S1; Lima and Kamada, 2009). Because this species seems to be developing into a “native invader” (Cunningham et al., 2016), and human infrastructure seems to facilitate its expansion in the native range, it is imperative to evaluate if C. albus is capable of establishing a viable population in Brazil. In order to do this, we developed an ecological niche model using data from the native range to identify potential suitable climate areas for population maintenance. In addition, we traced possible expansion routes of this species in Brazil based on the recent records of this species in the coast of São Paulo state. Finally, we also discuss the importance of these results to increase the current knowledge of the distribution of pied crow as a Neotropical invasive species.
MethodsStudied species and occurrence dataCrows and Ravens (Family: Corvidae) are medium to large birds reaching 69cm and 2000g. Crows are morphologically quite similar with a basic black plumage having strong beaks and legs (Anjos et al., 2009). C. albus, is a Pan-African large crow (45cm, 400g–700g), with head, neck, upper chest, upperparts, wings, and tail black or with nuances of black, blue and purple (Anjos et al., 2009). Different from other crows, these dark parts contrast with a white collar, which extends over the breast and underparts (see Fig. 5 in Lima and Kamada, 2009). This species lives in open areas, such as grasslands, open woodlands, forest edges and savannas. It can also be found in close proximity to aquatic environments, such as riverbanks and lakeshores (Anjos et al., 2009). Pied crows avoid dense forests and deserts, can withstand high altitudes (up to 3700m a.s.l.), but prefer lowlands where it is usually more abundant (Anjos et al., 2009). The species is also abundant in habitats associated with human settlements (Londei, 2010).
Native occurrence records (n=42,238) were obtained from the Global Information Facility Database (www.gbif.org). Duplicate records inside the same grid and points outside geographic boundaries were removed. The remaining occurrences (n=4482) were thinned using spatial filter analyses in order to reduce sampling bias (Boria et al., 2014), as implemented in the R package spThin (Aiello-Lammens et al., 2015). We used this procedure because biodiversity data is usually biased in areas of easy access. The thinning procedure was executed using 100 iterations and 10km thinning distance (i.e., minimum distance between occurrence points), which resulted in 2635 occurrences. As a final step, occurrences inside grids that lacked data for at least one of the environmental variables used were removed, resulting in a final data set of 1318 occurrences records (Fig. S1 and Supplementary Methods).
Environmental dataClimate data were obtained from the WorldClim database (Hijmans et al., 2005). In order to reduce over-fitting of the models, climatic variables were selected according to the ecology of C. albus. We also used a principal component analysis to reduce collinearity among WorldClim variables. This was done by keeping the variable with the highest loading when variables were correlated (see Fig. S2; Table S2; and Supplementary Methods for more details on variable selection). Due to the synanthropic nature of C. albus (Anjos et al., 2009), we included in the final model two variables that reflect human associated landscape changes: 1) global nightlight data (NOAA's National Geophysical Data Center; available at: http://ngdc.noaa.gov/eog/dmsp/); which measures human land use, infrastructure and human access; and 2) human foot print (Wildlife Conservation Society, Center for International Earth Science Information Network, 2005; available at: http://sedac.ciesin.columbia.), which is the Human Influence Index (HII). HII is determined using data on human population density, human land use/infrastructure and human access (roads, railroads, navigable rivers and coastlines). The extent of the native area used for modeling was: 37°20′58.632″N, 51°24′46.908″E, 46°58′44.148″S, 25°21′31.5″W. Final models were projected into South America (extent: 13°22′43.032″N, 29°21′35.028″E, 55°55′10.956″S, 109°26′56.94″W) using WGS84 projection. Grid cell resolution was 0.25° degrees, corresponding to ∼28km2 in each raster (Supplementary methods). We chose this resolution because of the species’ large native distributional range (Fig. S3), high densities of occurrence records for South Africa – thus indicating possible sampling biases (Fig. S1) – and the scale of our study, which is continental (model was made using occurrence data from Africa, which was then projected into South America).
Ecological niche models (ENM) and model calibrationWe used five different classes of algorithms to model the potential distribution of C. albus (see Supplementary methods). The algorithms were chosen according to different modeling approaches based on Bioclimatic Envelope, Distance and Machine Learning methods, which can be used to model distribution with presence only data (Franklin, 2010). These methods produce maps with continuous suitability values for each grid cell and rescale values from zero (i.e., grid unsuitable) to one (grid is suitable) when necessary. Models that presented a good fit (see below) were then combined to generate a single model (Araújo and New, 2007), which was then projected into South America to evaluate the potential invasiveness of C. albus. We used 75% of occurrence data (n=988) to calibrate the models and 25% (n=329) to validate the models, using a pseudo-absence approach to simulate absence of occurrence when necessary.
We used 100 iterations for each algorithm and used the partial area under the receiver operating characteristic curve (ROC) to measure model fit (Peterson et al., 2008). For this analysis the software “Partial ROC” (Barve, 2008) was used. The partial ROC approach is more suitable to evaluate environmental niche models because it allows one to reduce the biases generated by the pseudo-absence procedure (for details see Peterson et al., 2008). We used 10,000 iterations, and for each iteration, we re-sampled 50% of the test data (i.e. bootstrap) and accepted a 95% error (default settings). Models that had partial AUC ratio >1 were considered as being better than a random performance and were combined in one model (i.e., the ensemble approach sensu Araújo and New, 2007). We also used the multivariate environmental similarity surface (MESS) to evaluate how similar the non-native range projections were in comparison with the native training of the model (Elith et al., 2010). The MESS analysis was conducted using the dismo package (Hijmans et al., 2016) implemented in R software (R Core Team, 2015).
Potential suitable areas in South America and spatial analysisWe used a binary model to estimate the potential geographic region of C. albus occurrence in Brazil. For each acceptable model (pAUC ratio >1), a binary map was obtained using the sensitivity equal specificity threshold. We chose this threshold because it is an acceptable measure that minimizes mean positive and negative error rate (Liu et al., 2005). All models (see results) were considered adequate and their binary maps were pooled to obtain one single binary map. Grid cells were considered suitable for C. albus when at least two out of the five adequate models predicted its occurrence (i.e., grid cells sum values were ≥2). We chose this value because only two models (SVM and Mahalanobis) seemed to differ substantially from the other models. Because the binary model failed to include known occurrence records of C. albus in Brazil (see result), we calculated the minimum distance of the last occurrence record to the nearest suitable grid cell. This was done using the Haversine spherical trigonometry distance as implemented in the R package geosphere (Hijmans, 2015). To evaluate which Biome is more susceptible to the potential invasion of C. albus, we calculated the proportional area of the Biome that was predicted as suitable by the model (for details see Supplementary Methods). We also obtained the relative importance of each variable used in the model with the relaimpo R package (Grömping, 2006).
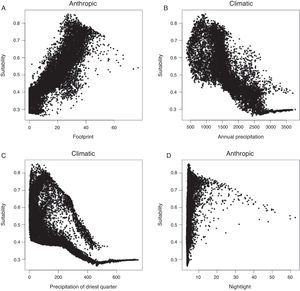
ResultsAll algorithms were considered as having a good model fit (Table 1). Variables in the model explained 85% of model variance. Variables that presented the highest values of relative importance were: Human Footprint, Annual Precipitation and, Precipitation of Driest Quarter (Table 1). Human Footprint was positively associated with suitability, while Precipitation and Driest quarter were negatively associated (Fig. 1 and Fig. S4). Atlantic Forest and Caatinga biomes were identified as the main regions to be affected with a potential invasion of the pied crow with 0.562 and 0.575, respectively, relative index. For potential distribution of the native range see Fig. S5.
Partial area under the curve (pROC) and threshold values (sensitivity=specificity) for the five algorithms used to model the potential distribution of Corvus albus (models were combined if pROC>1). Below this table is the relative importance of each variable used to model the distribution of Corvus albus.
| Model metrics | Bioclim | Domain | Mahalanobis | Maxent | SVM |
|---|---|---|---|---|---|
| pROC | 1.999±0.000 | 1.963±0.000 | 1.995±0.002 | 1.999±0.000 | 1.999±0.000 |
| Threshold | 0.072±0.007 | 0.672±0.006 | 0.207±0.058 | 0.332±0.020 | 0.630±0.035 |
| Relative importance of each variable | |||||
| Environmental layers | Relative importance | ||||
| Footprint | 0.296 | ||||
| Annual precipitation | 0.219 | ||||
| Precipitation of driest quarter | 0.116 | ||||
| Precipitation of wettest month | 0.094 | ||||
| Min temperature of coldest month | 0.069 | ||||
| Annual mean temperature | 0.067 | ||||
| Max temperature of warmest month | 0.064 | ||||
| Temperature annual range | 0.037 | ||||
| Nightlight | 0.033 | ||||
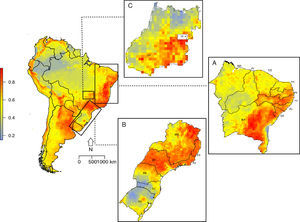
Suitable regions for the occurrence of C. albus in South America occur along the Atlantic coast and throughout the Andes, from north of Chile to Venezuela (Fig. 2). In Brazil, three main regions are highly suitable: i) the northeast with high suitability values for Bahia (BA), Alagoas (AL), Pernambuco (PE) and Paraíba (PB) (Fig. 2A); ii) southeast, with high suitability values in São Paulo (SP), Rio de Janeiro (RJ), Paraná (PR), Minas Gerais (MG) and Espiríto Santo (ES) (Fig. 2B); and iii) central, with high suitability values in Goiás (GO) and a small patch of high suitability in the Distrito Federal (DF) (Fig. 2C). Areas that had high suitability also presented high correlation in MESS, which indicates that the model did not extrapolate to novel climatic regions (Fig. S6).
Suitability map for Corvus albus occurrence in South America. (A) Northeastern coast of Brazil, (B) Southeastern coast of Brazil, and (C) central region of Brazil. Low suitability values are represented by blue color and high suitability values are represented by red color. State labels are: BA=Bahia, SE=Sergipe, AL=Alagoas, PE=Pernambuco, PB=Paraíba, RN=Rio Grande do Norte, CE=Ceará, PI=Piauí, MA=Maranhão.
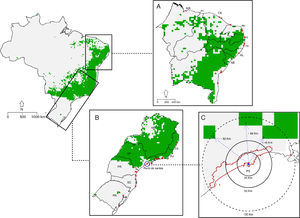
All known occurrence records in Brazil are located within the same grid cell showing a suitability value of 0.524 (Fig. S7). Combined binary maps show that 19.7% of the surface of Brazil (∼16,275km2) is suitable for the potential occurrence of C. albus (Fig. 3). Binary maps for the non-native range for each modeling algorithm are provided in Fig. S8. Five of the nine states in the northeast coast have active merchant ports in or near grid cells considered to be suitable for the occurrence of C. albus (Fig. 3A). In the southeast, there is a large suitable area in the interior of the states of Paraná, São Paulo, Minas Gerais and Rio de Janeiro and Espirito Santo, but only a small suitable area close to the coast of São Paulo state (Fig. 3B). Occurrence records for C. albus in Brazil fall outside predicted suitable areas, but are close to the port of Santos and the closest suitable area is 46km away (Fig. 3C).
Binary map for the occurrence of Corvus albus in Brazil. (A) Northeastern coast of Brazil, (B) Southeast coast of Brazil, (C) minimum distance to closest suitable area. Red stars are ship ports, the blue circle identifies Santo's port (Porto de Santos), while the blue dot is the last occurrence record of C. albus. In C, scale is provided as different circumference distances. Numbers 1, 2 and 3 and blue dot arrows indicates the nearest pixel and distance from the last occurrence respectively.
We found that anthropogenic landscape modification (Human Footprint) was the most important predictor for C. albus, where highly modified areas were positively correlated with suitability (Fig. S4). Despite the low relative importance, Nightlight also had a positive correlation with suitability (r=0.34; Fig. S4), reinforcing the idea that human landscape infrastructure are good predictors for this species. Human infrastructures, such as powerlines and transport networks, have already been shown to positively affect pied crow abundance in South Africa (Cunningham et al., 2016, Joseph et al., 2017), a region considered hot and dry (Metzger et al., 2013). We found that Annual Precipitation and Precipitation of Driest Quarter to be the main climatic contributors in predicting suitable areas for the pied crow. These climatic variables had a negative effect on suitability, which indicate that this species may favor dry regions. Therefore, our model is in accordance with previous studies and is an important tool to be used in identifying potential areas for the occurrence of the pied crows in Brazil.
Suitable areas in Brazil where mainly found in the Atlantic Forest biome, which has suffered tremendous landscape change. For example, estimates on forest cover ranges from 11.4% to 16%, with most forest fragments (80%) being smaller than 50ha (Ribeiro et al., 2009). This is problematic because human landscape modification (Human Footprint) was the main predictor for the occurrence of C. albus. Moreover, there is evidence that human infrastructure is facilitating the spread of C. albus in its native region (Cunnigham et al., 2015; Joseph et al., 2017). Therefore, the current landscape in the Atlantic Forest biome could facilitate the spread of C. albus in Brazil.
Current occurrence records for C. albus in Brazil are located outside predicted suitable areas for this species (Fig. 2C). So far, published records for this species in Brazil do not indicate that this species is spreading or that it has managed to establish a breeding population in Brazil (Lima and Kamada, 2009). The last known published record for this species occurred in 2008, which could lead to the conclusion that the species failed to establish a breeding population in Brazil. However, it is possible that population size is currently too small to draw attention of ornithologists and bird watchers. It usually takes some time for a species to become widespread and invasive (Simberloff, 2009). So it is possible that the current population of the pied crow in Brazil is small, local and innocuous.
The pied crow introduction in Brazil was not intentional, which means that more propagules could arrive in the future. Indeed, there was a recent record (i.e. photograph) for this species in 2014 (P.P. Rodrigues, Pers. Communication) in the municipality of Macéio in Alagoas (AL) State. Although there is no direct evidence of how C. albus arrived in Brazil, the most likely vector of introduction are merchant ships. Several ports in Brazil are in areas of high suitability or close to areas of high suitability for the occurrence of C. albus (Fig. 2). Ports are known hubs for the entrance of several invasive species (Lockwood et al., 2007) and it is plausible that more individuals could survive transportation and enter the country in the future. Although the species is large, it is capable of surviving transport in ships because of its behavioral flexibility, dietary plasticity and apparent human association (Anjos et al., 2009). The arrival of new propagules can reduce environmental and demographic stochasticity as well as introduce new genotypes to Brazil (Simberloff, 2009), which will increase the chances for the establishment and spread of C. albus in Brazil.
Corvids are known for their great learning abilities making them highly unpredictable when it comes to novel environmental conditions and resource use, as has already been shown for several species in this group (see Anjos et al., 2009). Moreover, behavior flexibility is an important predictor of invasion success in birds (Sol et al., 2002). The pied crow has already been called a “native invader” in its native range because of its great increase in abundance and potential negative impacts on the native fauna (Cunningham et al., 2016). Therefore, the pied crow is a potential threat to the native biota of Brazil and its recent arrival in Brazil should not be taken lightly. Our model predicted areas along the coast of Brazil that are highly suitable for the occurrence of C. albus. We recommend a higher surveillance of ports located in the Atlantic Forest region and efforts should be made to eradicate C. albus in Brazil before it has a chance to spread.
ConclusionIt is imperative for ornithologist and bird watchers to be on the look out for this species, especially in the southeast to make sure it has not managed to establish a breeding population. Although our model failed to predict its current occurrence points, records were only 46km away from suitable areas and in a region of Brazil that is known for its well-developed infrastructure. Because this species is a voracious predator of small mammals, passerines reptiles and amphibians and its current status of “native invader” in South Africa, we advocate that surveillance of this species is needed.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Fundação Araucária for providing a master's scholarship to JRP Adelino. Ld Anjos was supported by a CNPq fellowship. We would also like to thanks RF Souza for letting us use the facilities of the Laboratório de Bioinformática from Universidade Estadual de Londrina. We are also grateful for the valuable comments provided by three anonymous reviewers that greatly improved the final version of this manuscript.