Species are disappearing worldwide with increasing rates through the time, especially in the tropics. The human-induced impacts have a pivotal role in the discussion about the cause of these extinctions events. Although researchers are nearly unanimous about the current biodiversity crisis, there is still a debate about how these human-induced impacts affect biodiversity across different geographical scales. While our perception about landscape modification promoted by deforestation occurs at local scales, we treat the increase of greenhouse gas emissions and its consequences in a global perspective. This diffuse view about different processes but potential assembling properties illustrates the importance of combining small and broad-scales to improve our conservation actions and decelerate extinction events. Here, we propose an integrated framework (Ecoland) combining landscape ecology and macroecology for niche modelling applications, aiming to disentangle the effects of human-induced impacts on biodiversity and guide conservation tasks at multiple scales. We demonstrated the EcoLand application using a dataset of fruit-feeding butterfly species occurrence from the Atlantic Forest. Our findings highlight the impact of the land-use modification on the number of butterfly species. Finally, we discuss the method implications, its limitations and perspectives, and how it the Ecoland can help us to design conservation strategies aiming at the maintenance of biodiversity and its related policy practices.
Species are alarmingly disappearing worldwide (Dirzo and Raven, 2003) with the more pronounced rates in the tropics (Crutzen, 2006; Ceballos et al., 2015; Dirzo et al., 2014). Although researchers are nearly unanimous about the current biodiversity crisis (IPBES, 2016), there is still a hot debate about how human-induced processes affect the biodiversity across different geographical scales (Isbell et al., 2017; Opdam and Wascher, 2004). A macro-scale approach has broad applicability for understanding and dealing with global change. However, human impacts vary significantly by region, and they are subject to specific historical or local contextual factors according to a small-scale perspective. Since we currently have a critical scenario (Estrada et al., 2017), the development of conservation frameworks that combine different scales is urgently needed to understand how human-induced processes are causing the biodiversity crisis (Laurence, 2007).
Here, we discussed the urge to combining small and broad-scale processes to understand their impact on biodiversity distribution patterns. Additionally, we proposed an integrated framework for niche modelling analysis (Ecoland) that combines landscape ecology and macroecology to disentangle the effects of human-induced impacts on biodiversity and to guide conservation tasks at multiple scales. Specifically, we: (i) made a brief review of the processes acting on biodiversity at different scales; (ii) discussed the importance of combining them to understand the biodiversity distribution patterns and the eventual impacts on it; (iii) presented a methodological framework that takes into account the effect of small-scale processes on a broad-scale perspective; and (iv) empirically tested our modelling framework on the Atlantic Forest, a highly modified landscape.
Biodiversity: different perspectives, different processesProcesses occurring at different spatial and temporal scales determine the current biodiversity patterns (Willis and Whittaker, 2002). Following the community assembly rules, these processes may present hierarchical importance according to the respective occurring scale (Cornell and Harrison, 2014; Whittaker et al., 2001). Density-dependent processes, as biological interactions, shape biodiversity patterns at small-scales of space and time, whereas density-independent processes, as environmental filtering, drives the spatial organization of species at regional scales. Biogeographical processes such as speciation, extinction, and dispersal are the main factors driving biodiversity patterns at large scales of time and space (Cavender-Bares et al., 2009; Cornell and Harrison, 2014).
Community ecology studies focused on local scales utilizes relatively few species and predictor variables to determine the processes behind the assembly structure. However, transpose the same responses to regional scales is difficult due to the difference of importance between processes in both perspectives. Macroecology, otherwise, fills this gap by searching for general biodiversity patterns at large extents of geographical space through evolutionary time (Brown and Maurer, 1989). Nevertheless, a macroecological approach is only possible with interpolations, since full sampling data coverage on broad-scale seems improbable. The advances of niche-based modelling techniques have been allowing the prediction of biodiversity distribution using limited data coverage (Pearson et al., 2007), but it requires caution and attention (Jimenez-Valverde et al., 2008; Lobo et al., 2010).
Currently, macroecologists rely on different modelling techniques to predict biodiversity index in sites with no previous information at a broad-scale perspective (Guisan and Rahbek, 2011). One possibility is the macroecological models (MEMs) used to predict species richness based on theoretical background of small-scale community assembly (e.g. species × area relationship). This method gives species richness predictions at broad-scale according to macroecological constraints, even though the species occurrences from some local sites are unknown (Guisan and Rahbek, 2011). Another possibility is based on Stacked Species Distribution Models (SSDM) (Guisan and Rahbek, 2011). The SSDM is raised on predictions of species distributions modeled separately, followed by a posterior overlap among them, resulting in potential species richness maps and community composition at a broad-scale delimitation. However, SSDM overestimates local species richness and/or community composition by ignore community assembly mechanisms at small scales, such as biotic interactions (Mateo et al., 2017; Soberón and Nakamura, 2009). Thus, a Spatially Explicit Species Assemblage Modelling – SESAM – was proposed to infer models nearer to the real species-richness and species composition through SSDM, based on the biological constraints (e.g. biological interactions and species dispersal capacity) (Guisan and Rahbek, 2011) or trait-based approaches (Ackerly and Cornwell, 2007; Guisan et al., 2019), as assembly rules in modelling procedures.
Regardless of the employed modelling approach (MEMs or SSDMs), the broad-scale biodiversity inferences are raised in theoretical ecology. The theoretical basis of macroecological predictions take into account only the action of environmental filtering on species distributions, consequently considering biotic interactions as noise in coarse-grained resolutions (Eltonian Noise Hypothesis) (Pearson and Dawson, 2003; Soberón and Nakamura, 2009). Similarly, land-use modification and other small-scale processes have been neglected on macroecological perspectives.
Human impacts may affect the assembly of novel communities by allowing the encounter of species historically separated (Hoobs et al., 2009). Usually addressed as a small-scale process, human-induced modifications in landscape can influence: (i) population dynamics and local species persistence (Fahrig and Grez, 1996; Fahrig, 2003; Silva et al., 2015; Martello et al., 2016), (ii) local biological interactions (Nagy-Reis et al., 2017; Bello et al., 2015), and (iii) dispersal and species movement (Baum et al., 2004; Nathan, 2008). Dispersal capacity, for example, is a fundamental property to be included in predictions of species distributions at broad-scale (Zurell, 2017), and it has been affected by both landscape structure and configuration (Fahrig, 2003). The landscape modifications have been fragmenting continuous forest habitats in isolated patches, and negatively influencing the dispersal capacity of species. Moreover, these changes can also affect population dynamics, competition rates and permeability to alien species, possibly leading to indirect modifications on species composition (Vicente et al., 2011). These changes in species composition and the new biodiversity assembly rules are based on small-scale processes shaped by land-use modification, but it may cause direct effects on broader scale biodiversity distribution patterns.
To evaluate the effects of human land-use modification on biodiversity patterns, we need to consider that landscape configuration can play a role in species distributions (Fahrig, 2005). The landscape configuration refers to shifts between natural habitats and the composition of anthropogenic matrices (i.e. pasture, forestry, agriculture, urban areas and mining), which may severely affect the habitat quality and landscape connectivity. The degree in which species are impacted by land-use varies depending on their sensibility and requirements. In this sense, there are different landscape metrics that can be applied at broad-scale as surrogate of land cover modification and habitat quality, such as landscape connectivity (e.g. Ribeiro et al., 2009; Martensen et al., 2012), spatial heterogeneity (Moreira et al., 2015), edge effects (Martello et al., 2016), and measures of the impact that different categories of adjacent matrix can cause on natural habitats (Boesing et al., 2018). The landscape metrics can inform if changes in the landscape structure are causing small-scale biological constraints and having a high impact on broad-scale biodiversity distribution inferences.
Broad-scale conservation effortsConservation efforts should focus on reducing the impacts of anthropogenic land-use modification and the biodiversity crisis. Formerly, conservation issues were focused on local species richness and/or in rare species distribution (Noss, 1987). Nowadays, conservation efforts embrace all biodiversity complexities, linking genes to ecosystems functions into multiscale ecological patterns (Poff et al., 1997). The traditional inferences based on species richness are coarser biodiversity proxies in the highly human-modified world. Instead of relying only on the number of species, the conservation efforts should also consider combining this information with species composition, endemism, or abundance distribution (Fleishman et al., 2006). Moreover, the small-scale processes once considered as noise now turn into rules (Butchart et al., 2010), making the human-induced land-use be incorporated on broader biodiversity inferences and consequently in the conservation efforts (Pimm et al., 2014; Newbold et al., 2015). Below, we propose a modelling framework to predict broad-scale biodiversity patterns based on landscape modification and apply it to conservation planning.
Incorporating landscape metrics into broad-scale perspectivesNiche-based modelling can be used to test the effects of land-use on current species distribution and/or to predict broad-scale species richness patterns (SSDM). Nonetheless, different approaches have been designed to input landscape into niche-based inferences.
Some studies adopt only landscape metrics in niche-based models (Siqueira et al., 2009); however, this strategy has limitations by excluding broad-scale processes delineating species distribution (see review in Soberón and Nakamura, 2009). Consequently, niche-based modelling using just landscape metrics can generate unreal broad-scale predictions. Another strategy is to build niche-based models using only climate variables (broad-scale processes) and then cut the model predictions using a land-use categorization, as ‘forest remnants’ for example (Loiselle et al., 2010; Sobral-Souza et al., 2015; Paviolo et al., 2016; Hasui et al., 2017). Similarly, this approach does not combine the broad and small-scale processes; it predicts only the climate suitability within forest patches and does not directly consider the effects of human-induced landscape (re)configuration on broad-scale species dispersal.
Niche-based models combining climate and landscape, as broad and small-scale processes, can be useful to predict the effects of land-use on biodiversity patterns (Loiselle et al., 2010; Jorge et al., 2013; Paviolo et al., 2016; Hasui et al., 2017). However, models combining these variables neither address the relative contribution of broad and small-scale processes on biodiversity distribution nor highlight the isolated weight of land-use modification (small-scale processes) at broad view. Thus, the traditional approach does not incorporate the possibility to understand specific sites suffering effects of land-use modification, an important knowledge to define broad-scale conservation actions. The main interest to incorporate landscape metrics into niche-based models is inferring the effects of landscape in a broad-scale view using properties of landscape as a driver of local community assembly. Here we propose a new perspective to integrate landscape ecology into niche-based models, called EcoLand analysis.
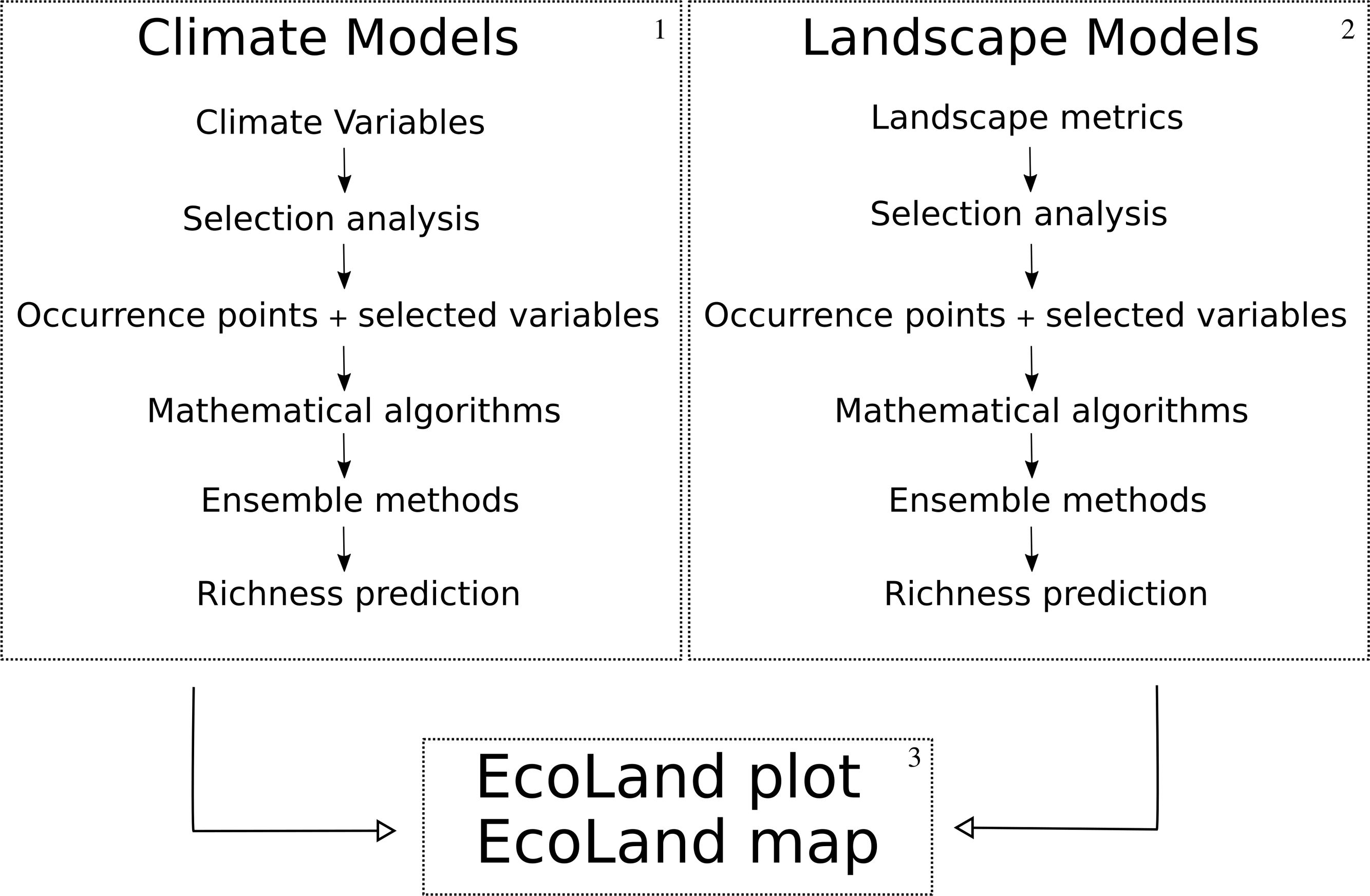
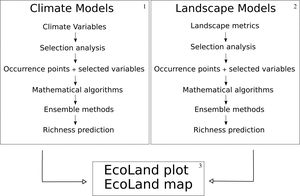
EcoLand analysisFirst, we propose to build niche models using climate variables (broad-scale processes) (Fig. 1, box 1). We suggest using climate variables from non-interpolated data (e.g. remote sensing variables instead of WorldClim, see Dablauwe et al., 2016) to decrease the probability of associated errors. After controlling the collinearity among climate variables (see De Marco and Nobrega, 2018 for details), the niche-based models must be built following recognized standards (Araujo et al., 2019). It is recommended to analyze and report the uncertainties in niche-based models by combining predictions from multiple algorithms (see Araujo and New, 2007). Finally, stack the species models to predict broad-scale biodiversity distribution (Fig. 2a).
EcoLand analysis framework. 1 — Building steps of climate niche-based models. 2 — Building steps of landscape niche-based models. In boxes 1 and 2 the niche-based models must be built following the same assumptions. 3 — EcoLand analysis for understanding the effects of landscape modification on biodiversity patterns at broad scale perspectives (see “Methods” Section). 4 — Geographical prediction of the EcoLand.
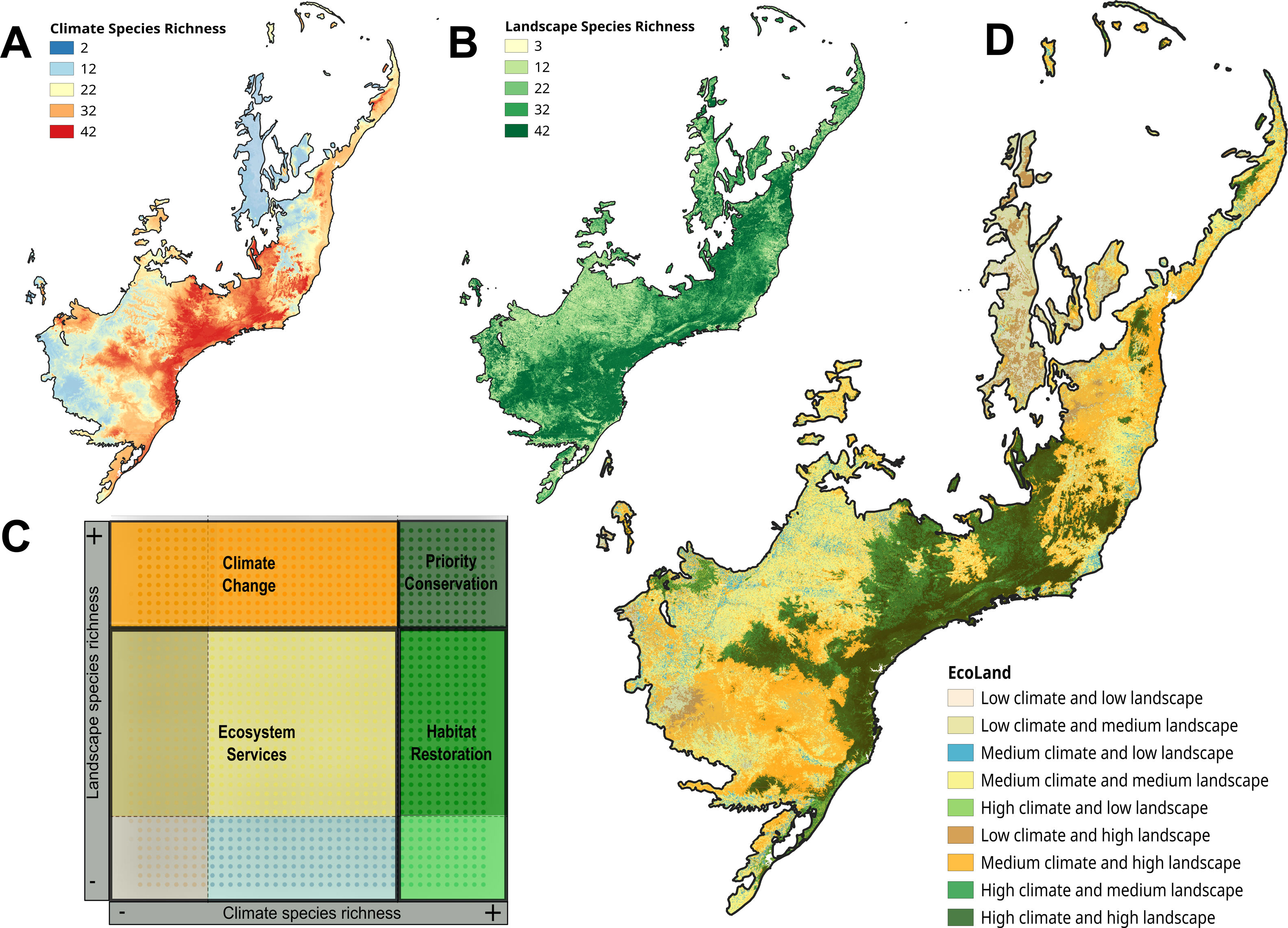
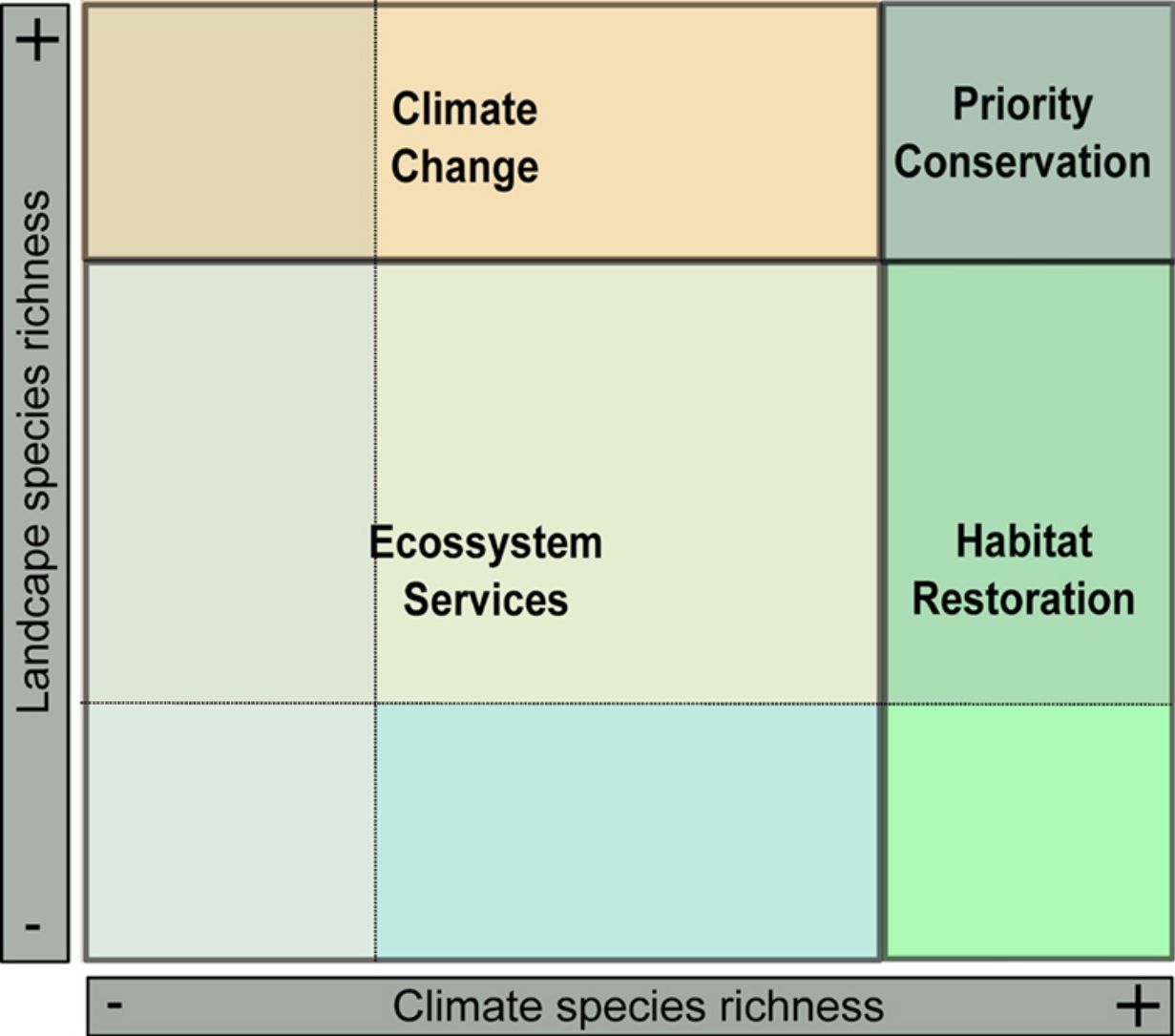
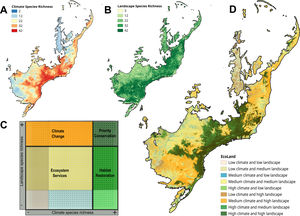
The EcoLand analysis. A — Prediction of climate species richness (broad-scale processes). B — Prediction of landscape species richness (small-scale processes) (see Section “Biodiversity: different perspectives, different processes”). C — The EcoLand plot applied to conservation actions (see Section “EcoLand analysis”). For each categorization class, a specific conservation management is suggested according to the species richness values. Thus, sites on the dark-green region can be considered priority to the creation of protected areas or reserves. On the light-green regions are the interesting sites to planning different habitat restoration programs, whereas orange spots are important sites to working on climate change scenarios. Finally, the yellow and sand colored regions are good sites to discuss ecosystems services applied to human well-being (see the main text). D — The EcoLand map highlighting each conservation categories applied to fruit-feeding butterflies of Atlantic Forest (see Section “The EcoLand analysis applied to broad-scale conservation efforts”).
Like the climate niche models, independent landscape niche models are built by using landscape predictions. It is also important to avoid associated errors with interpolated data. Landscape models must be built following the same standards of the climate models, selecting only ecologically scaled landscape variables (sensu Vos et al., 2001) (Fig. 1, box 2). Then, plot the prediction of species richness distribution based on landscape models (small-scale effects). (Fig. 2b).
The values of species richness based on both climate and landscape models are extracted and plotted in an XY dispersion graph (EcoLand plot), where X-axis corresponds to the climate predicted species richness, whereas the Y-axis represents the species richness from landscape models (Fig. 2c). The EcoLand plot is then categorized using a threshold-dependence criterion, based on the researcher's decision about defining high and low species richness categories. The choice of specific threshold depends on the study’s goal and the conservation priority. Here, we assume the classical quartiles (25% and 75%) as the threshold for low and high species richness, respectively (Fig. 2c). Thus, the EcoLand plot results in nine categories combining climate and landscape model predictions, as follows: (i) sites with high climate and landscape species richness predictions; (ii) sites with high climate and low landscape predicted richness; (iii) sites with high landscape and low climate predicted species richness; (iv) low climate and landscape predicted species richness; (v) low climate and intermediate landscape predicted species richness; (vi) low landscape and intermediate climate species richness predictions, (vii) intermediate climate and landscape species richness predictions; (viii) sites with high climate and intermediate landscape predicted species richness; and (ix) high landscape and intermediate climate predicted species richness (see Fig. 2c). Finally, these categories are plotted on geographical space (EcoLand map) to understand the geographical effects of landscape modification at a broad-scale perspective (Fig. 2d). The association between landscape and climate predicted species richness might indicate if there are negative effects of land-use on species richness.
Applying the EcoLand analysisHere, we used a subset from an extensive database of fruit-feeding butterflies (Charaxinae and Satyrinae: tribe Brassolini) from Atlantic Forest (see Santos et al., 2018) to apply the EcoLand framework. We modeled all 42 butterfly species based on landscape and climate conditions. These taxa are important bioindicators based on their sensitivity to forest-habitat fragmentation, thus, a good biological model to test the EcoLand framework. A large region at central Atlantic Forest present high species richness based on climate models (Fig. 2d). However, this region suffered high land-use modification decreasing the butterfly species richness. Thus, the EcoLand map showed only a continuous area, near to Atlantic coast area (Serra do Mar), as a region suitable for maintain high species richness based on both climate and landscape conditions (dark green spots — Fig. 2d). The neighbor areas in the interior, northern and southern Atlantic Forest suffered species richness decreasing due to climate and/or landscape conditions. Therefore, our results indicate a strong effect of land-use on species richness decreasing. Moreover, the EcoLand framework allows the combination of small and broad scale processes to help developing specific conservation tasks for each one of the models’ result to Atlantic Forest, an important necessity to broad scale conservation action.
The EcoLand analysis applied to broad-scale conservation effortsThe EcoLand analysis offers a protocol that potentially can be useful in different regions worldwide using distinct taxonomic groups as biodiversity surrogates. The categorization of classes combining climatic and landscape species richness generate criteria to be used in conservation decisions, mainly to guide the actions commonly addressed today, such as alien species management, protected areas selection and forest restoration using passive or active strategies (Guisan et al., 2013). By using the Ecoland analysis, it is possible to draw priority conservation sites based on broad and small-scale processes including the effects of land-use on species dispersion.
From our specific example of Ecoland application, sites presenting high species richness from both climatic and landscape predictions should receive special attention, being the priority areas to receive conservation actions (Figs. 2c and d — dark green spots). These areas would have high values of irreplaceability and vulnerability, fitting on biodiversity hotspots template according to the global biodiversity conservation priorities framework (Brooks et al., 2006). Therefore, considering these areas to the establishment of protected areas or conservation units would simply guarantee the habitat protection for the highest number of species as possible, increasing ecosystem services and functioning. However, it is noteworthy that using species richness distribution as a guidance for conservation actions may underestimate the threatened or rare species (Nobrega and De Marco, 2011; Veach et al., 2017). The rare species usually have a low number of records, so their inclusion on modelling processes may create biased distribution maps. Adding a complementary approach would prevent the non-consideration of data about these species and improve the discussion about the conservation goals (Fleishman et al., 2006). Examining the distribution of the species with low sampling representativeness or endemism, and checking their correlation to the richer areas, for example, is an inclusive way (see Santos et al., 2020).
The EcoLand analysis can also be useful to detect regions requiring the implementation of landscape management actions. The regions with high species richness predicted by climate, but low or medium richness predicted by landscape for example, would be possible targets to receive actions of ecological restoration processes (Fig. 2c and d — light green spots). These sites indicate the negative effects of land-use modification on species richness. The landscape configuration of these regions may increase the permeability to alien species invasions causing dramatic changes in ecological systems (Gurevitch and Padilla, 2004) and create a demand for special conservation action to protect the native biodiversity. Aiming to recover the habitat suitability for a higher number of species, a natural habitat restoration at small-scale or seed dispersers rewilding procedures (Sobral-Souza et al., 2017) are good opportunities for these sites. However, they may represent already quite modified landscapes, such as urban centers or any other land category of human exploitation. For some forested habitats, a careful evaluation based on the resilience capacity is a strategy for optimizing the efficiency and costs associated with forest restoration (Tambosi et al., 2014). In a broader sense, the success of a restoration process will depend on the capacity of reversing the state of the current landscape to another state with similar wild properties as the original. Thus, the closer a given landscape would be from its original natural state the more efficient its restoration will be. The context of forest recovery is the best approach for this specific example of the Ecoland application. Nevertheless, natural habitats elsewhere are not strictly represented by forests, but similar logic of landscape restoration could be designed to other natural ecosystems.
The Ecoland also can give support to predicting the climate change effects on biodiversity (Fig. 2c and d — orange spots). The areas with low climatic but high and medium landscape predicted species richness have suitable local habitats for most species even in unfavorable climatic conditions. In other words, the landscape features may improve the persistence of species in these areas, especially under climate change scenarios (Mclaughlin et al., 2017). The patterns of global biodiversity hotspots could be affected by climate change (Bellard et al., 2014). The recognition of prior landscapes for conservation under climate change scenarios is important since they may serve as a refuge to increase the species persistence in the future (Terribile et al., 2012). Therefore, the study of future patterns of species richness and biodiversity maintenance at regions of optimal landscape configuration is import task to conservation efficiency.
The areas that contain both climate and landscape low predicted species richness in Ecoland analysis are those in which conservation actions (focused on the specific biological indicator, at least) probably would be more expensive and less effective (Fig. 2c and d — yellow sand and blue spots). However, the fact that these areas are not species-rich for a specific surrogate does not mean they are not important for providing other ecosystem services. The biodiversity by itself has an intrinsic value (nature for nature), but in a perspective of nature contributions to people (NCP) it is also responsible for providing goods and services to society (Díaz et al., 2015; IPBES, 2016). The maintenance of the ecosystem services associated to “nature for society” values are critical for human well-being and good quality of life (Díaz et al., 2015; Pascual et al., 2017). Therefore, some attention is necessary to the maintenance of services related to society and human well-being, like the water supply and demand or pollination services.
Final considerationsHere, we proposed a framework for combining broad and small-scale processes, mainly related to landscape modification to predict conservation actions. The EcoLand framework is widely useful in any worldwide region, but here represented to hotspots regions with highly human-impacted indexes, as our results suggested to Atlantic Forest. Nevertheless, the EcoLand is not the endpoint, but simply starts integrating multiscale processes on broad-scale conservation planning. Further approaches are possible by using hierarchical models or the EcoLand results to infer species composition and turnover based on landscape influence. The species turnover and the emerging of new communities in response to landscape modification at both broad and small scales emerge as future next steps. The knowledge of new biodiversity (re)structuring at broad-scale can be applied to new conservation efforts aiming for the new demands of ecosystems services applied to human well-being.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We have special thank Julia Oshima for painstaking discussions along manuscript building. We also thank Levi Carina Terribile, João G. R. Giovanelli, Mauricio Vancine, Thiago Sanna Freire Silva for the comments on early versions of the manuscript. This work was suported by Fundação de Amparo à Pesquisa do Estado de São Paulo — FAPESP (2013/50421-2, process number (2020/1779-5)), Conselho Nacional de Desenvolvimento Científico e Tecnológico — CNPq (426785/2018-5, 312045/2013-1; 312292/2016-3, processes number 442147/2020-1 and 150178/2019-0), National Science Foundation (DEB-1256742), MCTIC/CNPq (465610/2014-5) and FAPEG (2018.1026.7000023), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, code 001).