Understanding the distribution and abundance of threatened species is crucial to elaborate effective management plans for wild populations; however, elusive species prove difficult to detect. To support conservation strategies for the Vulnerable Amazonian manatee (Trichechus inunguis), the only freshwater sirenian, we analyzed presence/absence data with hierarchical models based on imperfect detection to assess T. inunguis occupancy in a Sustainable Development Reserve, Brazilian Central Amazon. In parallel, we compared the effectiveness of direct and indirect sampling methods to provide occupancy (ψ) and detection (p) estimates. Combining both sampling methods’ presence datasets provided higher accuracy estimates. The Amazonian manatee’s detection probability had never been estimated before: surprisingly, it was high (p = 0.50, SD = 0.05) and positively related with macrophyte coverage. Results suggest that the studied communities resident impact is not affecting the manatee occupancy, with greatest probabilities closer to human settlements. The final occupancy estimate obtained (ψ = 0.85, SD = 0.12) can be a baseline to Amazonian manatee long-term monitoring studies, and provide support for decision makers and local communities to establish effective protection zones for the species. Our approach highlights the potential of hierarchical models to understand the distribution not only of T. inunguis in different habitats, but also of other threatened Amazonian aquatic mammals.
Understanding species distribution while identifying distinctive habitat components is crucial to elaborate effective management plans for endangered populations (Yoccoz et al., 2001; Pollock et al., 2002; Rushton et al., 2004). However, distribution and habitat use data can be highly demanding due to intense logistic effort and difficulties in detecting individuals (Thompson, 2004; Sewell et al., 2012; Castelblanco-Martínez et al., 2017). Gathering distribution data from fully aquatic mammals like sirenians is particularly challenging due to difficulties in detecting individuals at surface for scarce time (Marmontel et al., 2012).
The Amazonian manatee (Trichechus inunguis) is the only exclusively freshwater sirenian, and endemic to the Amazon river Basin (Best, 1984). Its movement and distribution are associated with the Amazon River’s flood pulse (Junk and da Silva, 1997), moving out of river channels and floodplain lakes onto adjacent flooded forests with the seasonal rise of river waters, and returning to deep-water and perennial lakes during the low river waters season (Arraut et al., 2010). So far, studies addressing T. inunguis distribution are scarce due the limitation of sampling methods (Timm et al., 1986; Reeves et al., 1996). Amazonian manatees are incredibly difficult to visually detect as they only briefly expose their snout when breathing at water surface. Furthermore, river turbidity associated with the animal’s dark skin color masks potential detections at subsurface (Rosas, 1994).
The species was extensively exploited, particularly at the Purus River (Pereira, 1944; Domning, 1982; Antunes et al., 2016), and has been under federal protection since 1967; currently listed as “Vulnerable” to extinction in the National List of Brazilian Fauna (ICMBio, 2018) and in the Red List by the International Union for Conservation Nature (IUCN) (Marmontel et al., 2016). Still, it is hunted for subsistence and illegal trade in popular Amazonian markets (Franzini et al., 2013; Souza, 2015), suggesting a severe population decline over last decades (Marmontel et al., 2016). Therefore, studies about anthropogenic and environmental factors influencing T. inunguis occurrence are key to support much-needed conservation efforts in Brazil and throughout the species’ occurrence range.
The importance of environmental factors in sirenian distribution has been particularly explored for the American manatee (Trichechus manatus) and the dugong (Dugong dugon) using multiple sampling methods, from satellite and GPS tracking (Sheppard et al., 2006) to aerial surveys (Marsh et al., 2004). For the T. manatus, distance to freshwater sources and depth variables are considered the most important to develop habitat models and support conservation strategies for this species (Alves et al., 2013; Landero et al., 2014; Favero et al., 2020). For the Amazonian manatee, availability of preferred food and aquatic space reduction and predator aggregation influence specie' habitat selection (Arraut et al., 2010). However, environmental factors influence not only the real distribution of individuals, but also affect individual detectability probability associated with different methods over space and time (Mackenzie, 2005; Luiselli, 2006). If unaccounted for, variability in detection probability may mask significant biological processes leading to inadequate management decisions (Yoccoz et al., 2001; Tyre et al., 2003; Issaris et al., 2012).
Current prevailing sampling methods are unable to detect all individuals occupying a study area (Mackenzie et al., 2002; Royle and Nichols, 2003). Thus, variable detectability and missed detections must be accounted for during data analysis. Nowadays, imperfect detection has been incorporated into occupancy models based on maximum likelihood (OMML) (Mackenzie et al., 2006), increasing precision and applicability of spatial distribution studies in wildlife management. OMML models are a low-cost alternative for viable estimates of species population trends and wildlife monitoring (Mackenzie and Kendall, 2002; Bailey et al., 2004; Guillera-Arroita et al., 2010) that has successfully been used to investigate for instance the status of river dolphins (Bashir et al., 2012) and dugongs’ occupancy (D’Souza et al., 2013).
Despite its historical relevance to the Amazon’s ecosystem and people, understanding how Amazonian manatees use habitats and identifying environmental and anthropogenic factors that affect their distribution in different scales has been a great challenge for the conservationists. The present study aimed to determine which factors are the most important to estimating Amazonian manatee’s occupancy and detection probabilities. Our hypotheses were: (1) manatee occupancy is affected by proximity to the human settlements, (2) manatee detection is driven by the depth of water body, and (3) the combined use of methods improves the species’ occupancy estimates.
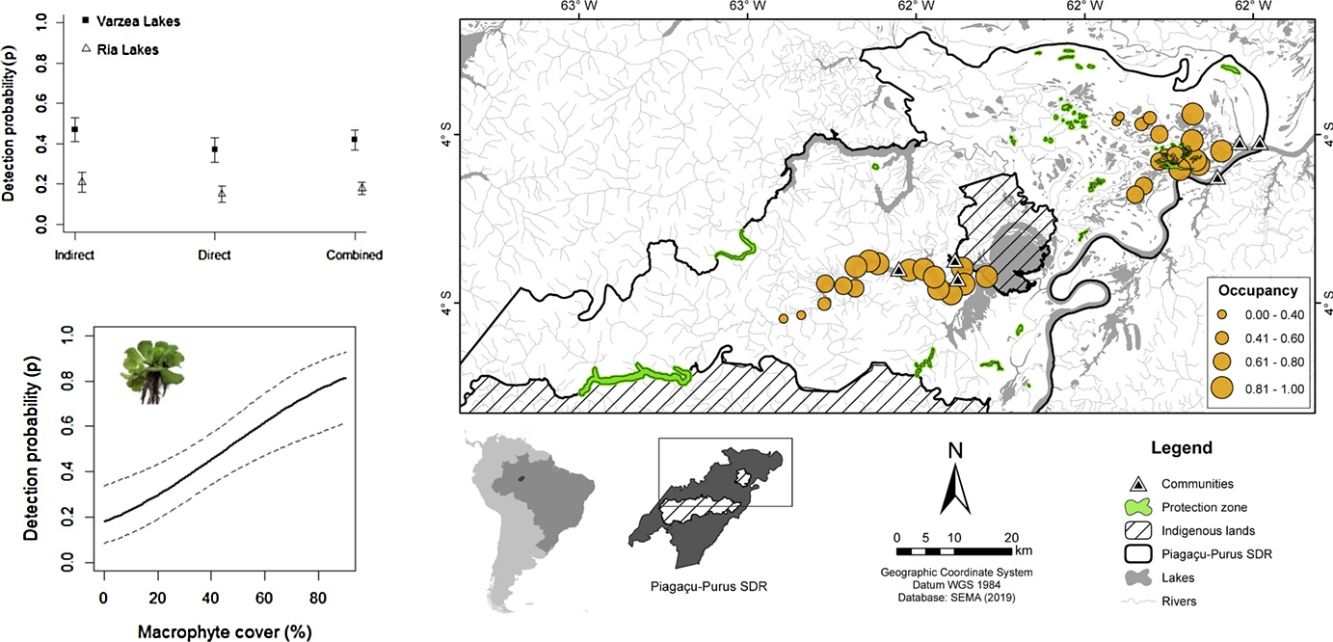
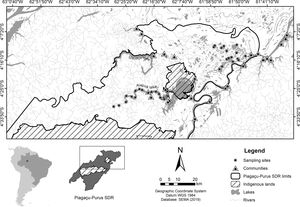
Materials and methodsStudy areaThe study was conducted in the Piagaçu-Purus Sustainable Development Reserve (hereafter PP-SDR) (4°05’, 5°35’S and 61°73’, 63°35’W), located in the Purus River basin, Brazilian Amazon (Fig. 1). The PP-SDR has an area of 834,245 hectares with approximately 4000 residents, who live in a traditional subsistence economy based on hunting and fishing that accompanies the Purus River’s flood pulse (IPI, 2010).
The study region comprises two aquatic landscapes with marked differences. At the edge of the floodplain, ria lakes (long, narrow lakes formed by the partial submergence of a river valley) (Arraut et al., 2010), are embedded in upland environments with well-developed riparian vegetation (Antunes et al., 2011). Its hydrological dynamics is strongly conditioned by the Purus river (Irion et al., 1997). The floodplain environments (locally known as várzea) in PP-SDR include the main river channel, connecting channels, floodplain lakes and the flooded forest, with a hydrological dynamic conditioned by Solimões and Purus rivers (Haugaasen and Peres, 2006).
The PP-SDR’s provisory management plan includes different human-use zones defined in the Reserve’s management plan, established to regulate the use of natural resources sustainably. The Protection zone was created to preserve natural resources and prohibits any human activity beyond surveillance and research, while the Use zone allows commercial fishing and subsistence activities for the resident’s livelihood (IPI, 2010). The region has intense fishery activities that supply the city of Manaus, the largest fish consumer market in the Amazon (Batista and Petrere, 2003).
SurveysAmazonian manatee presence was recorded over repeated visits to 33 sampling sites (Fig. 1) between August and November 2014 (low-water season). The study was carried out during the dry season because we expected the manatees not to be dispersed in the flooded forest, and we could obtain a more accurate estimation about the importance of each one of the monitored sites for manatee survival. Adittionally, in the dry season, the boundaries of the lakes are well-defined, facilitating the selection of sampling points. Sixteen sites were located in várzea habitat, 6 lakes of which were in Protection zone and 10 lakes in Use zone. The remaining 17 sites were ponds in the ria lake’s Use zone. Sampling sites were randomly distributed in the study area to account for heterogeneity in the natural environment and the Reserve’s categories. Sites were at least 3 km apart to avoid spatial autocorrelation. Each site was visited once every three weeks over the data collection period, totaling 44 days of manatee presence.
Manatee surveys were conducted during daytime by two experienced observers (the first author and a former manatee hunter) from a wooden canoe with paddles. Two sampling methods were used (Aragones et al., 2012) according to the local ecological knowledge about manatees. The direct evidence method consisted of active visual search for individuals while following random navigation routes. Each observer looked at the water naked eyed in opposite directions. Sightings were registered when the manatee surfaced. The indirect method consisted of following parallel transects from the margin to find indirect evidences of manatee presence according the skills and high identification experience of the local assistants (fresh scars on aquatic vegetation and floating feces at the water surface), and acoustic cues such as vocalizations (Sousa-Lima et al., 2002) and/or chewing sounds) (Kikuchi et al., 2014). Simultaneously, an omnidirectional hydrophone (Ocean ear mini, High Tech, Inc., EUA) with frequency responses between 20 Hz–20 kHz was used during the parallel transects to detect manatee sounds in loco based on the first author's previous experience with studies of the species in captivity (Kikuchi et al., 2014). Manatee sound is particular and easily distinguished from that of other aquatic organisms.
During the visits, both methods (active visual search and parallel transects) were used for one hour at each sampling site, without control of the travelled distance, executed in absolute silence to avoid animal dispersion. For every manatee detected, sampling method was recorded along with date, time and geographical position.
Statistical analysisManatee occupancy was investigated using a maximum likelihood approach. To test the effect of environmental variables on manatee detection (ρ, species’ probability of detection in a given location, conditioned by its occupancy), the following variables were used: mean depth (Depth); mean water transparency (Transp); perimeter of sample site (Peri); cover (Cover) and composition (Comp) of aquatic macrophytes at the margin; weather condition (Clim) and river state (River) for each visit in the study site.
Predictive variables used to explain manatee occupancy (ψ, species’ probability of occupying a given location) included non-linear distance to the nearest community (DistCom) and non-linear distance to the nearest protection zone (DistProt), both calculated as shortest distance between points. These metrics were calculated in Quantum GIS software. The categorical variable Habitat, defined as várzea or ria lake, was used to model occupancy and detection probabilities. All variables were selected from literature (Marmontel et al., 2012).
To estimate manatee occupancy and detection probability, a single-season occupancy model was used, assuming a constant ψ among sampling within sites. Manatee records were converted into a binary matrix of detection (1) and no detection (0) for each visit. Analysis was conducted in the PRESENCE 12.1 software (MacKenzie et al., 2002; Hines, 2006).
Method comparisonTo compare detection probability for direct and indirect methods, datasets were analyzed using an independent and combined approach (Bailey et al., 2007; Mattfeldt and Grant, 2007). In the independent approach, detection probability was modelled as a function of habitat (várzea or ria lakes) and method (direct or indirect), while occupancy was kept constant (model ψ [.], p [Habitat + Method]). This approach was used to identify potential detectability biases associated with habitat and the sampling method (Mattfeldt and Grant, 2007). In the combined approach, detection probability was modelled combining datasets, in which two or more recordings of the species, in the same sampling area during a visit, were considered a single detection in the presence/absence matrix.
Amazonian manatee occupancy estimate and detection probabilityPreviously, spearman correlation was used to test for significant correlation between variables. Statistical analysis was done using vegan package in R software. Continuous variables were z-normalized to standardize different metrics before modeling occupancy (Mackenzie et al., 2006). A 2-step approach was used to model manatee occupancy (Negrões et al., 2010; Sarmento et al., 2011). In the first step, occupancy was kept constant to test the effect of sampling variables on manatee detection probabilities (ψ[.], p[variable]). In the second step, the best-fit model in step one’s was combined to the predictive variables of species occupancy (Habitat, DistCom and DistProt) (Table 2). A null model (ψ and ρ kept constant), and a global model (occupancy variables) were also included in model comparison (Burnham and Anderson, 2002). Additionally, the best variable of detection in the first step was also considered affecting the occupancy.
The Akaike’s Information Criteria (AIC) was used to select the best models. Values ΔAIC ≤2 were used to select the model that better fit the data and their variables were considered the most important to estimate the species’ ψ and ρ. The AIC weight (ω) was used to determine the relative importance of each variable in the model. As no models obtained ωi ≥ 0.9, final species occupancy was estimated by model averaging values of β coefficients of the predictive variables from selected models. The 95% confident intervals were analyzed to determine if those crossed zero (Burnham and Anderson, 2002).
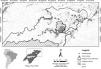
ResultsEfficiency of sampling methods in the manatee detection estimativeOverall, in 265 h of observations, 91 manatee detections (Table 1) were noted around the 33 sites. Detection probabilities were estimated at 0.33 (SD = 0.05) and 0.25 (SD = 0.04) for the indirect and direct methods respectively, using the independent approach. Detection probability estimates obtained using the indirect sampling method were significantly higher in várzea (p = 0.47, SD = 0.06) than in ria lake habitat (p = 0.21, SD = 0.05). Following the same trend, the detection probability estimate modelled using the direct sampling method was higher in várzea than in ria lake. Therefore, the ψ and p of T. inunguis in both habitats would be better modelled by combining the presence datasets from both sampling methods (combined approach). This produced more substantial estimates of detection probability and improved model convergence. As such, the combined dataset was used from this point forward.
Effort and number of manatee evidences by method and habitat in the study site.
| Sampling method (Evidence) | |||||||
|---|---|---|---|---|---|---|---|
| Habitat | Active search (Direct evidence) | Parallel transect (Indirect evidence) | |||||
| n = 34 | n = 57 | ||||||
| Number of sightings | Effort (hours) | Fresh scar | Feces | Acoustic | Effort (hours) | Total evidences per habitat | |
| Floodplain lake | 12 | 64 | 42 | 6 | 3 | 65 | 63 |
| Ria lake | 22 | 68 | 0 | 3 | 3 | 68 | 28 |
| Total | 34 | 132 | 42 | 9 | 6 | 133 | 91 |
Model selection results. Amazonian manatee occupancy was modelled as a function of occupancy variables (ψ) and the variable that better predicted detection probability (Cover - Macrophyte cover). Only the best fit models (ΔAIC ≤ 2) were used to make inferences over biological significance of results.
| Models | K | AIC | ΔAIC | ωAIC | Naive | Ψ (SD) | p (SD) |
|---|---|---|---|---|---|---|---|
| 1. Ψ(DistCom) p(Cover) | 4 | 156.97 | 0.00 | 0.25 | 0.80 (0.08) | 0.50 (0.05) | |
| 2. Ψ(DistProt) p(Cover) | 4 | 157.83 | 0.87 | 0.16 | 0.86 (0.10) | 0.48 (0.05) | |
| 3. Ψ(Habitat) p(Cover) | 4 | 158.32 | 1.36 | 0.12 | 0.99 (0.26) | 0.47 (0.05) | |
| 4. Ψ(Habitat + DistProt) p(Cover) | 5 | 158.53 | 1.57 | 0.11 | 0.84 (0.09) | 0.49 (0.05) | |
| 5. Ψ(Habitat + DistCom) p(Cover) | 5 | 158.59 | 1.63 | 0.11 | 0.81 (0.08) | 0.50 (0.05) | |
| 6. Ψ(DistCom + Cover) p(Cover) | 4 | 158.93 | 1.96 | 0.09 | 0.82 (0.08) | 0.50 (0.05) | |
| 7. Ψ(Cover) p(Cover) | 4 | 159.87 | 2.90 | 0.05 | 0.82 (0.08) | 0.46 (0.05) | |
| 8. Ψ(Habitat + Cover) p(Cover) | 5 | 160.29 | 3.32 | 0.04 | 0.81 (0.09) | 0.49 (0.05) | |
| 9. Ψ(Habitat + DistCom + DistProt + Cover) p(Cover) | 7 | 162.56 | 5.60 | 0.01 | 0.93 (0.12) | 0.50 (0.05) | |
| 10. Ψ(.) p(.) | 2 | 172.40 | 15.43 | 0.00 | 0.79 (0.08) | 0.47 (0.05) | |
| Model-averaging | 0.72 | 0.84 (0.12) | 0.48 (0.05) |
Note: Analysis considering the combination of direct and indirect methods. K: number of parameters; ΔAIC: difference between AIC values of each model in relation to the model with lower AIC value; ωAIC: model weight; Naïve: gross occupancy; ψ: occupancy estimate; p:detection probability; SD: standard deviation. DistCom = Nearest distance to the community; DistProt = Nearest distance to the protection zone; Habitat = Type of habitats (flooded forest “várzea” and ria lake); Cover = Macrophyte cover; Bold values = Final species occupancy.
T. inunguis was detected in 24 of the 33 sample sites, totalizing an occupancy value (naïve) of 0.72. In the analysis’ first step, the best model included the variable macrophyte cover (Cover) (ωAIC = 0.87) witch positively influenced manatee detection. The second step’s most parsimonious model was (ψ[DistCom], p[Cover]) (Table 2).
DistCom coefficient indicates a negative relationship between non-linear distance to the nearest community and manatee occupancy. Also, Cover and DistProt are negative correlated, but no Cover and DistCom. Nonetheless, six models with ΔAIC ≤ 2 showed a total AIC weight within 90%. As such, manatee ψ was dependent on all variables contained in these models (Cover in ρ and DistCom, DistProt, Habitat and Cover in ψ). The second-best model (ψ[DistProt], p[Cover]) suggests a negative effect of distance to protection zones and manatee occupancy. This is likely due to the negative correlation between DistProt and DistCom (rho = 0.47, p < 0.001), mainly influenced by the ria lakes sampling points (rho = -0.92, p < 0.001), where farther areas from communities showing the lesser protection zones. In the second step, although Habitat was present in three of the six top models, showing a AIC weight of 0.34, its importance to manatee occupancy remains unclear due to uncertainty around occupancy estimation.
No model alone had ωAIC ≥ 0.9. Therefore, manatee occupancy’s total value was calculated through model averaging. Final occupancy estimates (ψ = 0.84, SD = 0.12) represent an 17% increase in the species’ naïve occupancy (Table 2). The sum of weights (ωAIC) from most parsimonious models was used to make inferences about the species’ occupancy. The sum of ωAIC among models with the variable macrophyte cover was 0.84, indicating a 84% probability of it being the most important variable in predicting ρ for T. inunguis. Accordingly, the α coefficient estimate for macrophyte cover did not overlap zero (Table 3). The variable DistCom presented the higher importance to manatee occupancy, weighing 45%. Although the β estimated by model averaging superimposed zero, DistCom influence ψ. Habitat and DistProt showed a relative importance to influence the manatee occupancy. However, the β coefficients overlapped zero, indicating that these variables are not good predictors of ψ. Also, Cover variable affecting the species' occupancy corresponded only to the sixth best-model (Table 3).
Estimate of beta coefficients (β) are presented in logarithmic scale as result of the Model averaging. Confidence interval of the variables, considering the sum of models. Sum of weights (ω) = Sum of weight (ωAIC) of the variable between the best fit models (ΔAIC ≤ 2) for the manatee occupancy (ψ)**.
| Variables | Intercept | B DistCom | B1 DistProt | B2 Habitat | B3 Cover | α Cover |
|---|---|---|---|---|---|---|
| Model 1 | 1.40 [0.47; 2.70] | −0.99 [−2.21; 0.003] ¿ | 0.87 [0.41; 1.40]* | |||
| Model 2 | 1.84 [0.61; 5.83] | 1.10 [−0.11; 4.77] | 0.95 [0.49; 1.48]* | |||
| Model 3 | 2.31 [1.72; 6.86] | −6.37 [−3.13; 0.65] | 1.00 [0.54; 1.52]* | |||
| Model 4 | 1.73 [0.86; 8.80] | 3.30 [−1.02; 6.90] | 2.36 [−0.71; 5.05] | 0.89 [0.42; 1.42]* | ||
| Model 5 | 1.50 [0.51; 5.84] | −0.94 [−2.18; 0.002] ¿ | −0.33 [−4.36; 0.72] | 0.89 [0.42; 1.42]* | ||
| Model 6 | 1.48 [0.50; 2.95] | −0.99 [−2.25; 0.12] | −0.28 [−1.42; 0.72] | 0.89 [0.42; 1.41]* | ||
| Model 7 | 1.51 [0.51; 3.40] | −0.50 [−1.88; 0.47] | 0.93 [0.46; 1.46]* | |||
| Model 8 | 6.76 [0.66; 18.25] | −5.96 [−25.26; 0.79] | 0.14 [−1.50; 1.68] | 1.00 [0.54; 1.52]* | ||
| Model 9 | 1.47 [0.47; 6.78] | −0.49 [−2.26; 1.14] | 0.79 [−3.22; 15.30] | −0.23 [−9.67; 7.19] | 0.46 [−2.08; 2.94] | 0.85 [0.17; 1.51]* |
| Model averaging | 1.70 [0.13; 3.27] | −0.98 [−2.03; 0.006] ¿ | 2.00 [−1.59; 5.61] | −1.63 [−9.57; 6.31] | 0.34 [−1.72; 1.45] | 0.92 [0.42; 1.41]* |
| Sum weights (ω) | 0.45 | 0.27 | 0.34 | 0.19 | 0.84 |
*Indicates the coefficients of the confidence intervals of β that do not overlap with zero. [¿] Indicates the coefficients of confidence intervals of β that suggest a trend. **Analysis considering the combination of direct and indirect methods. β = value of beta coefficient for distance of community; β1 = value of beta coefficient for distance of the protection zone; β2 = value of beta coefficient for habitat type; β3 = value of beta coefficient for macrophyte coverage. α = beta coefficient value for macrophyte coverage in detection. Values in square brackets are the 95% confidence intervals.
Amazonian manatee detection probability in the wild had never been previously estimated. This study shows that the species was not always detected even though it could have been present in the sampling area. Surprisingly, T. inunguis probability detection was high (p = 0.50, SD = 0.05), despite cryptic species or with low population density usually being less likely detected (Mackenzie et al., 2002; Mackenzie and Royle, 2005). Macrophyte coverage was highly relevant in manatee detection probability. This variable reduced error associated with estimates and increased ρ in sampling areas where Cover was more abundant. It is beyond results here presented to directly explore the relationship between detection probability and population size.
Depth was not relevant to detect manatee presence. Deeper sites are related to be the main factor affecting sightings and habitat use of Amazonian manatees in low-water season (Rosas, 1994; Arraut et al., 2010; Marmontel et al., 2012). During this study, the water level was significantly different between months (Kruskall–Wallis H(3,N = 132) = 72.337, p < 0.001), and above the expected level, with the highest average recorded in August (9.8 ± 1.6 m) and the lowest in October (5 ± 1.6 m). This phenomenon may have favored individuals’ movement along all the region’s available habitats independently of depth. Therefore, a lack of relevance of depth to predict manatee detection observed here may be related to the study’s temporal extension. It is possible that depth will have greater effect to predict manatee detection in years with more severe droughts, or in larger temporal scales.
Arraut et al. (2010) using 10 radio-tagged male manatees, showed a migratory route between a floodplain lake (Mamirauá Reserve) and a ria Lake (Amanã Reserve), in which the tracked animals did not remain in the várzea habitat during the dry season. The authors suggests ria lakes as the main refuge for T. inunguis in the dry season. However, our results show that manatees are also occupying floodplain lakes during low water season in Piagaçu-Purus Reserve. Even with similar landscapes between the studies, other effects such as the largest distance for the ria lake and appropriate conditions (for example, largest lakes and reduced risk posed by humans) can maintain the animals in the várzea during dry season. According the perception of former manatee hunters living in the Piagaçu-Purus Reserve, the species remains during all phases of the flood pulse in the floodplain lakes, with individuals making short daily displacements between the main river channel and floodplain lakes (Souza, 2015). Out of 16 released and monitored manatees, only 18% (n = 3) moved to the ria lake during the dry season (Diogo Souza, com. pess., 2020), corroborating the local ecological knowledge of former manatee hunters (Souza, 2015).
The final manatee occupancy value increased the estimated occupancy by 17% (ψ = 0.84), suggesting an appropriate selection of the ecological variables and samples sites. In this study, DistCom was used as indicator for anthropogenic disturbance caused by manatee hunting historical locations, traffic of motorized vessels, and commercial fishing. Also, game species occurrence and abundance tend to decrease with proximity to human settlements, where hunting activities are generally concentrated (Robinson and Bennett, 2000; Peres and Nascimento, 2006). However, in contrast with previous sirenians studies (Lefebvre et al., 2001; Miksis-Olds et al., 2007; D’Souza et al., 2013) our results suggest that residents’ activities did not affect manatee occupancy. According to residents of PP-SDR, rates of T. inunguis hunting dropped in the last decades (Souza, 2015), which could partially explain these scenarios.
From a broader perspective, our results indicate that the best habitat for the Amazonian manatee includes important characteristics for the establishment of human communities. For instance, evidence strongly suggests that the acquisition of protein (Gross, 1975) and the availability of high-fertility land (e.g. várzea), along with strategic occupation in areas with rich springs (e.g. lakes and deep ponds) are extremely important factors for the settlements of the Amazon’s inhabitants (Meggers, 1992; Pantoja, 2005; Cruz, 2007). In addition, high manatee occupancy closer to communities may be associated with these areas’ greater connectivity to other water bodies, as well as proximity to the Purus River’s main channel. Occupying these areas benefits individuals by reducing geographic isolation and increasing potential mobility during the dry season, therefore increasing manatee survival in severe drought events in the Amazon such as with El niño (Malhi et al., 2008; Marengo et al., 2008; Marmontel et al., 2016).
The lower probability of manatee occupancy within and around protection zones of PP-SDR corroborates the former local manatee hunters, who reported absence or very low historical hunting events before the PP-SDR’s creation. They also reported that manatee occupancy is less frequent in protection zones (Souza, 2015). The model of aquatic zoning (Protection and Use zones) followed by Sustainable Development Reserves (SDR) is mainly based on the economic importance of fishing resource preservation. Nevertheless, it is still unclear if aquatic zoning of SDRs in the Amazonas state are effectively protecting the Amazonian manatee and other aquatic mammals. Therefore, studies addressing this issue must be conducted considering habitat characteristics, species occupancy patterns, local economic interest and ecological importance of species. In the PP-SDR’s case, more relevant areas should be considered in the Reserve’s management plan for the effective conservation of T. inunguis.
Efficiency of sampling methods to estimate Amazonian manatee’s ψ and pSampling methods differ in their species detection effectiveness (Bailey et al., 2004). The highest manatee detection probability by indirect method for both habitats may be related to the detection of accumulated feeding traces of the species in the wild. Timm et al. (1986) cited that feeding signals are easier to detect than direct T. inunguis sightings. Using the direct and indirect methods independently resulted in different detection and occupancy estimates for similar habitats. This evidence reinforces the importance of including different sampling methods to avoid bias when estimating detection probability of elusive species (Mattfeldt and Grant, 2007).
The present study demonstrated the efficacy of combining methods to investigate Amazonian manatee occupancy. To detect the Amazonian manatee’s presence, it is advisable to combine direct and indirect methods to minimize sampling errors and provide more accuracy to estimate the species’ occupancy. Searching for feeding traces in aquatic plants (n = 42; 73.7%) is an effective indirect method to detect the Amazonian manatee’s presence. In this study, feces samples (n = 9; 15.8%) and acoustic signals (n = 6; 10.5%) were considerably inefficient.
This study used simple and cost-effective methods to detect manatees, based mainly on the skills of experienced former manatee hunters in identifying the presence of the animals using direct and indirect evidences. Although new methodological approaches such as side-scan sonars (Gonzalez-Socoloske et al., 2009; Castelblanco-Martínez et al., 2017), drones (Landeo-Yauri et al., 2020), and e-DNA (Hunter et al., 2018) have emerged in the last decade with capability to study sirenians, their efficiency and accuracy to detect Amazonian manatee is unknown. These methods would be unsuitable and expensive as they require a longer term approach to train the team to use the equipment and analyze the field data. The traditional methods used in this study are effective to detect Amazonian manatee, having been successful to answer our research questions and enable the use of this low-cost methodology in Protected Areas in the Amazon under different financial support scenarios for manatee monitoring.
In contrast to studies reporting index of effort and abundance, spatial and temporal sampling provide systematic data of T. inunguis presence/absence which can be effectively analysed with occupancy models. By including environmental variables that may influence manatee occupancy patterns and by sampling throughout the river’s flood pulse, substantial inferences can be made to confirm detection changes over longer temporal scales. In the future, this kind of study may enable estimates of population recolonization rates and prediction of the species’ local extinction events in PP-DSR.
Amazonian manatee conservation and management implicationsThis is the first study applying occupancy models for T. inunguis showing that aquatic macrophyte cover influences the species’ detection. This approach has promising applications to long-term population monitoring not only for T. inunguis, but for other Amazonian aquatic mammals as well. It also contributes towards the ecological understanding of species by examining the relationships between distribution patterns and habitat characteristics.
Despite historical commercial exploitation that led to drastic reduction of the Amazonian manatee population in the Purus River (Pereira, 1944; Domning, 1982), the high detection and occupancy rates obtained in this study may suggest the species’ recovery within the study area. T. inunguis population recovery after the PP-SDR’s creation was suggested by former manatee hunters interviewed in the area (Souza, 2015). Moreover, genetic studies throughout the Brazilian Amazon found evidence of the species’ demographic expansion (Cantanhede et al., 2005). Results that we found may not reflect the reality of other Protected Areas in the Amazon where zoning rules are not effectively respected.
In the Amazon, SDR zoning plans have been important tools for biodiversity conservation (Arantes and Freitas, 2016; Campos-Silva and Peres, 2016; Campos-Silva et al., 2018). The creation of SDRs represents a governmental attempt to reconcile the resident human populations’ living conditions with the use of resources and biological diversity maintenance (MMA, 2011; Peres, 2011). However, to reach this goal, it is important to understand species’ habitat use so that appropriate conservation strategies can be proposed. Currently, Amazonian manatee conservation is poorly included in management plans and aquatic zoning of reserves in the Amazon. Knowledge about T. inunguis occupancy in PP-SDR can support future management strategies, such as the selection and delimitation of priority areas for the species’ conservation.
Our experience reinforced the importance of local participation during manatee monitoring, particularly former hunters, to ensure the high data quality to detect the species and also engaged local people for conservation studies. Locals residents can provide valuable skills and knowledge in terms of the species’ ecology, and a vested interest in monitoring the species for future managing. Several studies have shown that local people’s participation in monitoring projects can contribute in data collection and research design, as well as to conservation actions (Castello, 2004; Vieira et al., 2015; Campos-Silva et al., 2018).
Finally, our results contribute to meso-scale monitoring projects in the Amazon and highlight the applicability of occupancy modelling for T. inunguis. Occupancy estimates here presented can be used as baselines to future monitoring studies in the region to assess the species’ demographic trends. Our data will also provide support for managers and local communities to establish protection and water-use regulations in areas occupied by manatees, ensuring in the Reserve the effective conservation of this endangered species over time.
Author contributionsDAS, VMFS and EM conceived and designed the study. DAS collected the field data. DAS and ALSG compiled the database of available records and developed the hierarchical models and the spatial and statistical analyses. All authors contributed in the analysis, interpretation of results, and manuscript writing.
Data availability statementThe authors confirm that the data supporting this study’s findings are available within the article [and/or] its supplementary materials. Readers interested in other/further material can request them from the corresponding author [DAS].
FundingThis work was supported by the National Council for Scientific and Technological Development (CNPq) under a Master course scholarship grant for DAS. Financial support to the field work was also obtained from Petrobras Socioambiental (Projeto Mamíferos Aquáticos da Amazônia) and Associação Amigos do Peixe-boi – AMPA.
Competing interests statementThe authors declare no conflicts of interest.
We are especially thankful to riverine communities of Piagaçu-Purus Reserve, particularly the field assistants: José Francisco, Mario Jorge, Roque de Souza and Tiarle Vidinha. In addition, we thank the Aquatic Mammals Laboratory of the National Institute for Amazonian Research (INPA) and Piagaçu Institute (IPI) for their logistical support. We appreciate comments by Julia Dombroski, Kamilla de Souza and Marina Calderón in the early manuscript, and Thais Shepard for the English revision. We would like to thank the reviewers for their thoughtful comments and efforts towards improving our manuscript.