We thank Plumptre et al. (2023) for fostering discussion on the crucial topic of how to best identify priority areas for biological conservation. Our position largely aligns with theirs: we too advocate for effective tools that accurately map critical regions for biodiversity. We are not opposing the Key Biodiversity Area (KBA) initiative and highly commend the organization and its objectives.
Our publication (Farooq et al., 2023) was not an endeavour to identify nor delineate KBAs, but to assess the KBA framework’s sensitivity to specific criteria, notably the presence of unique and threatened species (criteria A and B). We explored and highlighted potential limitations with the existing KBA methodology, especially in the face of expanding biodiversity data and increasing threats to biodiversity. Our focus was to highlight a potential flaw: if all locations fit some of the KBA criteria, then selection might prioritize manageability over inherent biodiversity value.
Plumptre et al. (2023) raise three aspects of our study concerning (1) grid cell dimensions, (2) omission of manageability, and (3) neglect of population sizes. Taken together, the authors suggest that our analytical approach risks overestimating the number of potential KBAs. The initial two concerns, however, stem from misconceptions arising from our text. We acknowledge any ambiguity or lack of clarity in our publication and briefly clarify our approach below.
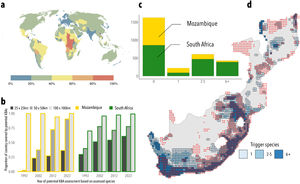
The dimensions of grid cellsWe agree that the smallest grids we used are larger than most KBAs (which we also showed in our paper) but KBAs of that size are not rare. In fact, countries such as Mozambique and South Africa have roughly 40% of their KBAs exceeding that size, and a third of all African countries surpass such a threshold (Fig. 1a), with some – like the Republic of Congo and Chad – having all of their KBAs exceeding it (BirdLife International, 2023). The fact that South Africa identified a greater number of potential trigger species than Mozambique (6539 compared to 180) (Plumptre et al., 2023), leading to more areas designated as KBAs, underscores our argument that more biodiversity surveys and Red List evaluations will result in an increase of species triggering KBAs. We also applied our methodology to identify potential KBAs using available IUCN assessments for the years 1992, 2002, 2012 and 2022, to show that the more species are assessed, the higher the proportion of Mozambique and South Africa that is triggered by the biological criteria of the KBA guidelines – a monotonic pattern that is robust across different grid sizes (Fig. 1b). Assuming that the trend identified across the range of cell sizes surveyed remains similar even for smaller KBAs, the approach in Farooq et al. (2023) was effective in identifying potential KBA locations and lends support to our conclusions.
An empirical evaluation of our approach in identifying potential Key Biodiversity Areas. a. Proportion of currently designated Key Biodiversity Areas exceeding the smallest grid size used in Farooq et al. (2023), corresponding to an area of approximately 625 km2. b. roportion of Mozambique and South Africa covered by potential KBAs based on potential KBA assessments using IUCN assessments available at four different years and at three different grid sizes. c. Number of cells triggered by different species. d. Visualization of the grid cells obtained from our methodology, coloured by the number of species triggering each cell and overlapped with a red dot whenever a real KBA occurs in such grid cell. Very few cells (18%) are triggered by a single taxon, which suggests that our analysis identifies KBAs reasonably well despite its simplifications of real-world conditions.
Rather than suggesting that most of the world could become KBAs, we suggested in Farooq et al. (2023) that most of the world fulfils the biodiversity criteria for becoming KBAs – a subtle but important difference. We defined such “potential” KBAs as “grid cells that can in theory trigger KBA status for criteria A1a), b), e) or B1 (the biological criteria)” and emphasised that, if so, manageability might become the primary determinant for KBA designation. Plumptre et al. (2023)’s assertion that we suggested that entire grid cells could be designated as KBAs is incorrect. Instead, we highlighted the risk of future KBA selections prioritizing manageability over biodiversity, potentially neglecting areas of high biological significance.
KBA criteria and population sizesPlumptre et al. (2023)’s third point about species population sizes seems to derive from the authors’ disagreement with the simplified assumptions we made in our exploratory analyses. We assumed an even distribution of individuals across the range of a species – a simplification of reality for the sake of standardising our analyses. Under this assumption, for the population of a wild species to not meet the reproductive units or adult numbers set in the criteria A1a and A1b, it would have to comprise fewer than 1000 individuals across the species’ entire range, or 100 individuals for criterion B1. Based on our experience, we consider this unlikely for most species. Even using mammals of the order Carnivora as an extreme example of low sparse population density – on average c. 0.01 individuals/km2 (Santini et al., 2022) – there would still be an average of 6.25 individuals in our smallest grid cell. This represents an exceptional case in our dataset – as carnivores only make up a small fraction of the species we analysed. These considerations suggest that Plumptre et al.’s third point, although possibly correct for some species and regions, should have a minor influence on our overall results and conclusions.
Fit to realityTo further explore the real-world significance of our findings – an overall concern in Plumptre et al. (2023) – we evaluated the ability of our statistical approach in Farooq et al. (2023) e actual KBAs (BirdLife International, 2023). As examples, we replicated the analysis for Mozambique and South Africa, the two countries highlighted in Plumptre et al. (2023). We found (1) similar percentages between our predicted grid cells and the cells with established KBAs: 27% versus 31% in Mozambique and 61% versus 40% in South Africa (Fig. 1c); (2) a significant positive association (p < 0.0001) between the number of species triggering cells and the presence of an actual KBA. Furthermore, over 60% cells in Farooq et al. (2023) were triggered by multiple species, implying that individual inaccuracies in our methodology should be offset by the extensive species count (Fig. 1d); and (3) our method effectively distinguished between KBAs and non-KBAs (AUC-ROC score: 0.68) while also maintaining a balanced accuracy in pinpointing KBAs (F1 score: 0.74) (see analysis in the Supplementary material).
Taken together, these results suggest that our approach in Farooq et al. (2023) was effective in identifying potential KBA locations, and lend support to our conclusions.
ConclusionsOne of our main points in Farooq et al. (2023) is that our maps were generated using less than 1% of the species expected to occur on Earth. On average, about 50 new species are scientifically described each day (Wheeler and Pennak, 2011). As more species are documented, our analyses indicate that the current KBA methodology as currently designed could start to fall short of its overall goals, because more species will trigger KBA status in nearly any new area thoroughly surveyed for its biodiversity. Furthermore, due to habitat loss, climate change and other threats to biodiversity, more species that do not currently trigger KBA status could over time become threatened and start triggering KBAs in new locations.
The take-home point of our study, which unfortunately was not addressed in Plumptre et al. (2023) critique, was that “as more species are considered when delineating KBAs, more territory meets the KBA biological requirements – a process that could continue to an extent where the biological features are no longer relevant, and manageability becomes the only factor determining whether an area should be a KBA.” We believe this conclusion remains strong and well-evidenced.
We hope the KBA secretariat could consider revising the KBA guidelines to ensure it becomes an even more robust and future-proof methodology that guides conservation in an increasingly data-rich and rapidly changing world.
FundingH.F is supported by The Danish Independent Research Council (grant no. 0165-00018B).
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.