A promising, but also controversial approach to ecological restoration is trophic rewilding, i.e., species introductions to restore top-down trophic interactions and associated trophic cascades to promote self-regulating biodiverse ecosystems. To provide historically-informed base-lines for trophic rewilding in the Neotropics, we aggregate data on late-Quaternary (last 130,000 years) large-bodied (megafauna, here: ≥10kg body mass) mammals to estimate two base-lines: megafaunas including historically (post-1500 AD) extinct species and accounting for regional extirpations of extant species (historic base-line), and megafaunas additionally including Late Pleistocene-Holocene prehistorically extinct species (prehistoric base-line). The historic base-line is less controversial, while the prehistoric base-line is more relevant from an evolutionary, long-term perspective. The estimated potential distributions indicate strong scope for trophic rewilding, with high levels for the prehistoric baseline (with >20 species missing in many regions and biomes), but also considerable values for the historical baseline. Many areas have strongly reduced diversities for a range of functional and phylogenetic subgroups. We discuss implications, highlighting the need for a more nuanced view on non-native megafauna species as they may sometimes represent taxon substitutions for missing species. We emphasize that trophic rewilding should be implemented flexibly and in dialogue with society, e.g., handling human–wildlife conflicts and ensuring benefits for local livelihoods.
Human impacts on ecosystem are intensifying, with increasing biodiversity losses and risk of 6th mass extinction as one of the major consequences (Barnosky et al., 2011). To overcome this challenge, there is a strong need to take a multifaceted approach to make best use of all possibilities to promote biodiversity conservation and restoration in the face of sustained human population growth and resource use. Optimistically, it appears clear that ecological restoration has strong potential to at least partially restore biodiversity and ecosystem functioning (Benayas et al., 2009). One recent, controversial approach to restoration goes under the term rewilding, and broadly speaking covers approaches to ecological restoration and conservation management that aim to promote self-regulating ecosystems (Sandom et al., 2013; Corlett, 2016; Svenning et al., 2016), i.e., with no or reduced needs for management. Importantly, rewilding if often focused on ‘future wildness’, not on recreating the past (Corlett, 2016).
The most widely considered version of rewilding is trophic rewilding, i.e., species introductions to restore top-down trophic interactions and associated trophic cascades to promote self-regulating biodiverse ecosystems (Svenning et al., 2016). This approach is inspired by increasing evidence (1) that top-down effects of carnivores and herbivores are often important for ecosystem functioning and biodiversity maintenance, (2) that large-bodied species (megafauna) have large ecological effects (Pedersen et al., 2017; Owen-Smith, 1988; Ripple et al., 2014, 2015), due to characteristic such as greater mobility and associated ability to disperse propagules and nutrients (Doughty et al., 2016b,c), and, sometimes, escape from top-down control themselves (Owen-Smith, 1988; Hopcraft et al., 2010), and (3) that megafauna species have been particularly subject to anthropogenic declines (defaunation) (Martin, 1967; Ripple et al., 2014, 2015; Sandom et al., 2014). In the Neotropics, there is little tradition for trophic rewilding, but several recent papers have called for it (Root-Bernstein et al., 2017; Galetti et al., 2017a). Trophic rewilding is defined to involve introductions (Svenning et al., 2016), but trophic restoration may sometimes be achieved via spontaneous wildlife comebacks (so-called passive rewilding) or via promoting recolonization, e.g., via corridors (borderline between trophic and passive rewilding).
Human impacts are now so pervasive and irreversible that historical baselines are often not feasible or even desired to reach, and future-oriented goals adjusted to the Anthropocene are needed (Corlett, 2016). Still, even under such a forward-looking agenda interventions should be historically informed, using historically knowledge to guide aims and actions. Notably, regional species pools will have evolved over long time scales, and the biotic and abiotic conditions over these time scales will reflect the environmental conditions to which these pools are adapted, hence inform us on the conditions that have allowed biodiversity to build up and be maintained (Corlett, 2016). This perspective links to the aspect of trophic rewilding that has been most controversial, namely Pleistocene rewilding, which proposes to look beyond recent historical base-lines to also consider Late Pleistocene and early Holocene faunas (ca. 5000–50,000 years BP) (Galetti, 2004; Donlan et al., 2005; Donlan et al., 2006). This idea received much discussion for North America and was based on the realization that the recent historical megafauna is massively impoverished relative to Pleistocene, with the losses linked to the immigration of Homo sapiens into the region (Donlan et al., 2005, 2006). There is still debate over relative role of climate and humans in driving these losses, but we believe the overall case for a dominant human role is clear (Sandom et al., 2014). However, this deeper-time baseline does not necessarily depend on whether the extinctions were anthropogenic, as a key point is that current biota has evolved under much more megafauna-rich conditions than historical base-lines and restoring these may promote greater capacity for biodiversity, including stopping likely ongoing slow declines of megafauna-dependent species (Doughty et al., 2016c). 5000 or 10,000 years is a considerable amount of time, but in terms of biota and their adaptations it is actually a short period, with most macroscopic species likely much older (e.g., Rull, 2008; Fernandes et al., 2014). Furthermore, it is important to note that the rich pre-extinction Pleistocene fauna represents a functional faunal state that has been typical across millions of years (e.g., Stegner and Holmes, 2013). Restoration of extinct species would have to be via functional analogues (Galetti, 2004; Hansen et al., 2010; Svenning et al., 2016), an aspect that has been particularly controversial (Oliveira-Santos and Fernandez, 2010), and careful risk assessment should always be done (Svenning et al., 2016).
The Pleistocene perspective on rewilding is highly relevant for the Neotropics, which is one of the regions that have experienced the strongest prehistoric late-Quaternary losses, having extremely rich megafaunas (as rich or richer than Africas, e.g., proboscideans, numerous ground sloths and giant armadillos, horses, rhino-like endemic ungulates, and giant camelids (Sandom et al., 2014; Faurby and Svenning, 2015b) through the Pleistocene, with losses starting some 12,000–15,000 years BP, but many taxa persisting until 7000–9000 years BP (Coltorti et al., 2012; Hubbe et al., 2013; Prado et al., 2015; Ubilla et al., 2017), i.e., well into the Holocene, and the coyote-sized canid Dusicyon avus surviving to within the last 500 years (Prevosti et al., 2015). Some extant species formerly had much wider ranges, e.g., Chacoan peccary (Catagonus wagneri) (Gasparini et al., 2012) and vicuña (Vicugna vicugna) (Weinstock et al., 2009). The latter range contractions may sometimes be linked to climate shifts, but this is not always obvious.
To provide historically-informed base-lines (sensu Corlett, 2016) for trophic rewilding in the Neotropical region, we here aggregate data on late-Quaternary (last 130,000 years) large-bodied (megafauna, here: ≥10kg body mass) mammals for the region: taxonomy, current and estimated present-natural distribution (distributions in the absence of any influence of humans, but adjusted to modern climate and other natural environmental conditions) (Faurby and Svenning, 2015b), diet (extant species following Kissling et al. (2014); extinct species manually scored for this study), and body size (Faurby and Svenning, 2016). We focus on two spatially explicit present-natural base-lines, namely megafaunas including historically (post-1500 AD) extinct species and accounting for regional extirpations of extant species (historic base-line), and megafaunas additionally including Late Pleistocene-Holocene prehistorically extinct species (prehistoric base-line, last 130,000 years). Both base-lines can be relevant to consider, with the historical base-line less controversial in general (Galetti et al., 2017a), but the prehistoric base-line more relevant from an evolutionary and long-term perspective and for this reason likely to have greater biodiversity benefits (Svenning et al., 2016). We provide estimates of current and present-natural species richness overall as well as for carnivores and herbivores and major body size subgroups, and for select important phylogenetic groups, separately. Based hereon, we estimate trophic rewilding potential as the deficit in species within a given group in the present compared to a given base-line.
Materials and methodsStudy areaThe study region is the Neotropical realm following Olson et al. (2001), but excluding the tip of Florida due to its geographic separation from the remaining region. Islands are not included in the mapping due to their disharmonic biota.
MethodsWe followed the taxonomy from Faurby and Svenning (2015a) combined with the ranges from Faurby and Svenning (2015b) using version 1.2 of both (available at http://bios.au.dk/en/about-bioscience/organisation/ecoinformatics-and-biodiversity/data/). In order to be conservative this taxonomy only includes species with reliable dates within the Late Pleistocene or Holocene. Different methods for range construction were used for different species depending on available data. Extant species ranges were generally reconstructed by a combination of info on historical range and ecological modeling based on info on historical range and climate in the current range. The ranges of extinct species were generally reconstructed based on a co-occurrence approach where likely current range was estimated based on the current range of the species that the extinct species co-existed with in fossil assemblages. Ranges were estimated at an approximate resolution of 110km×110km. For further details see Faurby and Svenning (2015b). Detailed species information is provided in Supplementary Table 1.
Following Owen-Smith (2013), we defined three body size groups for herbivores, mesoherbivores (10–99kg), macroherbivores (100–999kg), and megaherbivores (≥1000kg). Megaherbivores, due to their size, are generally not affected by top-down control, but only limited by resource supply and abiotic stresses, meaning that they can have strong effects on ecosystems (Owen-Smith, 1988). Macroherbivores can also have strong effects, and especially the larger species may not always be controlled by top-down predation, but this depends on environmental conditions and landscape settings (Hopcraft et al., 2010). Although often limited by predation, mesoherbivores can also have strong vegetation effects (van der Plas et al., 2016), with these sometimes shaped by interaction with larger herbivores (Lagendijk et al., 2015). Analogously, we also define three body size groups for carnivores (vertebrate eaters), mesocarnivores (10–21.4kg), macrocarnivores (21.5–99kg), megacarnivores (≥100kg). The 21.5-kg limit separate species that generally kill prey smaller than themselves from species that regularly kill prey larger than themselves (essentially megafauna) (Carbone et al., 1999). Megacarnivores separate out those carnivores that may prey on large macroherbivores and juvenile megaherbivores, and that may structure the rest of the carnivore community via top-down effects (Ripple et al., 2014). We considered species as carnivores if vertebrates constituted a major part of their diet, while herbivores were defined as species having plant material as a substantial part of their diet. Species solely eating invertebrates like giant anteater (Myrmecophaga tridactyla) were therefore not classified to any of these categories. Some species that did not have vertebrates or plants as the dominant food items were nevertheless included in one of the two categories, if either food category nevertheless had intermediate dietary importance, e.g., the primarily invertebrate-feeding giant armadillo (Priodontes maximus) which is reported to also include small vertebrates in its diet (Kissling et al., 2014).
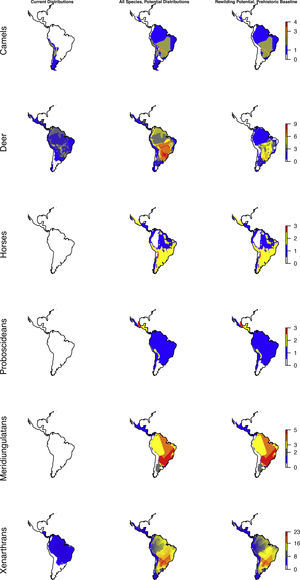
The highly diverse herbivore megafauna can be divided into a number of phylogenetic subgroups, representing different herbivore functionalities. Here, we considered the following select fully or largely herbivorous clades in more detail: camelids (Camelidae, guanacos and relatives), deer (Cervidae), horses (Equidae, with two genera represented Equus and the endemic Hippidion), elephant relatives (Proboscidea, gomphotheres, with mammoths and mastodons in the extreme north), the endemic Meridiungulata group (including rhino-like toxodonts and camel-like litopterns), and, finally, Xenarthra (including sloths, anteaters, and armadillos, hereunder a diversity of large to giant sloths and armadillos, including glyptodonts, but also a few insectivorous forms like giant anteater and the extinct armadillo genus Propraopus).
For the overall megafauna as well as each functional group and clade we mapped three diversities, (1) current diversity, (2) historical baseline diversity (only including estimated ranges of any species surviving with the Neotropics up to at least 1500AD), and (3) pre-historical baseline diversity (including estimated natural range for any species that has occurred within the Neotropics at any time within the last 130,000 years). We also calculated two rewilding potentials defined as the difference between the contemporary diversity and each baseline.
In order to assess the variation in rewilding potential between the region's major habitat types we also calculated rewilding potential based on either the historical or pre-historical baselines for each biome following the classification from (Olson et al., 2001).
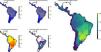
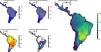
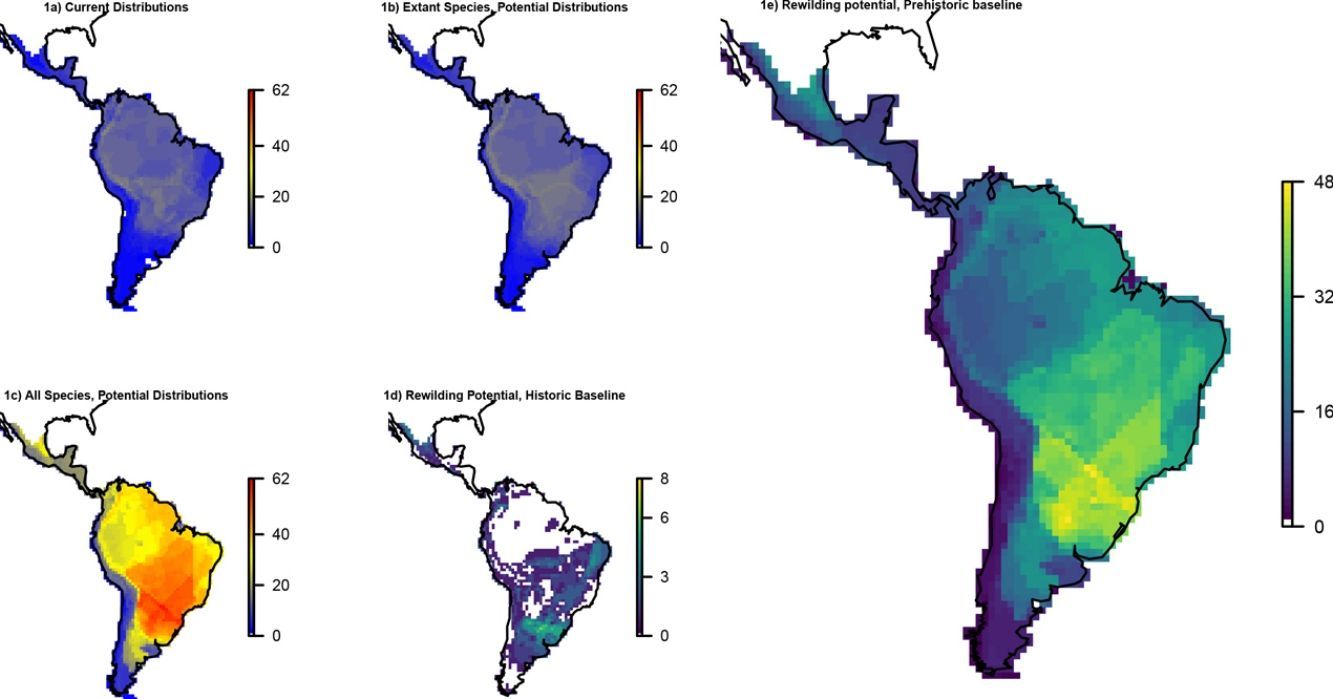
ResultsOverall patternsThe estimated present-natural distributions for megafauna species indicate high potential for trophic rewilding across all of the Neotropics (Fig. 1). This is always very high throughout the region for the prehistoric base-line estimates, which includes the numerous extinct species from the latest Pleistocene and early Holocene, with especially high potential in the region spanning eastern Brazil to northern Argentina (Fig. 1e). The extinct or extirpated species with large potential ranges in the region include several proboscideans, horses (including modern horse, Equus ferus), camelids, large deer, large canids, and ground sloths, as well as many other species (Table 1). However, it is also sizeable in many regions for the historical base-line estimates (Fig. 1d). The latter reflects large range contractions in species such jaguar (Panthera onca), marsh deer (Blastocerus dichotomus), and pampas deer (Ozotoceros bezoarticus) and well as more limited range contractions in other species.
Megafauna mammal species (body size ≥10kg) richness patterns across the Neotropics: (a) current situation, (b) level including historically (post-1500 AD) extinct species and accounting for regional extirpations of extant species, (c) level also including Late Pleistocene-Holocene prehistorically extinct species, (d) trophic rewilding potential, historic baseline (estimated as (b)–(a)), and (e) trophic rewilding potential, prehistoric baseline (estimated as (c)–(a)).
Status of large Neotropical mammals broken down by diet and size. Carnivores include all species with vertebrates as a major part of the diet, herbivores all species with as a major part of the diet. A few species appear in both lists and a few in neither. Species are listed as carnivores if vertebrates constitute a substantial part of the diet even if it is not the major part and the giant armadillo (Priodontes maximus) is for instance listed as a carnivore even though it main food item is ants because it also eats a moderate amount of herptiles.
| Extinct | Extant | |
|---|---|---|
| Carnivores | ||
| >100kg | Arctodus simusb, Arctotherium tarijense, Panthera atroxb, Smilodon populator, Smilodon fatalisb, Homotherium serumb,c | Panthera onca, Ursus americanusb, Ursus arctosb,c |
| 21.5–99.9kg | Canis dirus, Theriodictis tarijensis | Canis lupusb, Chrysocyon brachyurus, Priodontes maximus, Pteronura brasiliensis, Puma concolor, Tayassu pecari |
| 10.0–21.4kg | Cuon alpinusa,b, Dusicyon avus, Protocyon troglodytes | Leopardus pardalis |
| Herbivores | ||
| >1000kg | Camelops hesternusb, Cuvieronius hyodon, Doedicurus clavicaudatus, Eremotherium laurillardi, Glossotherium robustum, Glyptodon clavipes, Glyptotherium cylindricum, Glyptotherium floridanumb,c, Hemiauchenia paradoxa, Lestodon armatus, Mammut americanumb, Mammuthus columbib, Megatherium americanum, Megatherium tarijense, Mixotoxodon larensis, Mylodon darwinii, Notiomastodon platensis, Palaeolama major, Palaeolama weddeli, Panochthus tuberculatus, Paramylodon harlanib,c, Scelidodon chiliensis, Scelidotherium leptocephalum, Toxodon platensis, Trigonodops lopesi | No species |
| 100–999kg | Arctotherium tarijense, Catonyx cuvieri, Equus ferusa, Equus franciscib, Euceratherium collinumb,c, Eutatus seguini, Glyptodon reticulatus, Hemiauchenia macrocephalab,c, Hippidion devillei, Hippidion principale, Holmesina occidentalis, Holmesina paulacoutoi, Holmesina septentrionalisb,c, Hoplophorus euphractus, Macrauchenia patachonica, Megalonyx jeffersoniib, Neosclerocalyptus paskoenis, Neuryurus trabeculatus, Nothrotheriops shastenseb, Nothrotherium maquinense, Pampatherium humboldti, Pampatherium typum, Platygonus compressusb,c, Tapirus rondoniensis, Tapirus veroensisb,c, Tremarctos floridanusb, Valgipes deformis, Xenorhinotherium bahiense | Lama guanicoe, Tapirus bairdii, Tapirus pinchaque, Tapirus terrestris, Tremarctos ornatus, Ursus americanusb, Ursus arctosb,c |
| 10–99kg | Agalmaceros blicki, Antifer ultra, Arctotherium wingei, Caipora bambuiorum, Capromeryx minorb,c, Catagonus stenocephalus, Diabolotherium nordenskioldi, Morenelaphus brachyceros, Morenelaphus lujanensis, Mylohyus nasutusb,c, Neochoerus aesopi, Neolicaphrium recens, Oreamnos harringtonib, Pachyarmaterium brasiliense, Palaeolama mirificab,c, Paraceros fragilis, Protopithecus brasiliensis, Stockoceros conklingib,c, Tetrameryx shulerib,c | Antilocapra americanab,c, Blastocerus dichotomus, Brachyteles arachnoides, Catagonus wagneri, Chrysocyon brachyurus, Dinomys branickii, Hippocamelus antisensis, Hippocamelus bisulcus, Hydrochoerus hydrochaeris, Hydrochoerus isthmius, Mazama americana, Mazama bororo, Mazama bricenii, Mazama chunyi, Mazama gouazoubira, Mazama nana, Mazama nemorivaga, Mazama pandorab, Mazama rufina, Mazama temama, Odocoileus hemionusb,c, Odocoileus virginianus, Ovis canadensisb,c, Ozotoceros bezoarticus, Pecari tajacu, Tayassu pecari, Vicugna vicugna |
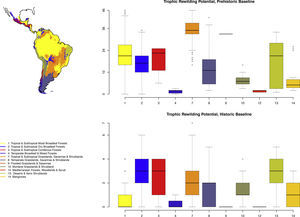
Rewilding potential varies among biomes, with the highest values for Tropical and Subtropical Grasslands, Savannas and Shrublands under the prehistoric base-line, but also generally high for the various tropical and subtropical forest biomes and for deserts and xeric shrublands (Fig. 2).
Boxplots of prehistoric and historic trophic rewilding potential (Fig. 1de) per major biome (following Olson et al., 2001). The median is indicated by a thick line, while the colored boxes delimit the 25% and 75% quantiles. The whiskers show the most extreme points within 1.5 times the interquartile range and the few data points outside this range are shown as small dots.
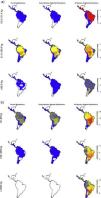
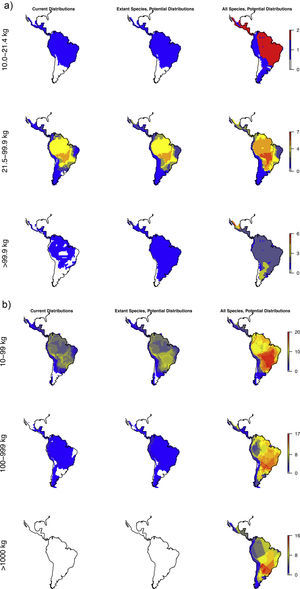
The estimated present-natural distributions show that many areas have reduced diversities of meso-, macro-, as well as megacarnivores relative to the prehistoric baseline, but also reduced diversities relative to the historic baseline (Fig. 3a). Notably, many areas are missing the region's only extant megacarnivore, jaguar (Panthera onca). Similarly, the estimated present-natural distributions show that many areas have greatly reduced diversities of meso-, macro-, and megaherbivores relative to the prehistoric baseline (Fig. 3b). While megaherbivores were diverse for the prehistoric baseline, with up to 16 co-occurring species and no major area without any, there are no megaherbivores were left today or for the historical baseline (Fig. 3b). Both meso- and macroherbivores also have reduced diversities relative to the historical base-line in many areas (Fig. 3b).
Like Fig. 1a,c and d, but separately for the two overall dietary classes, in three size classes each. Left: Carnivores: mesocarnivores (10–21.4kg), macrocarnivores (21.5–99kg), megacarnivores (≥100kg). Right: Herbivores: mesoherbivores (10–99kg), macroherbivores (100–999kg), megaherbivores (≥1000kg) (Owen-Smith, 2013).
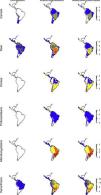
Considering major phylogenetic subgroups of herbivores (camelids, deer, horses, proboscideans, meridiungulates, and xenarthrans (sloths and armadillos)) all exhibit strong prehistoric rewilding potential across large parts of the Neotropics, albeit the exact spatial pattern varies (Fig. 4). Many regions show strong rewilding potential in most groups (Fig. 4), illustrating high deficits in herbivore species and functional diversity.
Species richness patterns across the Neotropics for select, largely herbivorous megafauna phylogenetic clades: Current situation, level in absence of Late Pleistocene-Holocene prehistoric extinctions and extirpations, and trophic rewilding potential (prehistoric base-line, estimated as (c)–(a)).
The estimated present-natural distributions for Neotropical megafauna indicate strong potential for trophic rewilding across all of the Neotropics. The potential is very high throughout the region for the prehistoric baseline estimates (with >20 megafauna species missing in many regions), but it is also considerable for the historical baseline estimates, with ≥3 species missing in much of eastern South America. The latter estimates shows that trophic rewilding with recently extirpated species, as recently outlined with special focus on Brazil's Atlantic forest (Galetti et al., 2017a), has much potential for refaunation defaunated ‘empty’ forests and other ecosystems across the Neotropical region, given favorable societal circumstances (see below). Importantly, the estimated rewilding potential is consistently high in most biomes (Fig. 2). The low values for a few biomes of restricted geographic occurrence may well simply represent uncertainties in estimating megafauna-associations to these areas due to their small size. The relatively low values for historical rewilding potential for Tropical and Subtropical Moist Broadleaf Forests reflect that much of this biome still has preserved most of its historical fauna, e.g., large parts of Amazonia. For the prehistoric base-line, the biome Tropical & Subtropical Grasslands, Savannas and Shrublands – including major savanna regions such the Cerrado, Gran Sabana, and the Chaco – stands out as having particularly high rewilding potential, typically more than 30 species (Fig. 2). Hereby, the prehistoric base-line for the Neotropics is consistent with current megafauna diversity pattern for Africa, with the highest richness is also in the savanna regions (e.g., Faurby and Svenning, 2015b), likely reflecting an overall greater capacity for megafauna in such semi-open habitats.
For carnivores, the prehistoric baseline indicates widespread elevated diversity in the carnivore guilds relative to the present-day and historical situation. Here, the mega-, macro- and mesocarnivores only have one widespread hypercarnivorous species each, namely the jaguar (Panthera onca), cougar (Puma concolor), and ocelot (Leopardus pardalis), respectively, with a few additional more specialized or omnivorous and more narrowly distributed species. The prehistoric baseline includes a high diversity of mega- and macrocarnivores, including many widespread species. These contribute important functional expansions to the carnivore guild, namely massive felids (sabertoothed cats, Smilodon spp.) capable of taking down very large prey (Morales and Giannini, 2014), potentially scavenging medium- to large bears (Arctotherium spp.) (Fiigueirido and Soibelzon, 2010) and a number of pursuit-hunting medium to large canids (e.g., Prevosti and Vizcaíno, 2006)), plus the jackal/coyote-like Dusicyon avus. These findings underscore that the current carnivore communities in the region, even prior to recent defaunation, are impoverished both in species and functionally relative to the longer-term Pleistocene base-line. This impoverishment is likely to have impacts on the functioning of the extant ecosystems via trophic cascades, but with complex effects via interactions with herbivore losses. Hence, carnivore restorations should also consider the herbivores.
The patterns for the herbivores are quite similar to the carnivores, with the prehistoric baseline indicating widespread highly elevated diversity in macroherbivore guild, with less diverse, but ubiquitous megaherbivores and moderately elevated mesoherbivore diversity as well. Notably, the present-day situation and the historical baseline include no megaherbivores. Today, these only occur in parts of Africa and southern Asia. This is a key contrast, given their particular role of ecosystem engineers via strong vegetation effects due to their near-immunity to top-down control by predators, their size-driven broad dietary niche, their intake of large amounts of plant material, and the physical strength associated to their size (Owen-Smith, 1988). Considering the different major herbivore groups, it is clear that the prehistoric baseline represents much greater functional diversity among herbivores throughout the Neotropics beyond simply megaherbivores. Within the megaherbivore group, more than 20 species are recorded from the region, compared to 10 extant species globally, and these come from phylogenetically divergent groups: proboscideans (gomphotheres), large-bodied camelids, some-what rhino-like toxodonts, as well as a large number of giant edentates, ground sloths as well as glyptodonts. These are unlikely to have had equivalent functions. Also considering the smaller herbivores, it is clear that the prehistoric baseline involves higher diversity in a number of groups, likely providing differing functions. França et al. (2015) provide an overview of the feeding ecology of the Late Pleistocene large herbivores in South America, showing a varied representation of browsers, grazers and mixed-feeders among the groups, with at least some species exhibiting broad flexible dietary niches. Among the horses, these were grazers (Equus), similar to extant horses, or mixed-feeders (Hippidion). Likely the most special aspect of the prehistoric baseline is the large numbers of edentates (sloths and armadillos including glyptodonts) among mega- as well as macroherbivores (Vizcaíno and Bargo, 2014). These likely provided functions that partially deviated from any similarly sized extant herbivores, due to their many peculiar characteristics (Vizcaíno and Bargo, 2014). For example, many of these large edentates were good diggers (Vizcaíno et al., 2001), similar to the present 15–80kg Priodontes maximus (giant armadillo), which for this reason is considered an ecosystem engineer (Desbiez and Kluyber, 2013). The high diversity and functional types of large herbivores throughout the region, hereunder a range of megaherbivores, suggest that effects on ecosystem structure end functions must have been large and at least on par with the extant situation in non-defaunated African savanna and forest ecosystems. Indeed, recent studies have estimated that the greater tree density in Neotropical savannas relative to African savannas can be explained by the missing large herbivores, and that the reductions in the herbivore guild have decreased seed and nutrient dispersal rates (Doughty et al., 2016a,c).
Ecological relevancy of historical vs. prehistoric base-linesGiven the widespread current and historical defaunation across the region, trophic rewilding with a historical base-line is relevant as part of ecological restoration efforts throughout the region, if poaching and other defaunation drivers can be controlled. As outlined for Brazil's Atlantic Forest (Galetti et al., 2017a), many small- to moderately-sized extant vertebrate species (above and below the 10-kg threshold used in our study) are relevant to consider and relatively unproblematic to integrate into restoration projects. However, considering the biogeographic history of the region is also clear that even pre-defaunation historical state (pre-Columbian) is a recent (last 7000–14,000 years, depending on the area) and evolutionarily unusually megafauna-poor state. Most macroscopic species have evolved prior to end-Pleistocene and early Holocene megafauna extinctions (e.g., Rull, 2008; Fernandes et al., 2014) and hence have evolved under megafauna-rich conditions, with many species likely exhibiting adaptations to such settings (Janzen and Martin, 1982; Galetti et al., 2017b), and it is under these conditions that the Neotropics’ staggering biodiversity has evolved (Rull, 2008). Some species are likely to have adaptations that are ecological anachronisms and may be in decline due to lost interactions (exhibiting extinction debts) (Janzen and Martin, 1982; Doughty et al., 2016c) or may have shifted interactions to humans and our domesticates (Galetti et al., 2017b). Hence, a strong ecological case can be made for an end-Pleistocene and early Holocene base-line as a starting point for considering restoration actions in the region, as already discussed by Galetti (2004) for the Cerrado and the Pantanal more than a decade ago.
Trophic rewilding potential – biological and societal possibilities and constraintsThe extent to which our estimated historical or prehistorical trophic rewilding potential can be realized depends on a range of biological and societal possibilities and constraints. Key factors will be the societal circumstances in a given area (hunting pressure, land abandonment, conservation status, potential/realized human–wildlife conflicts), availability and ecological functionality and requirements of species for rewilding introduction, as well as biological risks associated with the introductions. Trophic rewilding can only be a successful restoration approach if it is compatible and accepted by society. First of all, rewilding introductions only make sense when the processes that have driven defaunation are under control, or can be expected to come under control. This especially concerns poaching, which may feedback with the social acceptance of a given rewilding project. A related key issue is human-wildlife conflict, e.g., between ranchers and large carnivores (Quiroga et al., 2016). Such conflicts or potential conflicts need to be carefully addressed. Also key for societal acceptance, any rewilding project will need to consider not just their compatibility with existing land uses, but also if they can offer new ways of improving the local population's livelihoods, e.g., via ecotourism. Furthermore, landscape circumstances will strongly affect if and how rewilding can be implemented, with the amount of area available and its protection status as key factors. Areas undergoing large-scale land abandonment offer particularly large opportunities for rewilding (Navarro and Pereira, 2012), and are increasingly widespread in the Neotropics (Aide and Grau, 2004; Hecht, 2014). Trophic rewilding will be easier to implement in large protected areas, although other settings are also possible, especially if some minimal ongoing management is allowed. Importantly, it should be assessed if an area has potential to harbor a minimum viable population of a species, or, especially if this is not the case, if a population with the required ecosystem functions could still be established with some level of ongoing population management, e.g., using a managed metapopulation approach as done for several large carnivores in South Africa (Miller et al., 2015). In Europe, there are many examples of rewilding projects in relatively small areas (<100ha to <1000ha), where such minimal ongoing population management is part of the implementation (J.-C. Svenning and S. Faurby, pers. obs.).
Given that most of the prehistoric megafauna losses from the region involve global extinctions, sometimes not just of species, but also of functional types (e.g., ground sloths), detailed restoration toward a prehistoric base-line is not feasible from a biological, let alone a societal perspective. In contrast, reintroductions toward a historical baseline are more simple in that the species are generally extant and often in nearby areas, typically providing much more ecological background information and easier social acceptance (Galetti et al., 2017a). Hence, a logical focus for rewilding projects would be on re-establishing historically extirpated species, as has already been done in a number of projects, e.g., giant anteaters (Myrmecophaga tridactyla) to the Iberá wetlands in northern Argentina (Di Blanco et al., 2017). Nevertheless, it is worth considering if the massive prehistoric losses could also be partially overcome – typically via taxon substitutions – to restore lost functional types and their ecological effects (e.g., Galetti, 2004). This will require careful assessment and experimentation with ecosystem and socio-ecological effects of potential substitutes to minimize risks (e.g., Oliveira-Santos and Fernandez, 2010), e.g., competitive exclusion or disease transfer to resident native species as well as human–wildlife conflicts. Here, it will also be important to benchmark risks against realistic alternatives, as no land management scenario will be risk free. Further, it would be ideal to develop a systematic framework for identifying candidates for taxon substitution, based on functional and phylogenetic criteria (Svenning et al., 2016). Additionally, for any concrete area an assessment should be made of geophysical and societal constraints, to inform the selection of species to be restored, e.g., in terms of habitat requirements, population size potential, and any required ongoing management.
Many large mammals have been introduced from other continents to various areas of the region for hunting or accidentally (Merino et al., 2009) and it would make sense to more carefully consider their ability to substitute lost megafauna effects and balance these against any negative effects they might have, rather a priori viewing them negatively. Some of the species that should be re-evaluated include various exotic deer (which might to some extent substitute extinct large deer and other mesoherbivores), feral cattle and water buffaloes (which may substitute extinct large mesoherbivores, despite bovids having never occurred south of Central America), feral pigs and boar (rapidly expanding in Argentina and Brazil), feral dogs (which may substitute extinct large canids), and the hitherto only case of a megaherbivore introduction, namely drug lord Pablo Escobar's famous escaped hippopotamuses in Colombia (Novillo and Ojeda, 2008; Vásquez, 2012; Pires et al., 2014; Ballari et al., 2015; Pedrosa et al., 2015; Lessa et al., 2016; da Rosa et al., 2017). A particularly interesting case concerns horses, as horses have been extremely widespread and common across the region for several millions years, hereunder with taxa conspecific with or extremely closely related to the extant horse (Equus ferus) (Orlando et al., 2008). Hence, horses constitute a particularly obvious candidate for trophic rewilding in the region in addition to the historically native species (Naundrup and Svenning, 2015). After European colonization, horses have been reintroduced and feral populations now exist in many areas (Naundrup and Svenning, 2015). These are generally viewed as cases of exotic species introductions and the horses treated as invasive species, and sometimes sought to be exterminated, or at least be subject to population reductions (Scorolli, 2016). However, given the history it would be more meaningful to manage them as natives throughout the Americas (Naundrup and Svenning, 2015). As there is no guarantee that native species – especially those with strong ecosystem effects – can never have negative effects, especially in interaction with anthropogenic effects, such as habitat reduction and trophic down-grading, viewing horses as natives does not argue against assessing their effects in different kinds of ecosystems and landscape settings, nor that some level of active management may be beneficial in some circumstances. More generally, contrary to popular belief, effects of exotic megafauna are not always negative. For example, a detailed study of niche overlap in feral pigs (Sus scrofa), collared peccaries (Pecari tajacu), and white-lipped peccaries (Tayassu pecari) in the Pantanal concluded that due to low niche overlap feral pigs are not currently a direct threat to the peccaries in the study area (Desbiez et al., 2009). Similarly, a study from high Andean desert in northern Chile concluded low potential for competition between taruka (Hippocamelus antisensis) and feral donkeys (Equus asinus) due to differing habitat preferences (Fuentes-Allende et al., 2016). Feral horses in a Pampas area in Argentina were found to promote some native grasslands birds, while having negative effects on others (Zalba and Cozzani, 2004), while moderate cattle densities were suggested to benefit raptor diversity in a Peruvian forested area (Piana and Marsden, 2014). We are not suggesting that exotic species are not sometimes problematic, as there are certainly cases where there appear to be strong negative effects on resident native species (e.g., Martin-Albarracin et al., 2015), or societal costs may be high, e.g., from risk of disease transfer to domestic animals (e.g., Pedrosa et al., 2015). In contrast, we are simply suggesting that the effects of exotic megafauna should be carefully evaluated in the light of the high megafauna deficits in the region relative to a prehistoric baseline.
Conclusion and perspectiveThe estimated present-natural distributions for Neotropical megafauna indicate high potential for trophic rewilding across the region both considering recently (post-1500 AD) extirpated species and species lost earlier in the Holocene or latest Pleistocene. Notably, the potential is very high throughout the region for the prehistoric baseline (with >20 megafauna species missing in many regions), with eastern savanna regions (e.g., Cerrado and Chaco regions) of South America having particularly high values. This recent prehistoric perspective is important to consider for ecological restoration in the region, as it represents the ecological conditions under which the region's rich biodiversity has evolved, in contrast to a recent historical base-line. Still, the latter can also be useful, notably due to the often greater ecological knowledge available and easier societal acceptance (Galetti et al., 2017a). We highlight the ecological impacts of already established populations of non-native megafauna species should be re-assessed in the light that they may represent taxon substitutions for extinct species and need not have negative effects on local biodiversity. We emphasize that trophic rewilding should be implemented flexibly and in dialogue with society, as it can only be a successful restoration approach if it is compatible with and accepted by society. Hence, it is key to carefully assess risks and develop solutions, e.g., to handle human–wildlife conflicts and ensure benefits to local livelihoods.
Conflicts of interestThe authors declare no conflicts of interest.
J.-C.S. was supported by the European Research Council (ERC-2012-StG-310886-HISTFUNC) and the Carlsberg Foundation (“Semper Ardens” grant: CF16-0005-MegaPast2Future). J.-C.S. also considers this work a contribution to his VILLUM Investigator project “Biodiversity Dynamics in a Changing World” (grant 16549).