We examine the feasibility of improving connectivity between of two dryland woodland types (“espinal”, a savanna type habitat, and sclerophyllous forest) in the Mediterranean climate zone of Chile, for restoration purposes. The restoration approach we examine involves the reintroduction of guanacos (Lama guanicoe) in a “transhumant rewilding” model. The guanacos stimulate the growth of Acacia caven in espinal through browsing, and also provide seed and nutrient dispersal and connectivity between the two woodland types. As in many drylands, degradation is problematic in this region, but clear criteria or maps of degradation are lacking. Thus to assess where restoration is required, how degradation has varied with ENSO across years, and how degraded areas are distributed across the region, we construct an index of woodland condition from Normalized Difference Vegetation Index (NDVI), Net Primary Production (NPP) and evapotranspiration (ET), derived from MODIS data at 1km resolution for each year between 2000 and 2013 inclusive. We construct a least-cost path for each year, which models the possible transhumant movements of herded guanacos between low and high quality woodlands each year. We find highly fragmented areas of espinal, sclerophyllous forest, and overlap between these, which we identified as sucessional habitat. We also found a fully-connected network linking low-quality and high-quality fragments of all types of woodland in each year. This shows that guanaco transhumant herding has the capacity to be used to create regional connectivity and restoration services.
Restoration of dryland forests around the world is an important challenge for conservation and sustainable development (Maestre et al., 2012; Newton et al., 2012; Crouzeilles et al., 2016). Woodlands in semi-arid habitats play significant roles in absorbing global atmospheric carbon and safeguarding the livelihoods of human populations (Michon, 2011; Michon et al., 2013; Maestre et al., 2012; Gamfeldt et al., 2013; Rockström et al., 1999). Tree diversity in woodlands contributes to soil carbon storage, hydrological cycling, and food security (Gamfeldt et al., 2013; Rockström et al., 1999). In some cases, as in the Mediterranean habitats, they also have high biodiversity and endemism (Cowling et al., 1996; Underwood et al., 2009). The combined effects of climate change, land cover change and widespread anthropogenic disturbances are expected to severely impact the resilience of many ecosystems (Scheffer et al., 2009). Seasonally dry and dryland forests of South America are no exception, suffering from overgrazing by livestock, overhunting of forest species, deforestation and land-cover change (Redford et al., 1990; Leal et al., 2005; Newton et al., 2012; Ribeiro et al., 2015; Salazar et al., 2015; Hernandez et al., 2015). Water limitation, especially when variable, may reduce the ability of semi-arid habitats to recover from overgrazing, woodcutting, fire, biopedturbation, erosion, and other anthropogenic or non-anthropogenic disturbances (Holmgren et al., 2001). Consequently, understanding how to avoid or recover from woodland degradation, and how to improve resilience to degradation, is an important challenge for conservation ecology. Novel restoration and conservation models designed for the socio-ecological contexts of dryland forests are needed to stimulate the development of sustainable futures.
Measuring woodland degradation and resilience is difficult. Degradation is generally understood as a reduction in productivity due to human disturbance or land-use change (Gibbs and Salmon, 2015). There are several global measures of degradation that use different proxies of degradation (Gibbs and Salmon, 2015). Similarly, woodland resilience, which prevents degradation, is hard to measure due to multiple concepts of what resilience means (Newton and Cantarello, 2015). Newton and Cantarello (2015) make several practical suggestions about how to manage for different resilience-related restoration goals in woodlands. These include supporting forest succession processes to achieve ‘engineering resilience’ or time to return to a single stable state, reintroducing species whose ecological functions improve capacity to self-organize under changing conditions (‘adaptive capacity’), increasing resistance to disturbances through e.g. increasing disturbance-tolerant species (‘resistance’), and at a landscape scale, increasing connectivity and seed dispersal (‘landscape resilience’). Here, we look at the connectivity that could be gained through reintroducing a species with important ecological functions in central Chile.
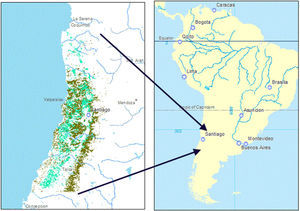
Central Chilean habitats, including sclerophyllous forest, matorral (shrub habitat), and espinal, suffer from inadequate conservation, high fragmentation and land-cover change (Armesto et al., 2010; Schulz et al., 2010; Hernandez et al., 2015). Estimates of rates of deforestation and conversion to agriculture in central Chile vary considerably at a sub-regional scale, with some pointing to net deforestation and lack of forest recovery (Schulz et al., 2010; Van de Wouw et al., 2011), while others observe land abandonment and recovery of sclerophyllous forest (Hernandez et al., 2015). Patterns of change in land cover in central Chile over the past 50 years can be traced to changes in government intervention and economic drivers for different agricultural products, primarily for export (Hernandez et al., 2015; Kurtz, 2001).
Climate variation in the form of the El Ñiño Southern Oscillation (ENSO) is also likely to interact with anthropogenic factors to affect the ecological condition or degradation of central Chilean woodlands. In central Chile, warm ENSO phases lead to increased rainfall and cold ENSO phases lead to drought. ENSO precipitation pulses affect tree, shrub, and herbaceous recruitment patterns (Gutiérrez et al., 1997; Gutiérrez and Meserve, 2000; Meserve et al., 2003; Holmgren et al., 2001; Holmgren et al., 2006; López et al., 2008). Fragmentation is likely to further impact woodland degradation and slow forest recovery by preventing the exchange or circulation of ecosystem functions such as seed dispersal between matorral and espinal (Root-Bernstein and Jaksic, 2015) or between espinal and sclerophyllous forest (Root-Bernstein et al., 2017).
Restoration of sclerophyllous forests should consider the successional processes that lead to its formation (Newton and Cantarello, 2015). Sclerlophylous forest and espinal (Fig. 1) have recently been shown to be linked by successional processes (Root-Bernstein et al., 2017), although it is not yet clear whether they form a continuously shifting mosaic or whether the sclerophyllous forest represents a natural historical stable state. The long-term precipitation instability caused by ENSO, along with a prehispanic history of probable human disturbance, argues for a shifting mosaic (Holmgren et al., 2001; Root-Bernstein et al., 2017).
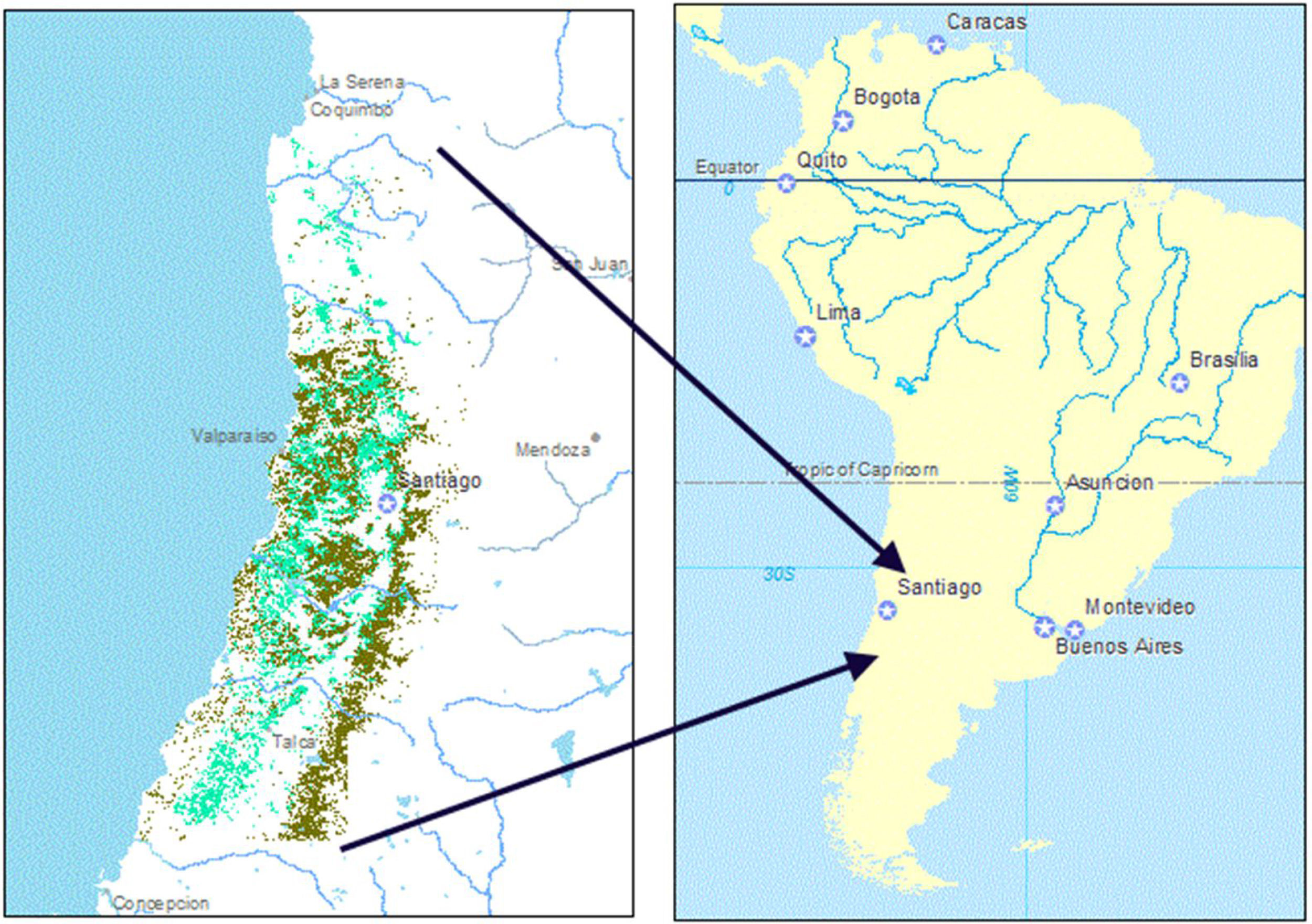
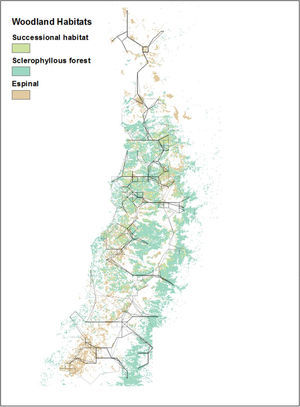
Distribution of espinal (light green) and sclerophyllous forest (dark green) within the study region (IV Region (Coquimbo) through the VII Region (Maule)), corresponding to central Chile, shown in the South American context. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
One approach to restoration of schlerophyllous and espinal woodlands that shares several features with the approach recommended by Newton and Cantarello (2015), is rewilding, or the (re-)introduction of species for ecosystem restoration via their trophic and non-trophic interactions, usually under passive management (Svenning et al., 2016; Lorimer et al., 2015). Camelids have been identified as excellent candidates for dryland rewilding (Root-Bernstein and Svenning, 2016). Guanaco (Lama guanicoe) rewilding in central Chile could provide regional-scale connectivity between sclerophyllous woodland espinal and facilitate successional processes, thus potentially addressing three of Newton and Cantarello's (2015) types of resilience to degradation, through facilitating succession, increasing connectivity, and reintroducing species with key ecological functions.
The transhumant rewilding model of guanaco reintroduction is based on the evidence that guanacos and their recent direct ancestors formerly lived in semi-arid woodlands in central Chile, and throughout South America (Root-Bernstein and Svenning, 2016). We hypothesize that their loss from central Chilean woodlands due to overhunting during the colonial period (about 500 years ago) may have contributed to altered successional dynamics and slow recovery from degradation (Van Uytvanck and Verheyen, 2014; Root-Bernstein et al., 2016; Root-Bernstein et al., 2017). However, the contemporary landscape is significantly altered. Due to considerable woodland fragmentation due to anthropogenic land conversion in the region (Schulz et al., 2010; Hernandez et al., 2015; Hernández et al., 2016), we argue that wild populations of guanacos would be exposed to human-wildlife conflict, and that moving down the gradient between “wild” and “domestic” to a management relationship similar to reindeer herding may be more feasible (hence “transhumant rewilding” Root-Bernstein et al., 2016). Specifically, the model we propose involves herding guanaco to espinals that require restoration in winter, when the Acacia caven trees have no or few leaves and compensatory growth should be stimulated most strongly, and to espinals in better condition in summer, when the trees have leaves (Oba et al., 2000; Cromsigt and Kuijper, 2011). The reverse phenology of A. caven and the herbaceous understory ensure that the guanacos always have complementary food sources. Guanacos are generalists and feed on both leaves and branches of trees (including Acacia caven, Peumus boldus, Cryptocarya alba, Lithraea caustica, and Colliguaja integerrima in central Chile, Root-Bernstein et al. unpublished results; as well as Nothofagus pumilio (Cavieres and Fajardo, 2005), various tree seeds including Fabacaea seeds (Campos et al., 2008), as well as herbaceous species (Puig et al., 2001), grasses and shrubs (Rivals et al., 2013). Guanacos carry out multiple ecosystem functions, including seed dispersal within their territories and potentially during natural seasonal migration, stimulation and alteration of tree growth through browsing, and formation of paths and dung middens (Fuentes et al., 1989; Fuentes et al., 1986; Root-Bernstein et al., 2016; unpublished results Root-Bernstein et al.). Guanacos can thus be thought of as mobile link species, whose historical intra-range and migratory movements would have provided large-scale connectivity in these habitats in the past (Lundberg and Moberg, 2003; Kremen et al., 2007). We thus speculate that guanaco reintroduction can improve successional processes, connectivity, and potentially improved drought recovery or resistance to drought due to past growth, in both sclerophyllous and espinal woodlands.
Here, we examined the effect of ENSO-related climate variation on woodland ecological condition across fragments of both woodland types, and on the inter-year connectivity of the proposed transhumant rewilding network. We thus expand our focus from espinal (Root-Bernstein et al., 2016) to include sclerophyllous forest, and use a more-complete and higher-resolution data set compared to our previous study. This is significant because the successional links between espinal and sclerophyllous forest are only beginning to be understood and have not yet been integrated into restoration recommendations. We approximate woodland degradation as “ecological condition” using productivity-related proxies, but due to a lack of a baseline or historical reference state for these woodland habitats, we answer the baselines question “degraded compared to what?” by comparing across the region and across ENSO conditions.
MethodsWe obtained woodland distribution data inclusive of the IV Region (Coquimbo) through the VII Region (Maule), corresponding to central Chile and including the Mediterranean-climate zone. We used a map of espinal previously derived from the Chilean Forestry Corporation CONAF's data on tree species distributions, by mapping areas where A. caven occurs (Root-Bernstein et al., 2016). To find the distribution of sclerophyllous forest, images of the map of “native forest and plantations” were captured from CONAF's online map service (sit.conaf.cl, visited May 2016). These images were then georeferenced and the distribution of native (sclerophyllous) forest was extracted. Additionally, where espinal and sclerophyllous forest overlapped, we extracted a vegetation type distribution that we identify as successional habitat between espinal and sclerophyllous forest, following Root-Bernstein et al. (2017). These data were updated in different years: Coquimbo in 2008 (only in biodiversity priority sites, otherwise in 2003), in Valparaiso in 2001, in the Región Metropolitana in 2001, in O’Higgins in 2005, and in Maule in 2009 (Ruiz-Tagle et al., 2011). Yearly land use change data are not available. Although land conversion is ongoing in central Chile, the rates of woodland cover loss vary widely between sub-regional areas (Hernandez et al., 2015; Schulz et al., 2010; Van de Wouw et al., 2011). Furthermore, although woodland may be lost abruptly due to land-use conversion, gains in woodland area and transitions between woodland types are slow, at a 20–50 year timescale (Root-Bernstein et al., 2017; Hernandez et al., 2015; Schulz et al., 2010; Van de Wouw et al., 2011). It is unlikely that there are woodlands missing from our maps, although the maps may overestimate woodland area in some places.
Normalized Difference Vegetation Index (NDVI), Net Primary Production (NPP) and evapotranspiration (ET) were derived from MODIS data at 1km resolution for each year between 2000 and 2013 inclusive. The NDVI provides a measure of plant photosynthetic activity or vegetation greenness. NPP is a measure of biomass creation minus plant respiration. Evapotranspiration is the transfer of water to the air, in this case from plants and the soil. Evapotranspiration is necessary for photosynthesis, which is necessary for primary production. However, plants can be more or less efficient at different steps in converting energy and water into biomass, so the three measures give different views of this overall process. Evapotranspiration MODIS data (MOD16A3) was downloaded from ftp://ftp.ntsg.umt.edu/pub/MODIS/NTSG_Products/MOD16/MOD16A3.105_MERRAGMAO/. NDVI MODIS data (MOD13Q1) was downloaded from http://e4ftl01.cr.usgs.gov/MOLT/MOD13Q1.006/. NPP MODIS data (MOD17A3) was downloaded from http://e4ftl01.cr.usgs.gov/MOLT/MOD17A3H.006/. NDVI was split between summer (December) and winter (June). This was done in order to distinguish between the herbaceous understory production, which peaks in winter-spring, from the A. caven canopy production in espinal and successional habitat, which has an opposite phenology and peaks in summer. Different processes (e.g. overgrazing) and periods of rainfall and drought may affect the condition of the understory vs. the canopy, although a good-condition canopy should improve the condition of the understory (Olivares, 2006). Sclerophyllous forest tree species are either evergreen or semi-deciduous, having the same phenology as the understory (Hoffmann, 2012). The remote sensing data was unavailable or incomplete for 2002 and 2007, so those years were excluded from analysis.
Our index of habitat condition consisted of a weighted sum of NDVI, NPP and ET, with a range of 0–400. We constructed an annual index, which consisted of ET+NPP+Dec NDVI+June NDVI. The annual index included both seasons’ NDVI to reflect the contribution of both trees (December NDVI) and herbaceous layers (June NDVI) to high or low ecological condition and need for restoration. By contrast, the summer index is intended to identify the highest quality habitats not requiring restoration, during summer when water is limiting and these habitats may be particularly susceptible to degradation from overgrazing, erosion, drought, and fire. The summer index thus was defined as ET+NPP+2* Dec. NDVI. The summer NDVI is weighted ×2 in order to maintain the same relative weighting of ET, NPP, and NDVI data as in the annual index.
Least cost paths represent physical paths across a landscape, which minimize movement costs or barrier-crossing. We used the same cost raster (spatially defining the costs and barriers to be avoided) as Root-Bernstein et al. (2016), which combined high elevation, urban areas and roads as features to be avoided. We do not take into account rivers as a barrier, as in central Chile they are shallow and highly variable in flow volume. Not all the cost raster inputs were available at 1-km resolution. Consequently, we retained the 8-km resolution data. This means that the least cost paths are also at the coarser, 8-km resolution. We calculated least cost paths linking woodland fragments with low-quality annual indices of ecological condition, defined as ≤200, with woodland fragments with high-quality summer indices of quality ≥250. Value cut-offs were defined to exclude the middle two standard deviations of values across years, rounding to the nearest unit of 50. The least cost paths connect areas of contiguous “low quality” raster cells to areas of contiguous “high quality” raster cells. These clusters, while defined from 1-km resolution data, varied in size. To understand how these woodland fragments might be connected by the least-cost paths, we extracted a 10-km buffer around the paths and looked at the area of each woodland type within that buffer, accessible from the least-cost path. We interpret this approach as producing “main arteries” that would be fed by smaller-scale subsidiary paths connecting woodlands within a 10km radius. Those smaller paths are not modeled. We believe that the main artery approach is in any case more appropriate for answering our research questions since we are unable to take into account numerous fine-scale and contextual details such as fences, land tenure, temporal variation in impassibility of rivers and streams, and actual land use change. 1km scale least cost paths would thus be highly hypothetical, while 8km scale paths with a buffer give a general overview.
The goal of the analysis was twofold: first to find low-quality espinals and sclerophyllous woodlands where guanaco browsing and other ecological roles could contribute to restoration, and second to find high-quality espinals and sclerophyllous woodlands where guanacos could feed to ensure their body condition and provide connectivity between better condition and worse condition areas. Browsing is expected to be most effective when A. caven has no leaves (winter), so we look for sites to be restored using the annual index, which guanacos could visit in winter. We then look for sites with high-quality summer indices where guanacos could go to feed, providing landscape-level connectivity, in summer.
We calculated the total obtained areas for each habitat type in central Chile. We then asked whether summer NDVI, primarily representing tree canopy in espinals, and winter NDVI, primarily representing the herbaceous layer in espinals, are correlated within years, and how this depends on ENSO. ENSO condition was classed according to the dominant state or pair of states during the year (since ENSO phases do not usually accord with the calendar year), including dry, wet, and normal (non-ENSO), using data from http://www.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ensoyears.shtml (accessed May 2016). Warm ENSO phases lead to increased rainfall in Chile, while cold ENSO phases lead to drought. We then asked how ecological condition of woodlands is distributed geographically and across woodland habitat types. Finally, we asked how many independent (non-connected) least cost path networks were formed each year, how much they overlap across years, and how much of each woodland habitat type are included in the buffers around the main artery paths each year.
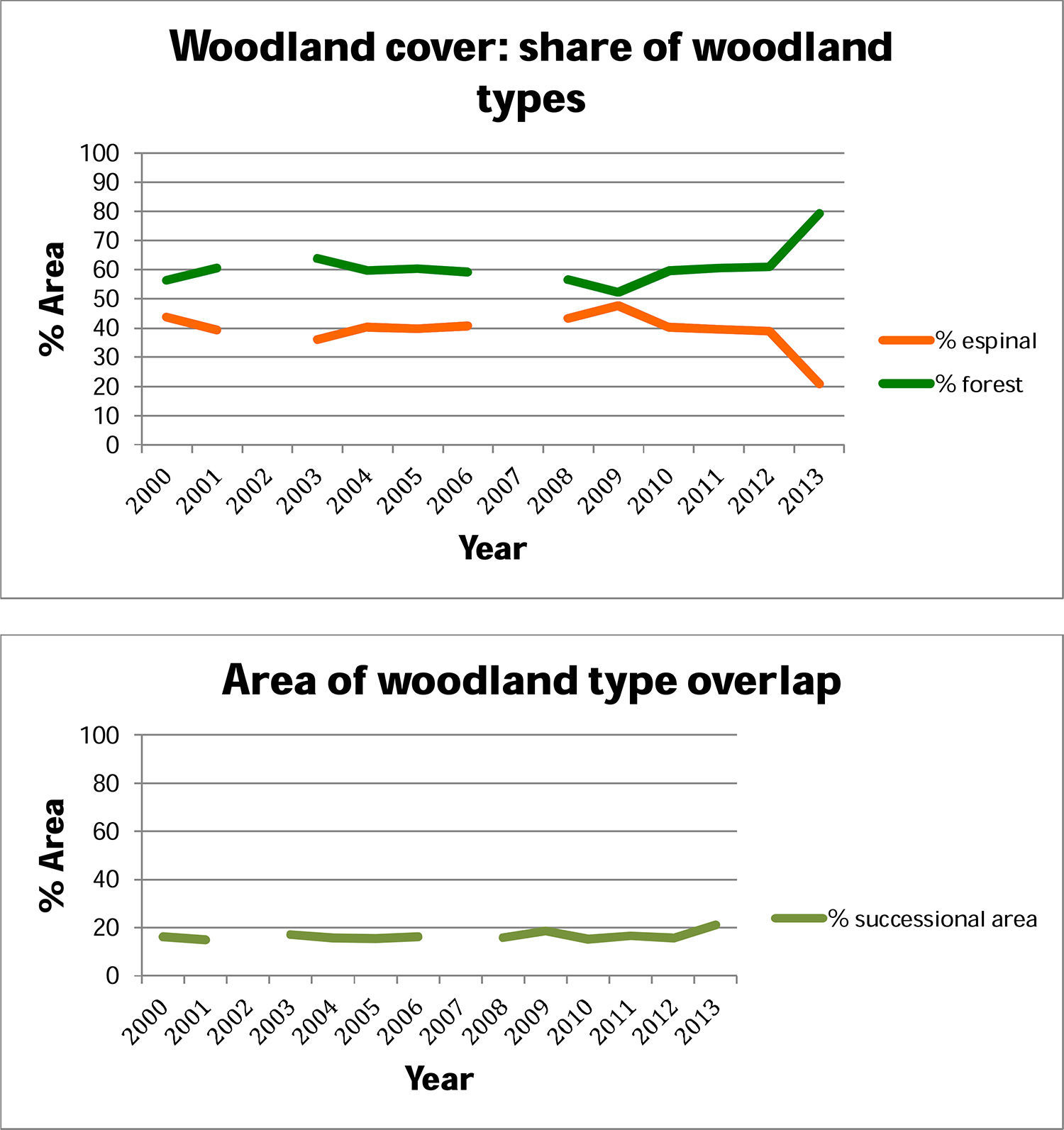
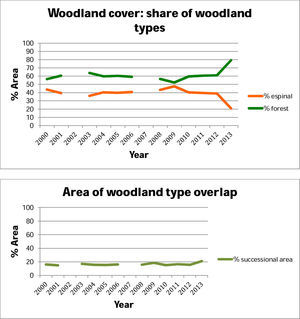
ResultsWe found a total area of espinal of 13,734km2, with a mean fragment size of 0.25km2, and sclerophyllous forest of 24,942km2, with a mean fragment size of 0.09km2. In the overlap of these, we found successional habitat of 4448km2, with mean fragment size of 0.16km2.
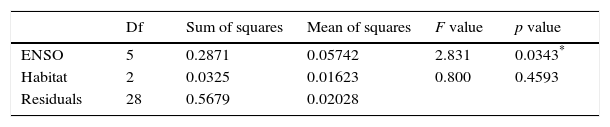
The correlation between winter (June) and summer (December) NDVI showed a large range across years (correlation coefficient 0.2–0.8). ENSO condition, but not habitat type, explained the variation in correlation between winter and summer NDVI (Table 1). A Tukey post hoc test showed that normal-wet years (normal phase followed by wet phase) had significantly higher winter-summer correlations than dry-normal years (dry phase followed by normal phase) (normal-dry years: mean correlation 0.54 SE 0.05; normal-wet years: mean correlation 0.75 SE 0.03).
Results of the analysis of variation in correlation between winter and summer NDVI across years (correlation coefficients range 0.2–0.8, n=12 years).
| Df | Sum of squares | Mean of squares | F value | p value | |
|---|---|---|---|---|---|
| ENSO | 5 | 0.2871 | 0.05742 | 2.831 | 0.0343* |
| Habitat | 2 | 0.0325 | 0.01623 | 0.800 | 0.4593 |
| Residuals | 28 | 0.5679 | 0.02028 |
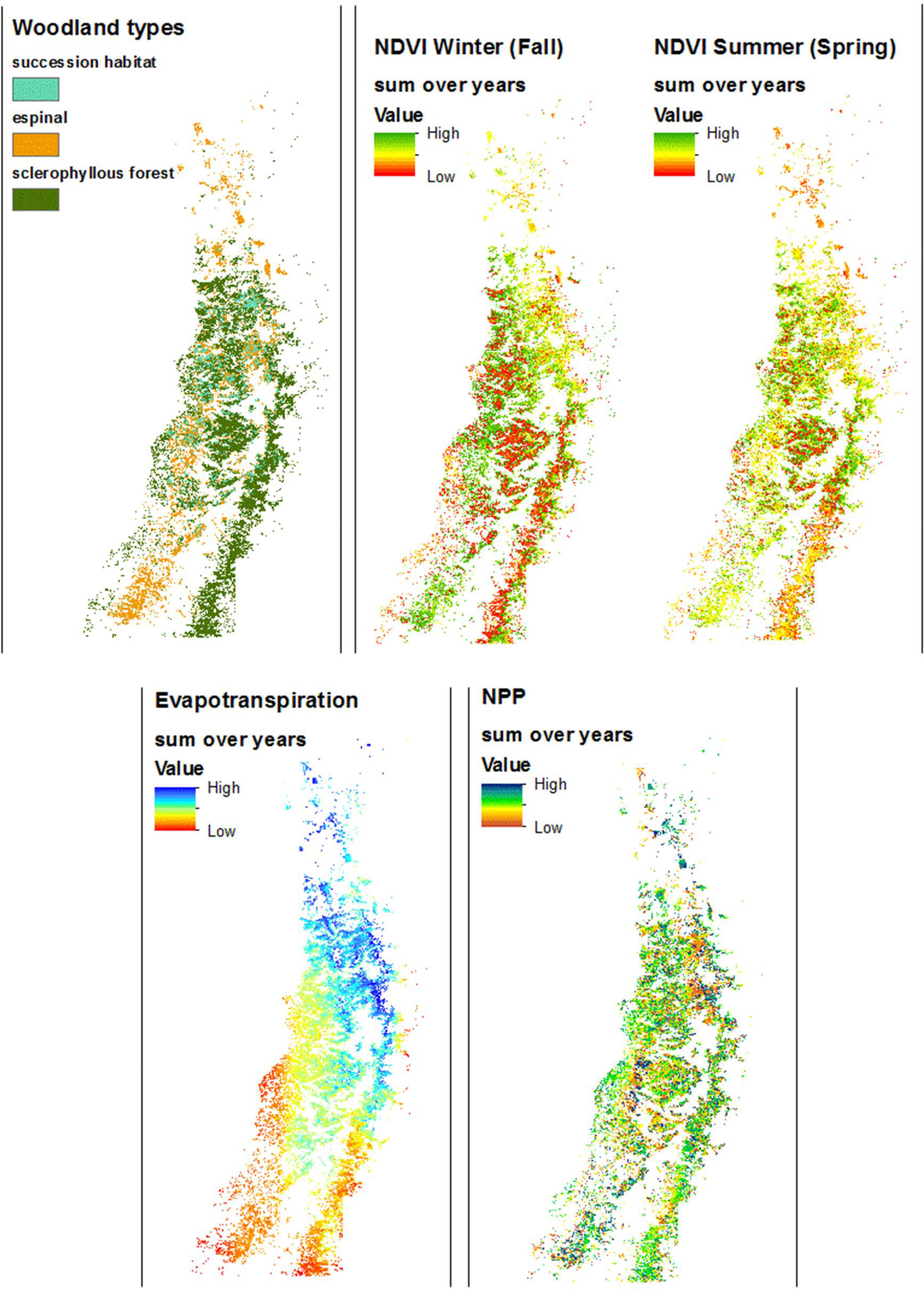
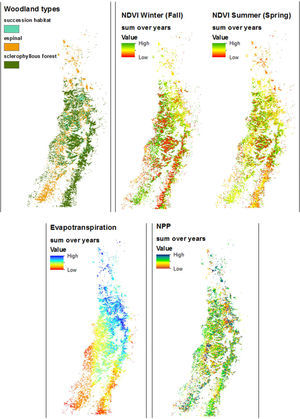
Spatial trends characterized ET and NDVI, but not NPP across the region (Fig. 2). ET shows an obvious gradient from NE to SW. Winter NDVI is clearly higher in espinal habitat and outside the northern third of the woodland distribution. Summer NDVI in the northern half of the distribution is lower in espinal and higher in sclerophyllous forest, while this pattern is inverted at the southern end such that it is higher in espinal and lower in sclerophyllous forest.
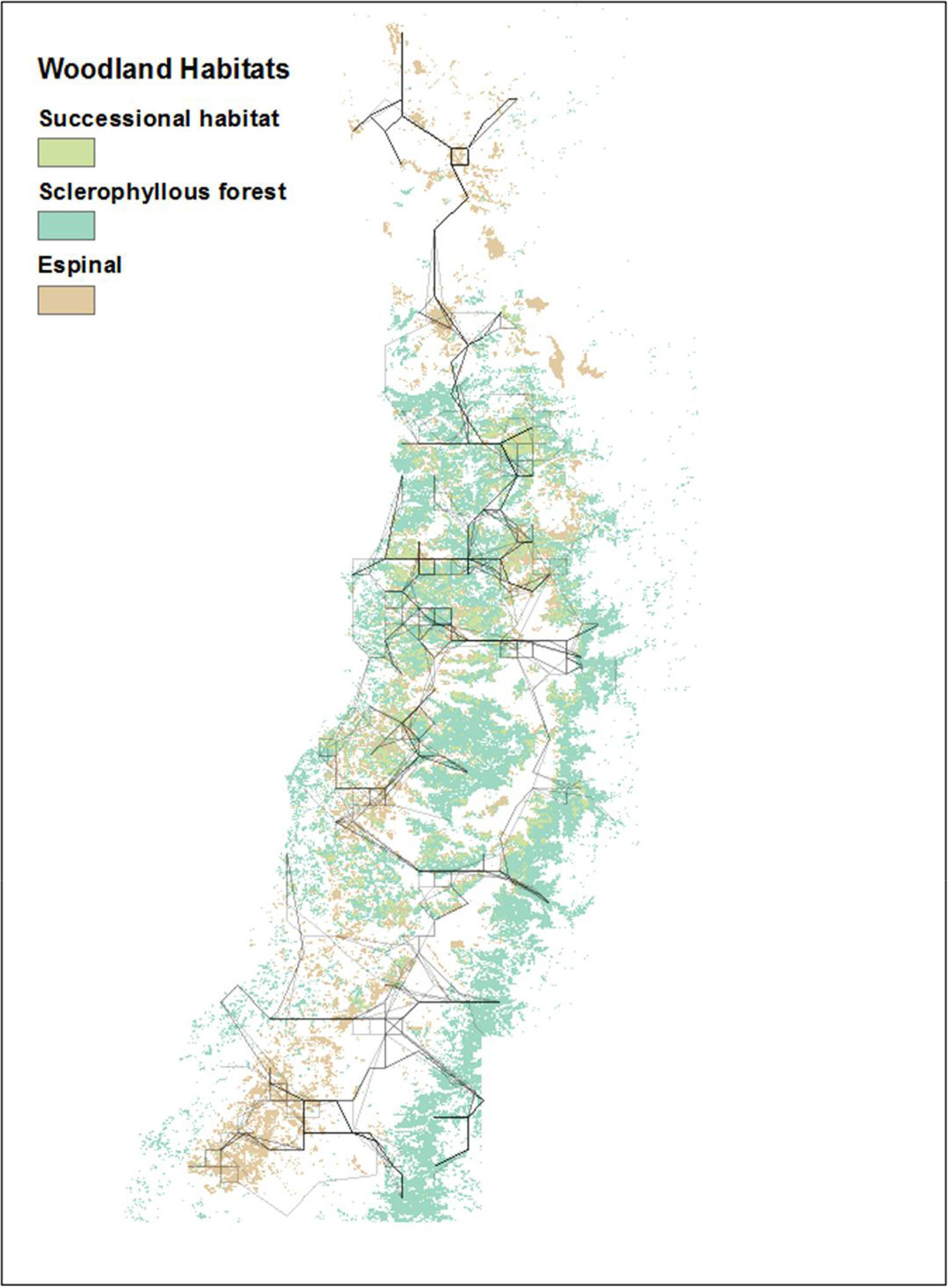
Least-cost paths from high-condition index summer woodland fragments to low-condition index winter woodland fragments (and vice versa) for each year ranged from 3 paths to 10 paths, where all continuously connected lines are considered a single path, and showed a high degree of spatial overlap (Fig. 3). The resulting regional network is fully connected when integrating across years, with six or seven main compartments or horizontally-oriented subsets, linked by fewer vertical paths. In all years, 10-km buffers around the main paths included espinal, sclerophyllous forest, and successional habitat (Fig. 4).
All obtained least-cost paths linking high-quality ecological condition woodlands (in summer) with low-quality ecological condition woodlands (in winter), overlapped (including years 2000–2001, 2003–2006, 2008–2013). Costs, or landscape features to be avoided, were a combination of high elevation, urban areas and roads.
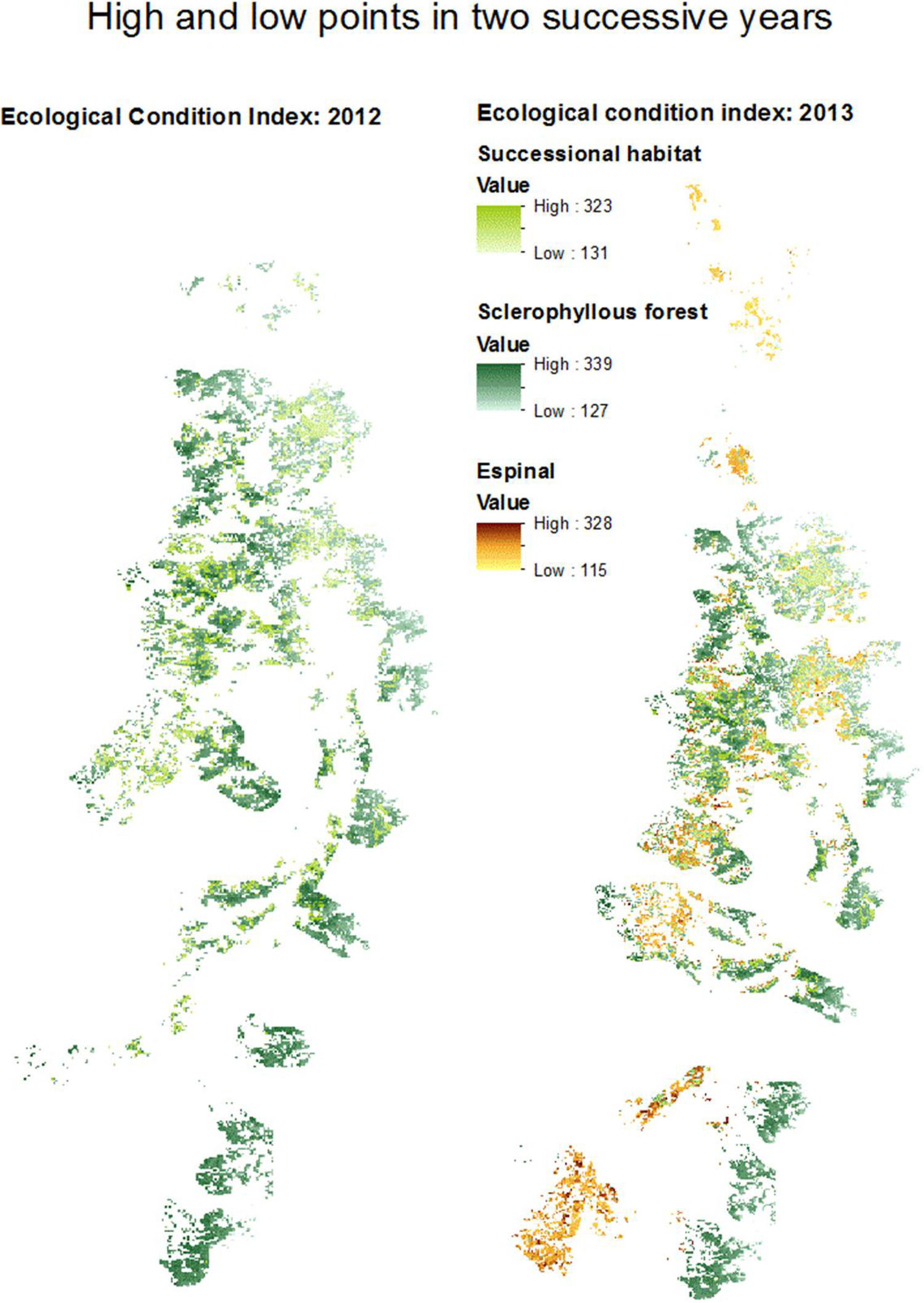
Above, percent woodland that is espinal and sclerophyllous fores, within the 10km buffers around the main arteries for guanaco transhumant rewilding. Below, the overlap between these woodland categories in each year, which we identify as successional habitat, within the 10km buffers around the main arteries.
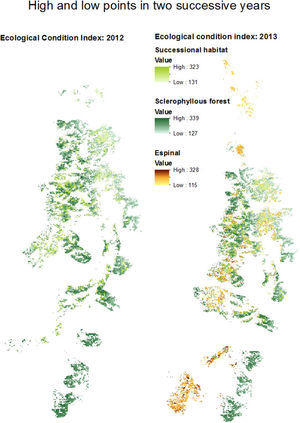
The mean woodland condition values within the buffers around the main arteries showed almost no variation between habitat types and years (data not shown). The buffer area around the main arteries is shown for 2012 and 2013, as an example (Fig. 5). 2012 was a dry ENSO-then-normal year, and 2013 was not an ENSO year (normal). In 2012, all the selected espinal habitat within the buffer area served by the main arteries was also identified as successional habitat (compare Fig. 4).
DiscussionAlthough we found an extent of sclerophyllous forest twice as large as that of espinal, the fragments of sclerophyllous forest were only half the size, on average, compared to espinal. Successional habitat fragments were intermediate in size. Woodlands in low ecological condition and likely to be degraded were found throughout the region. Some of the spatial trends in woodland condition could be explained by climate and geography. The distribution of NPP across years showed strong variation at small scales. Evapotranspiration showed a clear geographic trend, while NDVI varied across seasons and geographically, due largely to the opposite phenologies of A. caven in espinal, and deciduous sclerophyllous trees in forest. Of the Chilean sclerophylous trees, about half are deciduous and half are evergreen (Hoffmann, 2012). Dry ENSO phases led to a greater within-year variation in NDVI than did wet ENSO phases, suggesting that central Chilean woodlands show greater response to drought than to high precipitation. Thus we observe that woodland fragment condition does not simply track ENSO but shows inter-fragment variability especially in NDVI and NPP. The NDVI results suggest that woodlands in need of restoration can be identified not by the condition of the woodland after high precipitation, but to its condition following drought.
Potential main arteries linking high and low quality woodlands for guanaco transhumant rewilding linked espinal to sclerophyllous forest. There was variation in the locations of high and low quality sites between years, which is at least partly ENSO-related, via the NDVI portions of the ecological condition index. A regional fully-connected network of main arteries emerges over the period 2000–2013, while individual sub-networks are apparent in each year. This suggests that real-time monitoring of woodland condition could be used to target areas for restoration with guanaco browsing in winter, and high-quality habitats to “rest” the guanacos in summer, within sub-regional organizational units. Other possible benefits of guanaco transhumant rewilding could include improving connectivity via the transport of seeds and nutrients between woodland fragments (e.g. Poschlod and Bonn, 1998; Kremen et al., 2007), as well as the creation of suitable habitat for other species through the guanacos’ disturbance activities (Root-Bernstein and Ebensperger, 2012; Pringle, 2008). In particular, since we find that both sclerophyllous and espinal woodlands could be connected to each other via guanaco transhumance, the successional processes linking these habitats may be favored, leading to the creation of more successional habitat, and, in the long term, sclerophyllous forests (Root-Bernstein et al., 2017).
The connectivity observed also suggests that it could be feasible to maintain a widely interbreeding guanaco population, with transhumant movements across the landscape on scales similar to natural migratory behavior of guanacos (Ortega and Franklin, 1995; Puig et al., 2011; Marino and Baldi, 2014). The transhumant rewilding concept assumes that regional-scale agricultural land abandonment in central Chile is unlikely in the foreseeable future (Newton et al., 2011; Schulz et al., 2011; Hernández et al., 2016; Root-Bernstein et al., 2016), and that guanacos would live in proximity to humans and livestock. It seems unlikely, therefore, that the identified paths could be converted to wildlife corridors along which guanacos and other species could disperse and migrate without human interaction (Rouget et al., 2006; Sawyer et al., 2009; Rabinowitz and Zeller, 2010; Poor et al., 2012; Fremier et al., 2015). A wildlife corridor solution would both help Chile to meet its protected area commitments under the Convention on Biological Diversity in the Mediterranean-climate area of the country (Tognelli et al., 2008; Squeo et al., 2012), and would more closely resemble a standard rewilding approach in which ecological processes and large species are restored in areas without anthropogenic activity (Ceausu et al., 2015). From an ecological perspective, however, transhumance of semi-wild large herbivores has very similar effects to large-scale ungulate migrations (Coughenour, 1991; Avgar et al., 2013). We believe that with attention to preventing and resolving human-wildlife conflicts within a framework of sustainable development (Webber et al., 2007; Treves et al., 2009; Sayer et al., 2013), rewilding can be integrated into human land use practices in anthropogenic landscapes that might otherwise be left to ongoing degradation and land conversion (Lindon and Root-Bernstein, 2015; Root-Bernstein et al., 2016).
MR-B and J.-C.S. were supported by the Danish National Research Foundation Niels Bohr professorship project Aarhus university Research on the Anthropocene (AURA), and MR-B by a FP7 Marie Curie Agreenskills+fellowship. JCS also considers this work a contribution to his VILLUM Investigator project “Biodiversity Dynamics in a Changing World” funded by VILLUM FONDEN.